Abstract
Background and purpose
Capecitabine can be used as first-line treatment for advanced breast cancer. However, real-world data on efficacy of capecitabine in this setting is sparse. The purpose of the study is to evaluate outcomes of patients with Human Epidermal Growth Factor Receptor (HER2)-normal advanced breast cancer treated with capecitabine monotherapy as first-line treatment.
Material and Methods
The study utilized the Danish Breast Cancer Group (DBCG) database and was conducted retrospectively across all Danish oncology departments. Inclusion criteria were female patients, with HER2-normal advanced breast cancer treated with capecitabine monotherapy as the first-line treatment from 2010 to 2020. The primary endpoints were overall survival (OS) and progression-free survival (PFS).
Results
A total of 494 patients were included. Median OS was 16.4 months (95% confidence interval [CI]: 14.5–18.0), and median PFS was 6.0 months (95% CI: 5.3–6.7). Patients with estrogen receptor (ER)-positive disease had significantly longer OS (median: 22.8 vs. 10.5 months, p < 0.001) and PFS (median: 7.4 vs. 4.9 months, p = 0.003), when compared to ER-negative patients. Stratifying by age, patients under 45 years displayed a median PFS of 4.1 months, while those aged 45–70 years and over 70 years had median PFS of 5.7 and 7.2 months, respectively (p = 0.01).
Interpretation
In this nationwide study, the efficacy of capecitabine as a first-line treatment for HER2-normal advanced breast cancer is consistent with other, mainly retrospective, studies. However, when assessed against contemporary and newer treatments, its effectiveness appears inferior to alternative chemotherapies or targeted therapies.
KEYWORDS: Breast cancer, advanced breast cancer, HER2-normal, Capecitabine, chemotherapy, overall survival, progression free survival
Introduction
Every year, approximately 4,500 Danish women are diagnosed with breast cancer, with around 5% of these women presenting with primary metastatic disease (de novo), and a further 10%–30% experiencing a systemic relapse within 10 years of their initial breast cancer diagnosis [1, 2].
Individuals diagnosed with breast cancer can be categorized into subtypes based on their hormonal and molecular characteristics. These categories include estrogen receptor (ER)-positive, Human Epidermal Growth Factor Receptor 2 (HER2)-positive, and triple-negative breast cancer (TNBC), with TNBC distinguished by the absence of both ER expression, progesterone receptor expression, and HER2 amplification. In a study involving 22,000 patients with metastatic breast cancer, the distribution of these subtypes was found to be 62%, 18%, and 13%, respectively [3]. Treatment options for advanced breast cancer rely on chemotherapy, targeted therapy (e.g. HER2-directed therapy, Cyclin-dependent Kinase 4/6 (CDK4/6) inhibitors, antibody-drug conjugates), endocrine therapy, immunotherapy, or combinations of these therapies [4, 5].
Capecitabine, an oral pro-drug of fluoropyrimidine, was approved by the European Medicines Agency (EMA) in 2001 for the treatment of advanced breast cancer [6, 7]. Initially, it was approved as a monotherapy option after failure of prior taxane and anthracycline or as combination therapy with docetaxel after prior anthracycline therapy failure. Clinical trials, including prospective randomized phase II/III trials, have demonstrated the antitumour activity of capecitabine as a first-line treatment for advanced breast cancer. It has been studied as monotherapy and in combination with other agents [8–11]. First-line capecitabine monotherapy has shown a favourable safety profile, with no significant myelosuppression or alopecia [12, 13]. Capecitabine is available in tablet form and can be administered long-term without the cumulative toxicity associated with other chemotherapy agents.
While capecitabine is not considered the standard-of-care first-line treatment for any subtype according to the guidelines of the American Society of Clinical Oncology (ASCO) or the European Society for Medical Oncology (ESMO), it is mentioned as one of several options for first-line treatment in patients with ER-positive disease in specific circumstances outlined by both guidelines. These circumstances may include viscerally dominant disease (ASCO) or visceral crisis (ESMO) [4, 5, 14–16]. The standard first-line treatment for patients with metastatic ER-positive, HER2-normal breast cancer (and no imminent organ failure) is combination therapy with CDK4/6 inhibitors and endocrine therapy. Results from the PEARL study indicated no significant enhancements in progression-free survival for patients with aromatase inhibitor-resistant metastatic ER-positive, HER2-normal breast cancer. However, the CDK4/6-inhibitor, palbociclib, combined with endocrine therapy demonstrated a superior safety profile and enhanced quality of life compared to capecitabine treatment [17]. Consequently, international guidelines do not endorse the utilization of capecitabine as the standard-of-care first-line treatment. Nevertheless, it remains one of the preferred treatment options used in Denmark.
Although capecitabine was approved over 20 years ago based on clinical trials, recent knowledge about capecitabine derives from its use as a control arm in studies evaluating newer chemotherapies and biological agents, either alone or in combination with capecitabine [17, 18].
The current literature lacks real-world evidence on first-line capecitabine as treatment for patients with HER2-normal advanced breast cancer. Our study aims to provide a description of outcomes of patients who received capecitabine as first-line treatment for their advanced breast cancer, utilizing a nationwide database.
Material and methods
Study design
This is a nationwide, retrospective, observational real-world evidence study involving all Danish departments of oncology.
Patient selection
All women at least 18 years old who initiated treatment with capecitabine monotherapy as first-line treatment for HER2-normal metastatic or locally advanced breast cancer between January 1, 2010, and December 31, 2020, were eligible for inclusion. Patients were excluded if they had received treatment with another agent prior to or concurrently with capecitabine in the first-line.
