Abstract
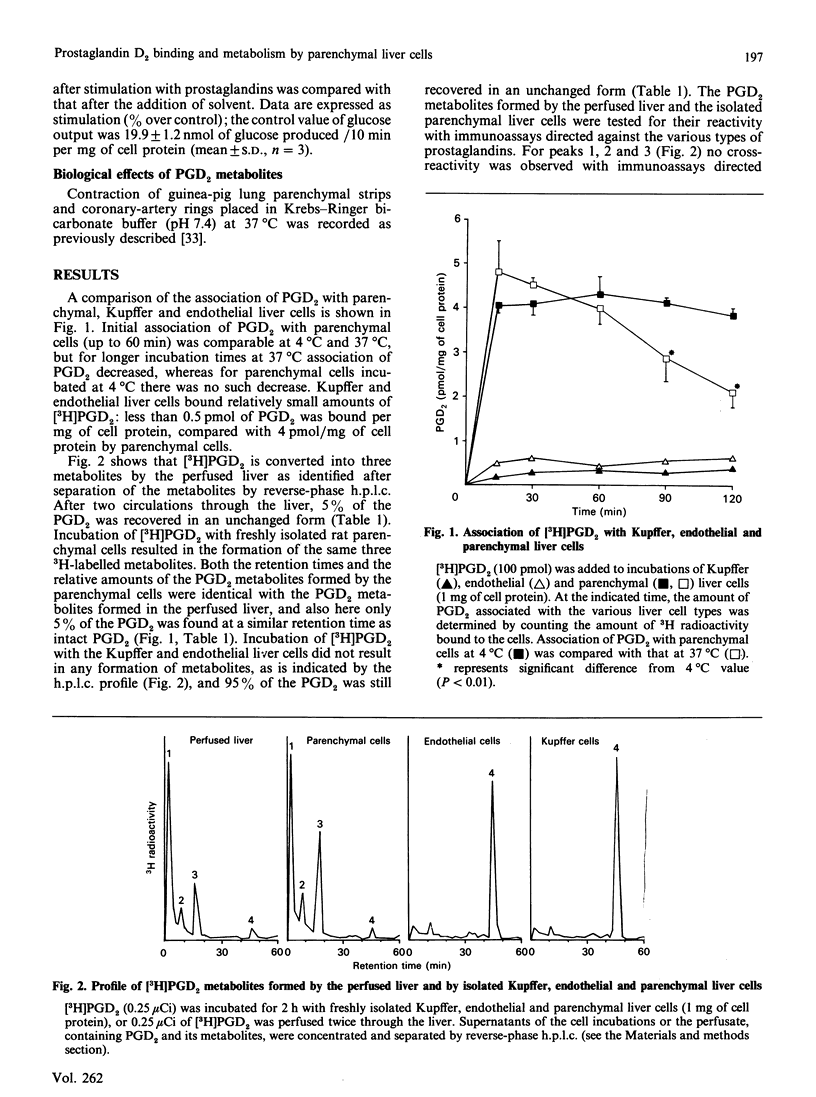
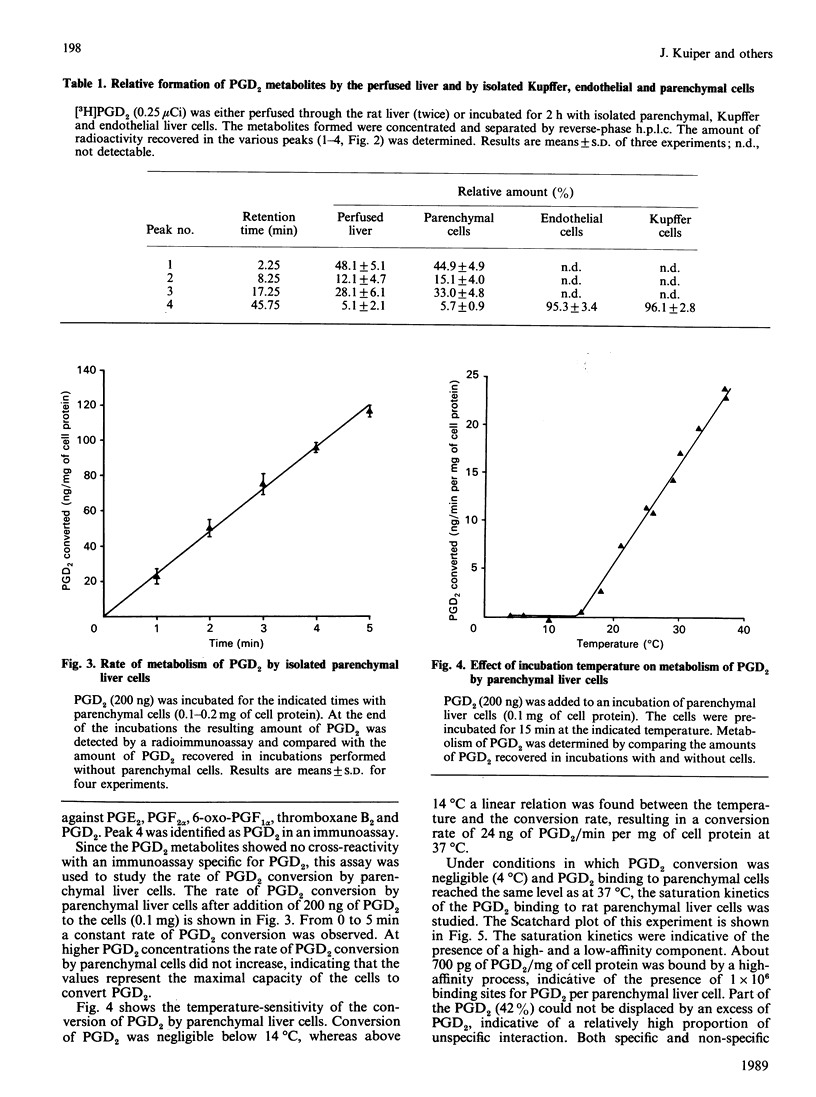
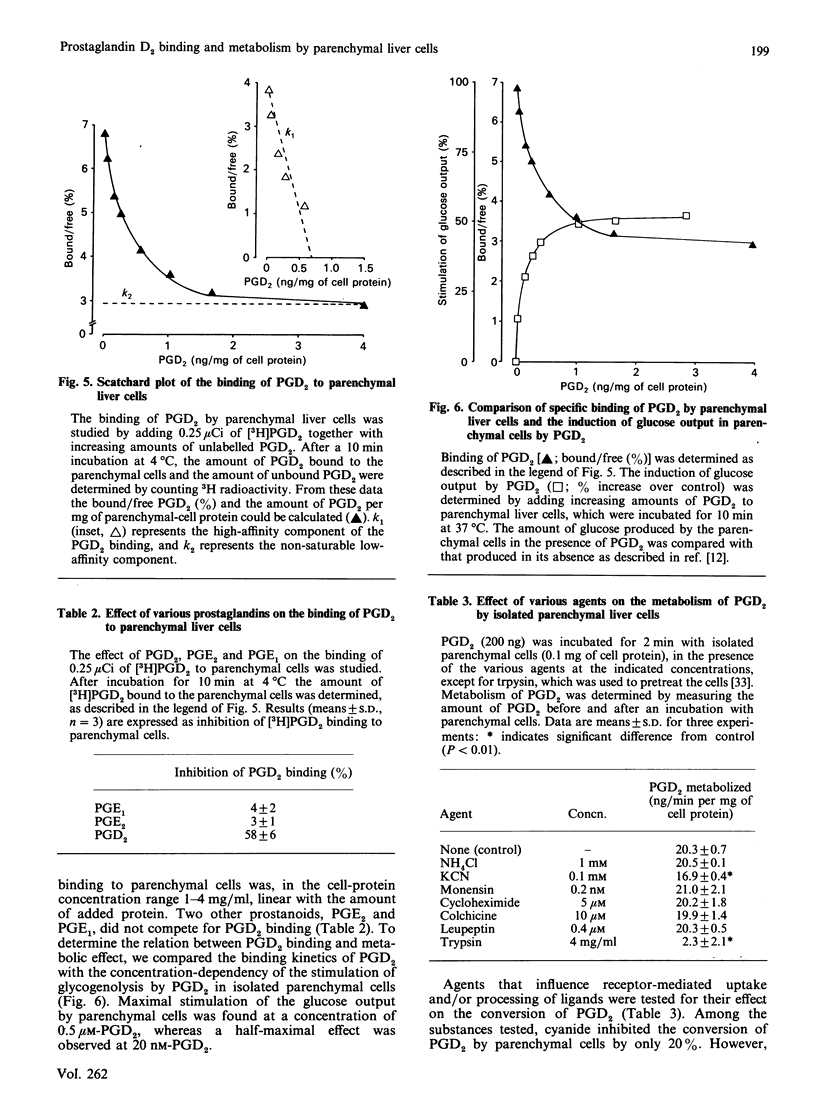
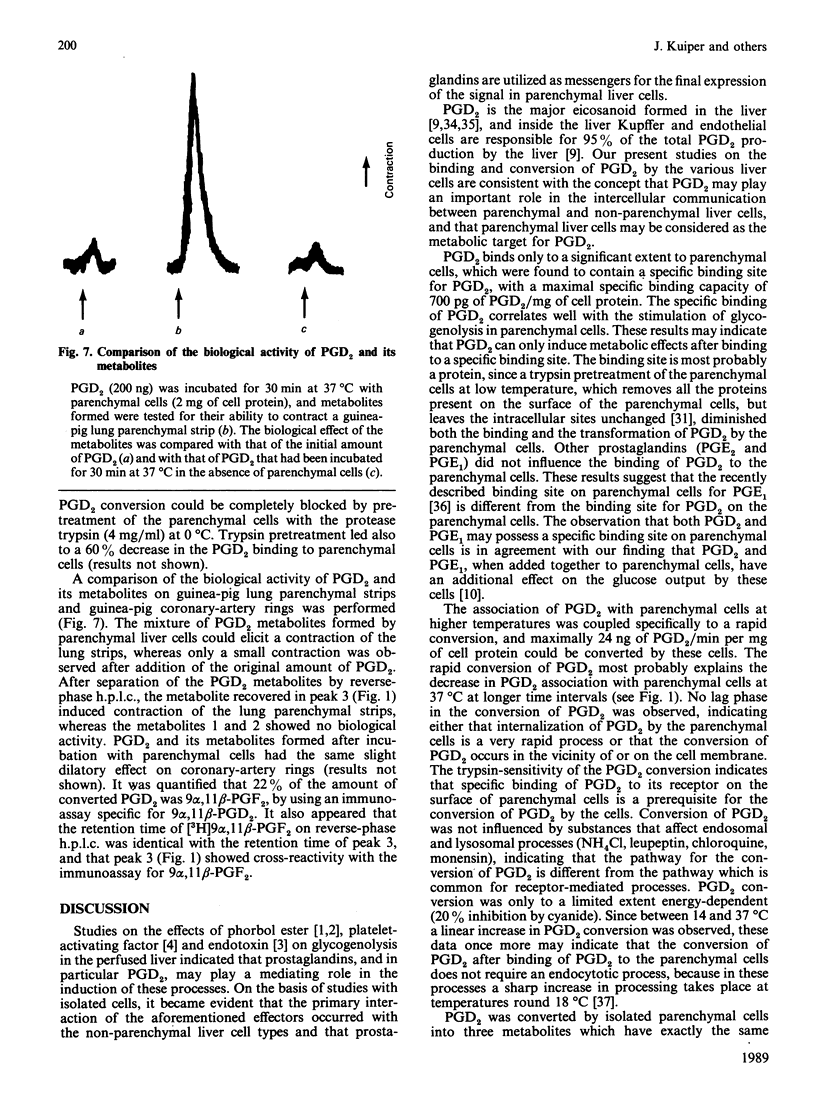
The major eicosanoid produced within the rat liver, prostaglandin (PG) D2, wa studied for its ability to interact with the various liver cell types. It appeared that PGD2 bound specifically to parenchymal liver cells, whereas the binding of PGD2 to Kupffer and endothelial liver cells was quantitatively unimportant. Maximally 700 pg of PGD2/mg of parenchymal-cell protein could be bound by a high-affinity site (1 x 10(6) PGD2-binding sites/cell). The recognition site for PGD2 is probably a protein because trypsin treatment of the cells virtually abolished the high-affinity binding. High-affinity binding of PGD2 was a prerequisite for the induction of a metabolic effect in isolated parenchymal liver cells, i.e. the induction of glycogenolysis. High-affinity binding of PGD2 by parenchymal cells was coupled to the conversion of PGD2 into three metabolites, whereas no conversion of PGD2 by Kupffer and endothelial liver cells was noticed. The temperature-sensitivity of the conversion of PGD2 was consistent with a conversion of PGD2 on or in the vicinity of the cell membrane. One of the PGD2 metabolites could be identified as 9 alpha, 11 beta-PGF2. It can be calculated that the conversion rate of PGD2 by parenchymal liver cells exceeds the production rate of PGD2 by Kupffer plus endothelial liver cells, indicating that PGD2 is meant to exert its activity within the liver. The present finding that PGD2 formed by the non-parenchymal liver cells is recognized by a specific receptor on parenchymal liver cells and that binding, conversion and metabolic effect of PGD2 are interlinked by this receptor provides further support for the specific role of PGD2 in the intercellular communication in the liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Dieter P., Bygrave F. L. Evidence that Ca2+ fluxes and respiratory, glycogenolytic and vasoconstrictive effects induced by the action of platelet-activating factor and L-alpha-lysophosphatidylcholine in the perfused rat liver are mediated by products of the cyclo-oxygenase pathway. Biochem J. 1987 Jul 1;245(1):145–150. doi: 10.1042/bj2450145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass E. P., Beyerinck R. A., Garrity M. J. Inhibition of prostaglandin E2 catabolism and potentiation of hepatic prostaglandin E2 action in rat hepatocytes by inhibitors of oxidative metabolism. Biochem Pharmacol. 1988 Apr 1;37(7):1343–1349. doi: 10.1016/0006-2952(88)90792-7. [DOI] [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., Van Rooij H. C., Kamps J. A., Koster J. F., Van Berkel T. J. Endotoxin stimulates glycogenolysis in the liver by means of intercellular communication. J Biol Chem. 1988 May 25;263(15):6953–6955. [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., Van Rooij H. C., Kamps J. A., Koster J. F., Van Berkel T. J. Prostaglandin D2 mediates the stimulation of glycogenolysis in the liver by phorbol ester. Biochem J. 1988 Feb 15;250(1):77–80. doi: 10.1042/bj2500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., Van Rooij H. C., Koster J. F., Van Berkel T. J. Conditioned media of Kupffer and endothelial liver cells influence protein phosphorylation in parenchymal liver cells. Involvement of prostaglandins. Biochem J. 1988 Jun 1;252(2):601–605. doi: 10.1042/bj2520601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., van Rooij H. C., Kamps J. A., Koster J. F., van Berkel T. J. Hormonal control of glycogenolysis in parenchymal liver cells by Kupffer and endothelial liver cells. J Biol Chem. 1988 Feb 25;263(6):2699–2703. [PubMed] [Google Scholar]
