Abstract
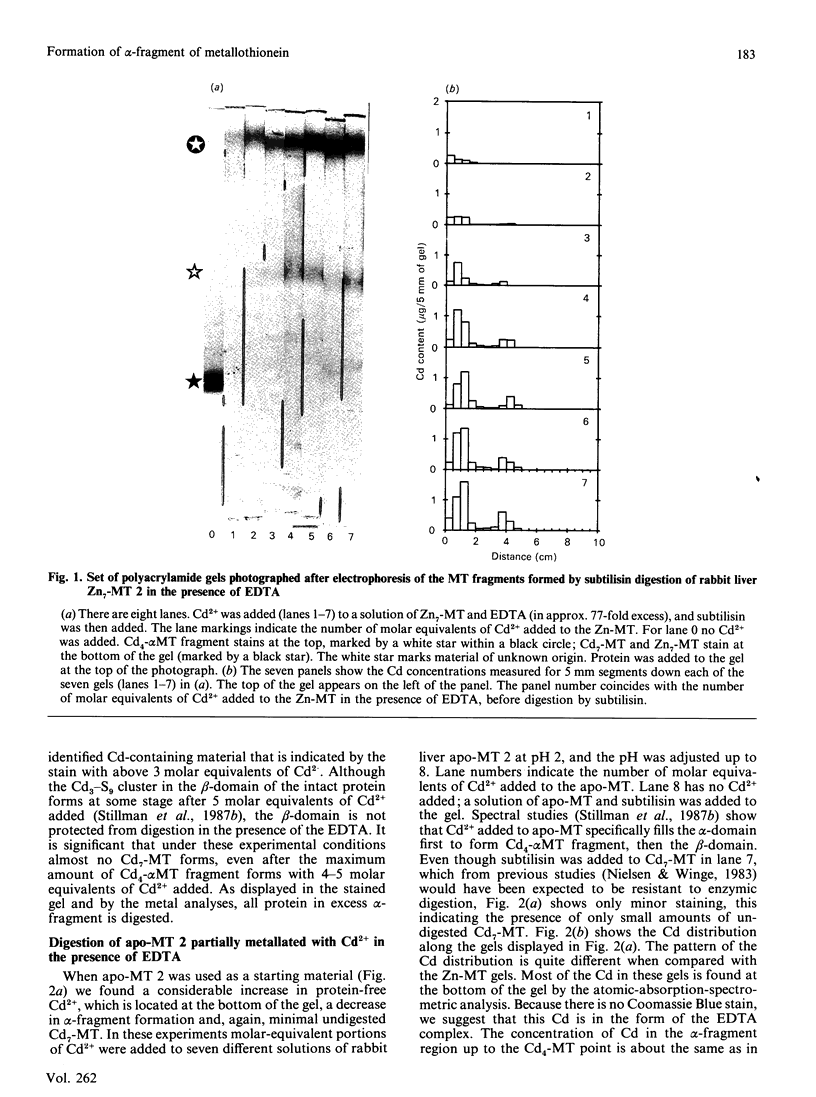
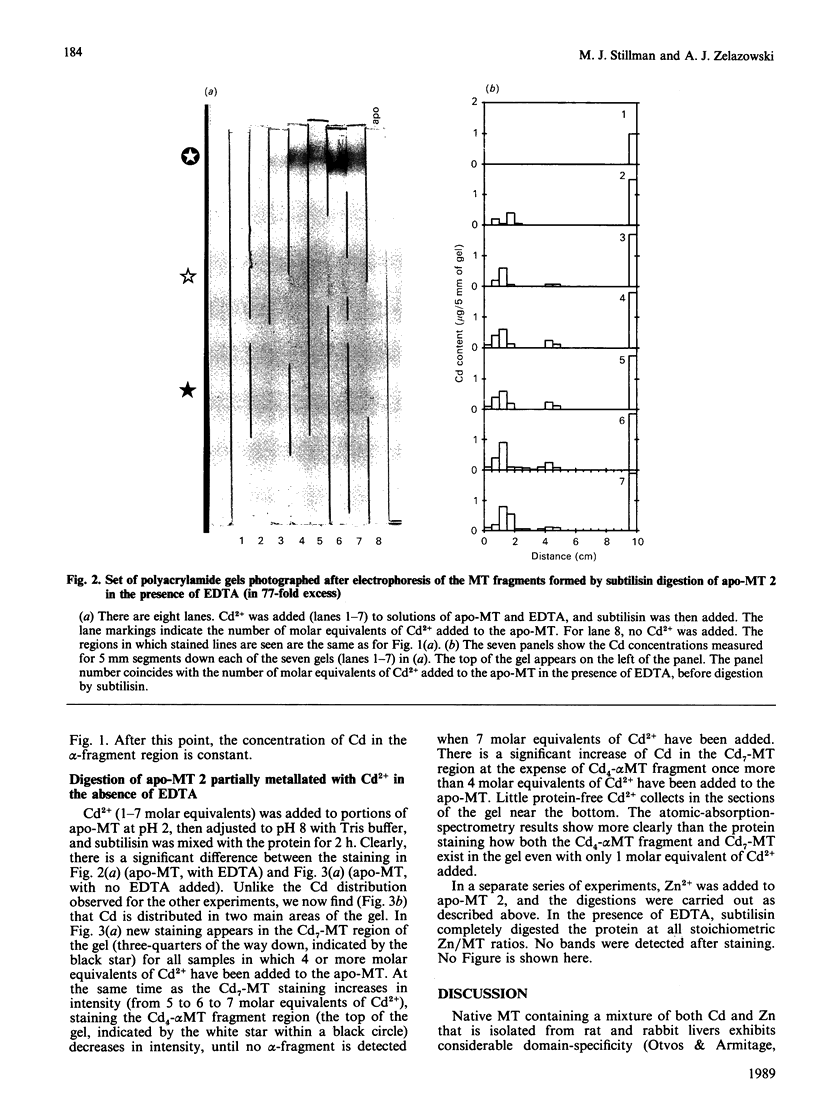
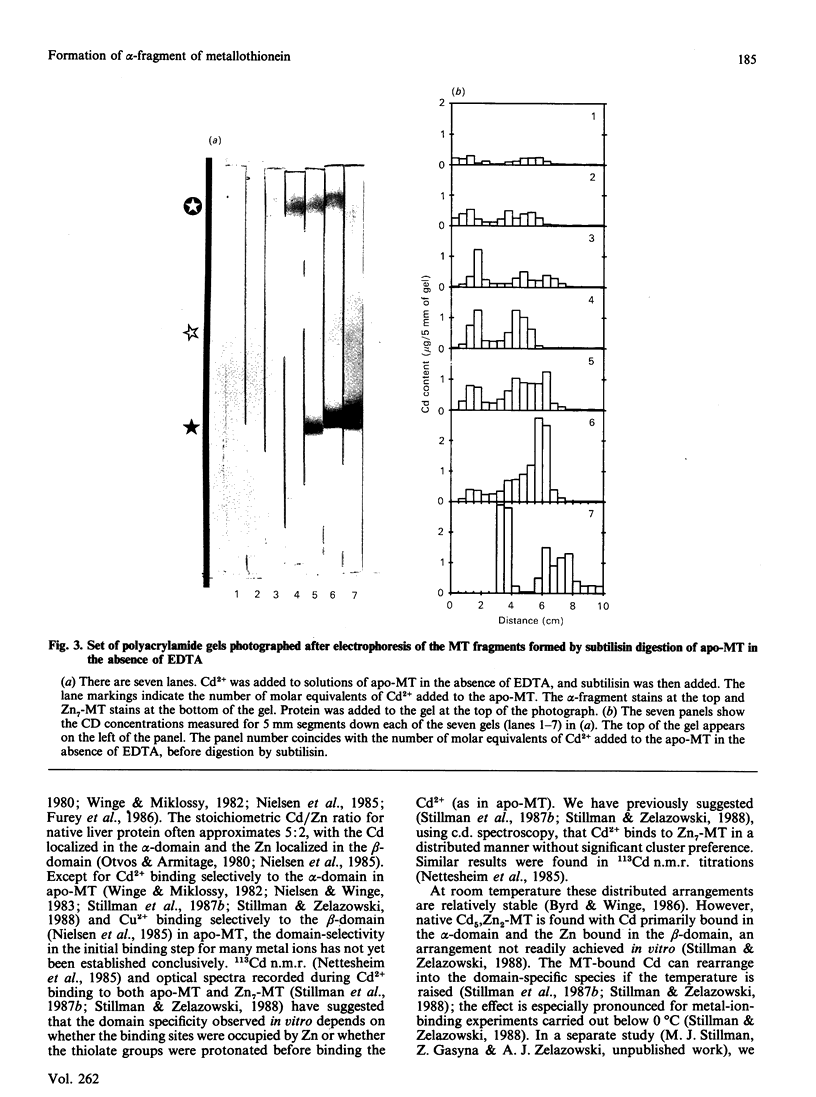
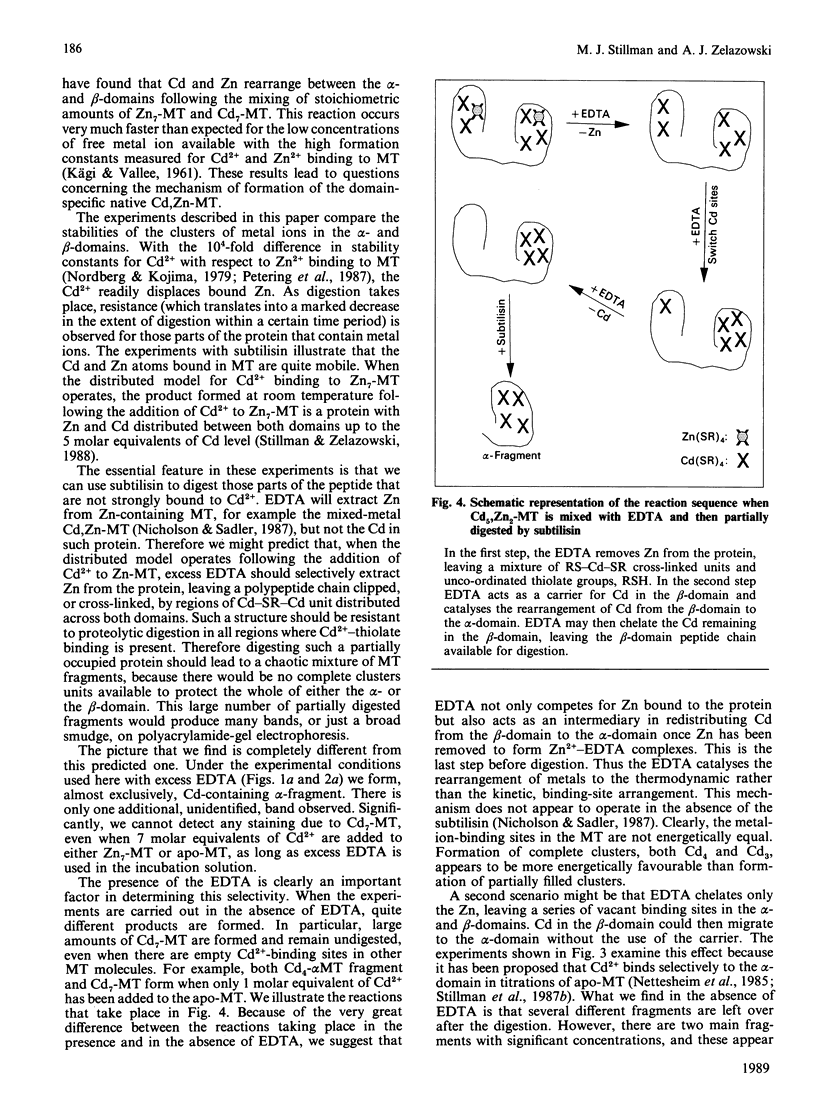
The yield of the alpha-fragment of rabbit liver metallothionein 2 was used to test the domain-specificity and mobility of Cd2+ and Zn2+ when bound to metallothionein. Increasing molar ratios of Cd2+ were added to either Zn7-metallothionein or the metal-ion-free apo-metallothionein. The enzyme subtilisin was used to digest those parts of the peptide chain that were not bound to Cd2+. Analysis of the digestion products was carried out by separation by polyacrylamide-gel electrophoresis. The chelation agent EDTA was used as a competitive chelator. It was found that the presence of excess EDTA greatly enhances the formation of the Cd4-metallothionein alpha-fragment, and catalyses the complete digestion of all other the metal-ion-containing peptides, so that even Cd7-metallothionein, formed when 7 molar equivalents of Cd2+ are added to Zn7-metallothionein, is digested to the alpha-fragment. These results suggest that the Cd2+ bound in the beta-sites is very labile, much more labile than the kinetics of the off-reaction would suggest. The observation of significant amounts of alpha-fragment on the gels, even when the stoichiometry of the metal ions initially present in the protein should not have resulted in much concentration of Cd4-alpha-fragment clusters, indicates that as the digestion proceeds the metal ions move to sites that form complete clusters and therefore selectively protect that part of the peptide chain from digestion. We also find that rabbit Cd4-metallothionein 2 alpha-fragment stains near to the top of the gel, in complete contrast with the location of rat Cd4-metallothionein 2 alpha-fragment. This difference in the mobilities suggests that the alpha-fragment prepared from rabbit metallothionein 2 is much less negatively charged than the analogous protein fragment prepared from rat liver metallothionein 2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrd J., Winge D. R. Cooperative cluster formation in metallothionein. Arch Biochem Biophys. 1986 Oct;250(1):233–237. doi: 10.1016/0003-9861(86)90721-6. [DOI] [PubMed] [Google Scholar]
- Furey W. F., Robbins A. H., Clancy L. L., Winge D. R., Wang B. C., Stout C. D. Crystal structure of Cd,Zn metallothionein. Science. 1986 Feb 14;231(4739):704–710. doi: 10.1126/science.3945804. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Klauser S., Kägi J. H., Wilson K. J. Characterization of isoprotein patterns in tissue extracts and isolated samples of metallothioneins by reverse-phase high-pressure liquid chromatography. Biochem J. 1983 Jan 1;209(1):71–80. doi: 10.1042/bj2090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. Y., Kraker A. J., Shaw C. F., 3rd, Petering D. H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettesheim D. G., Engeseth H. R., Otvos J. D. Products of metal exchange reactions of metallothionein. Biochemistry. 