- Casteleijn E., van Rooij H. C., van Berkel T. J., Koster J. F. Mechanism of glucagon stimulation of fructose-1,6-bisphosphatase in rat hepatocytes. Involvement of a low-Mr activator. FEBS Lett. 1986 Jun 9;201(2):193–197. doi: 10.1016/0014-5793(86)80607-x. [DOI] [PubMed] [Google Scholar]
- Chiabrando C., Noseda A., Novella Castagnoli M., Salmona M., Fanelli R. Characterization of arachidonic acid metabolic profiles in animal tissues by high-resolution gas chromatography-mass spectrometry. Biochim Biophys Acta. 1984 Jul 6;794(2):292–297. doi: 10.1016/0005-2760(84)90158-9. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970 Oct;47(1):247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sáinz J. A., Hernández-Sotomayor S. M. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) via cyclooxygenase products. Biochem Biophys Res Commun. 1985 Oct 15;132(1):204–209. doi: 10.1016/0006-291x(85)91008-3. [DOI] [PubMed] [Google Scholar]
- Garrity M. J., Brass E. P., Robertson R. P. Kinetics of prostaglandin E metabolism in isolated hepatocytes. Biochim Biophys Acta. 1984 Nov 14;796(2):136–141. doi: 10.1016/0005-2760(84)90340-0. [DOI] [PubMed] [Google Scholar]
- Harford J., Klausner R. D. Biochemical methods for the study of receptor-mediated endocytosis. Methods Enzymol. 1987;149:3–9. doi: 10.1016/0076-6879(87)49039-3. [DOI] [PubMed] [Google Scholar]
- Harper T. W., Garrity M. J., Murphy R. C. Metabolism of leukotriene B4 in isolated rat hepatocytes. Identification of a novel 18-carboxy-19,20-dinor leukotriene B4 metabolite. J Biol Chem. 1986 Apr 25;261(12):5414–5418. [PubMed] [Google Scholar]
- Hayashi H., Ito S., Watanabe K., Negishi M., Shintani T., Hayaishi O. Metabolism of prostaglandin D2 in isolated rat lung: the stereospecific formation of 9 alpha,11 beta-prostaglandin F2 from prostaglandin D2. Biochim Biophys Acta. 1987 Feb 23;917(3):356–364. [PubMed] [Google Scholar]