1985 Nov 19;24(24):6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- Neuhaus D., Wagner G., Vasák M., Kägi J. H., Wüthrich K. Systematic application of high-resolution, phase-sensitive two-dimensional 1H-NMR techniques for the identification of the amino-acid-proton spin systems in proteins. Rabbit metallothionein-2. Eur J Biochem. 1985 Sep 2;151(2):257–273. doi: 10.1111/j.1432-1033.1985.tb09096.x. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Sadler P. J., Vasák M. Probing the reactivity of the zinc and cadmium ions bound to rabbit liver metallothioneins with EDTA. Experientia Suppl. 1987;52:191–201. doi: 10.1007/978-3-0348-6784-9_12. [DOI] [PubMed] [Google Scholar]
- Nielson K. B., Atkin C. L., Winge D. R. Distinct metal-binding configurations in metallothionein. J Biol Chem. 1985 May 10;260(9):5342–5350. [PubMed] [Google Scholar]
- Nielson K. B., Winge D. R. Order of metal binding in metallothionein. J Biol Chem. 1983 Nov 10;258(21):13063–13069. [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering D. H., Krezoski S., Villalobos J., Shaw C. F., 3rd, Otvos J. D. Cadmium-zinc interactions in the Ehrlich cell: metallothionein and other sites. Experientia Suppl. 1987;52:573–580. doi: 10.1007/978-3-0348-6784-9_59. [DOI] [PubMed] [Google Scholar]
- Piotrowski J. K., Szymańska J. A., Mogilnicka E. M., Zelazowski A. J. Renal metal binding proteins. Experientia Suppl. 1979;34:363–371. doi: 10.1007/978-3-0348-6493-0_30. [DOI] [PubMed] [Google Scholar]
- Stillman M. J., Cai W., Zelazowski A. J. Cadmium binding to metallothioneins. Domain specificity in reactions of alpha and beta fragments, apometallothionein, and zinc metallothionein with Cd2+. J Biol Chem. 1987 Apr 5;262(10):4538–4548. [PubMed] [Google Scholar]
- Stillman M. J., Zelazowski A. J. Domain specificity in metal binding to metallothionein. A circular dichroism and magnetic circular dichroism study of cadmium and zinc binding at temperature extremes. J Biol Chem. 1988 May 5;263(13):6128–6133. [PubMed] [Google Scholar]
- Stillman M. J., Zelazowski A. J., Gasyna Z. Luminescent Ag12-metallothionein: dependence of emission intensity on silver-thiolate cluster formation. FEBS Lett. 1988 Nov 21;240(1-2):159–162. doi: 10.1016/0014-5793(88)80359-4. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]
- Zelazowski A. J., Szymanska J. A., Law A. Y., Stillman M. J. Spectroscopic properties of the alpha fragment of metallothionein. J Biol Chem. 1984 Nov 10;259(21):12960–12963. [PubMed] [Google Scholar]
- Zelazowski A. J., Szymańska J. A., Witas H. W. Purification of low molecular weight metal-binding proteins by preparative polyacrylamide gel electrophoresis: properties of electrophoretically purified rat liver (Cd, Zn) - metallothioneins. Prep Biochem. 1980;10(4):495–505. doi: 10.1080/00327488008061746. [DOI] [PubMed] [Google Scholar]