- Kuiper J., Casteleyn E., Van Berkel T. J. Regulation of liver metabolism by intercellular communication. Adv Enzyme Regul. 1988;27:193–208. doi: 10.1016/0065-2571(88)90017-9. [DOI] [PubMed] [Google Scholar]
- Kuiper J., Kamps J. A., Van Berkel T. J. Induction of ornithine decarboxylase in rat liver by phorbol ester is mediated by prostanoids from Kupffer cells. J Biol Chem. 1989 Apr 25;264(12):6874–6878. [PubMed] [Google Scholar]
- Kuiper J., Zijlstra F. J., Kamps J. A., van Berkel T. J. Identification of prostaglandin D2 as the major eicosanoid from liver endothelial and Kupffer cells. Biochim Biophys Acta. 1988 Mar 25;959(2):143–152. doi: 10.1016/0005-2760(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Liston T. E., Roberts L. J., 2nd Transformation of prostaglandin D2 to 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9 alpha, 11 beta-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6030–6034. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Okumura T., Nakayama R., Sago T., Saito K. Identification of prostaglandin E metabolites from primary cultures of rat hepatocytes. Biochim Biophys Acta. 1985 Nov 14;837(2):197–207. [PubMed] [Google Scholar]
- Okumura T., Sago T., Saito K. Prostaglandin E1 binding protein on the surface of primary cultured hepatocytes. Biochem Int. 1987 Mar;14(3):443–449. [PubMed] [Google Scholar]
- Okumura T., Saito K. Degradation of prostaglandin E2 in a primary culture of adult rat hepatocytes. J Biochem. 1984 Aug;96(2):429–436. doi: 10.1093/oxfordjournals.jbchem.a134854. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Johnson C. J., Brown T. A. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. Evidence for reversible binding at the cell surface without internalization. J Biol Chem. 1987 Feb 15;262(5):2405–2410. [PubMed] [Google Scholar]
- Pugliese G., Spokas E. G., Marcinkiewicz E., Wong P. Y. Hepatic transformation of prostaglandin D2 to a new prostanoid, 9 alpha,11 beta-prostaglandin F2, that inhibits platelet aggregation and constricts blood vessels. J Biol Chem. 1985 Nov 25;260(27):14621–14625. [PubMed] [Google Scholar]
- Reingold D. F., Kawasaki A., Needleman P. A novel prostaglandin 11-keto reductase found in rabbit liver. Biochim Biophys Acta. 1981 May 14;659(1):179–188. doi: 10.1016/0005-2744(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Sago T., Nakayama R., Okumura T., Saito K. Metabolism of prostaglandins D2 and F2 alpha in primary cultures of rat hepatocytes. Biochim Biophys Acta. 1986 Dec 5;879(3):330–338. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seibert K., Sheller J. R., Roberts L. J., 2nd (5Z,13E)-(15S)-9 alpha,11 beta,15-trihydroxyprosta-5,13-dien-1-oic acid (9 alpha,11 beta-prostaglandin F2): formation and metabolism by human lung and contractile effects on human bronchial smooth muscle. Proc Natl Acad Sci U S A. 1987 Jan;84(1):256–260. doi: 10.1073/pnas.84.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene D. O., Murphy R. C. Metabolism of leukotriene E4 in isolated rat hepatocytes. Identification of beta-oxidation products of sulfidopeptide leukotrienes. J Biol Chem. 1988 Feb 25;263(6):2773–2778. [PubMed] [Google Scholar]
- Tran-Thi T. A., Gyufko K., Henninger H., Busse R., Decker K. Studies on synthesis and degradation of eicosanoids by rat hepatocytes in primary culture. J Hepatol. 1987 Dec;5(3):322–331. doi: 10.1016/s0168-8278(87)80038-7. [DOI] [PubMed] [Google Scholar]
- Uehara N., Ormstad K., Orning L., Hammarström S. Characteristics of the uptake of cysteine-containing leukotrienes by isolated hepatocytes. Biochim Biophys Acta. 1983 Jul 13;732(1):69–74. doi: 10.1016/0005-2736(83)90187-6. [DOI] [PubMed] [Google Scholar]
- Ujihara M., Urade Y., Eguchi N., Hayashi H., Ikai K., Hayaishi O. Prostaglandin D2 formation and characterization of its synthetases in various tissues of adult rats. Arch Biochem Biophys. 1988 Feb 1;260(2):521–531. doi: 10.1016/0003-9861(88)90477-8. [DOI] [PubMed] [Google Scholar]
- Uzumaki H., Yamamoto S., Kato R. Effects of lipoxygenase and cyclooxygenase inhibitors on the induction of ornithine decarboxylase by 12-O-tetradecanoylphorbol-13-acetate and isoproterenol in mouse tissues in vivo. Carcinogenesis. 1986 Feb;7(2):289–294. doi: 10.1093/carcin/7.2.289. [DOI] [PubMed] [Google Scholar]
- Wendelborn D. F., Seibert K., Roberts L. J., 2nd Isomeric prostaglandin F2 compounds arising from prostaglandin D2: a family of icosanoids produced in vivo in humans. Proc Natl Acad Sci U S A. 1988 Jan;85(2):304–308. doi: 10.1073/pnas.85.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Y., Lee W. H., Quilley C. P., McGiff J. C. Metabolism of prostacyclin: formation of an active metabolite in the liver. Fed Proc. 1981 May 15;40(7):2001–2004. [PubMed] [Google Scholar]
- Wong P. Y. Purification and partial characterization of prostaglandin D2 11-keto reductase in rabbit liver. Biochim Biophys Acta. 1981 May 14;659(1):169–178. doi: 10.1016/0005-2744(81)90281-3. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Vincent J. E. Determination of the ratio of the contractions, induced by thromboxane A2 in the guinea pig lung parenchymal strip after exogenous administration and formation in the tissue. Prostaglandins Leukot Med. 1984 Aug;15(2):143–146. doi: 10.1016/0262-1746(84)90170-7. [DOI] [PubMed] [Google Scholar]
- van Rooijen L. A., Uijtewaal B., Bisschop A. Inhibition by indomethacin and 5,8,11,14-eicosatetraynoic acid of the induction of rat hepatic ornithine decarboxylase by the tumor promoters phenobarbital and 12-O-tetradecanoylphorbol-13-acetate in vivo. Carcinogenesis. 1987 Jan;8(1):191–192. doi: 10.1093/carcin/8.1.191. [DOI] [PubMed] [Google Scholar]