Data source
The primary data source for this study was the DBCG’s database. The database includes data on diagnosis, patient demographics, tumour features, treatments, and follow-up. Both the organisation of the DBCG and its database have been described in detail before [19, 20]. Information on vital status was acquired from the Civil Registration System with complete information until September 1, 2023.
Endpoints
The primary endpoints of this study were overall survival (OS) and progression free survival (PFS).
Measures
De novo metastatic breast cancer refers to patients who are diagnosed with confirmed metastatic disease either at the time of their initial diagnosis or within a maximum of 90 days following surgery. The index date was defined as the date of diagnosis of metastatic/locally advanced disease. ER- and HER2-status were determined from metastatic site biopsies or primary tumour data when metastatic site information was unavailable. In Denmark, patients diagnosed with tumours that are ER-positive (≥10%) or have 1–9% ER positivity and exhibit the luminal A/B subtype (e.g. as determined by Prediction Analysis of Microarray 50 [PAM50]), are deemed to be ER-positive. HER2 status was evaluated via immunohistochemistry (IHC), where scores of 3+ were classified as HER2-positive, while tumours scoring 0 or 1+ were categorized as HER2-normal. For tumours scoring 2+ on IHC, Fluorescence in situ Hybridization (FISH) analysis was performed. Tumours found to be amplified during FISH were defined as HER2-positive. Disease progression was assessed according to RECIST 1.1 criteria, using radiological and clinical examinations conducted by the treating departments every 8–12 weeks [21].
Statistical analysis
PFS was defined as the time from index date to either progression, death from any cause, or end of clinical follow-up; whichever occurred first. OS was defined as the time between the index date and death from any cause or end of clinical follow-up; whichever occurred first. OS and PFS were estimated using the Kaplan–Meier method. Subgroup analysis of OS and PFS was done by disease presentation (de novo metastatic vs. recurrent breast cancer), ER-status (positive vs. negative), age (< 45 vs. 45–70 vs. > 70), and adjuvant chemotherapy (yes vs. no). Formal statistical test was conducted for subgroup-analyses of OS and PFS using Log-Rank method. Estimated potential follow-up was computed using the reverse Kaplan–Meier method, wherein the roles of event status and censored data were inverted. Follow-up time was subsequently reported in medians, along with confidence intervals (CIs). Testing was done by Wilcoxon rank sum test, Pearson’s Chi-squared test, or Fisher’s exact test when subgroups were compared by patient characteristics.
Results
Patient population
From January 1, 2010, to December 31, 2020, 494 patients were identified, starting capecitabine monotherapy as first-line treatment for HER2-normal advanced breast cancer (Figure 1).
Figure 1.
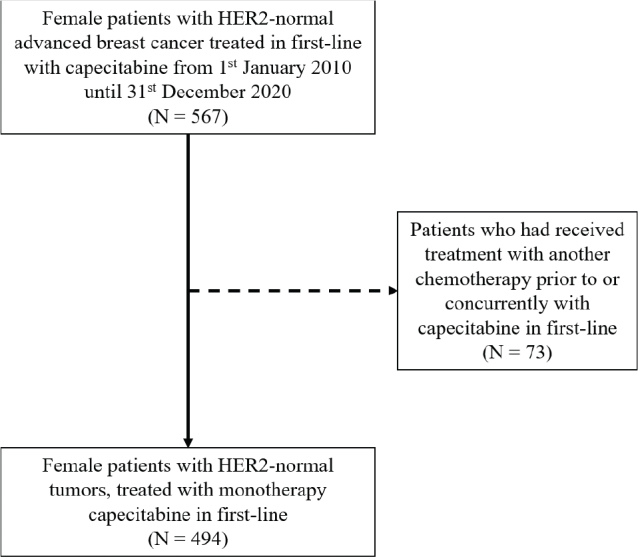
Flowchart of patient selection.
HER2: human epidermal growth factor receptor 2.
Patient characteristics are presented by ER-status in Table 1 and by disease presentation in Table 2.
Table 1.
Patient characteristics stratified by estrogen receptor status.
| Characteristic | Overall, N = 494 | ER-positive, N = 252 | ER-negative, N = 242 | p-value |
|---|---|---|---|---|
| Age 1 | 64 (52, 73) | 63 (51, 72) | 65 (53, 74) | 0.01 |
| <45 | 55 (11%) | 30 (12%) | 25 (10%) | |
| ≥45 & ≤70 | 265 (54%) | 140 (56%) | 125 (52%) | |
| >70 | 174 (35%) | 82 (33%) | 92 (38%) | |
| Disease presentation | 0.009 | |||
| Recurrent BC | 448 (91%) | 237 (94%) | 211 (87%) | |
| De novo mBC | 46 (9.3%) | 15 (6.0%) | 31 (13%) | |
| Metastatic site | ||||
| Visceral | 319 (65%) | 175 (69%) | 144 (60%) | 0.02 |
| CNS metastases | 36 (7.3%) | 16 (6.3%) | 20 (8.3%) | 0.40 |
| Number of sites | 0.02 | |||
| 1–2 | 270 (55%) | 151 (60%) | 119 (49%) | |
| ≥3 | 224 (45%) | 101 (40%) | 123 (51%) |
Median (IQR); n (%).
ER: estrogen receptor; BC: breast cancer; mBC: metastatic breast cancer; CNS: central nervous system.
Table 2.
Patient characteristics stratified by disease presentation.
| Characteristic | Overall, N = 494 | Recurrent, N = 448 | De novo, N = 46 | p-value |
|---|---|---|---|---|
| Age1 | 64 (52, 73) | 62 (52, 72) | 74 (68, 79) | <0.001 |
| <45 | 55 (11%) | 51 (11%) | 4 (8.7%) | |
| ≥45 & ≤70 | 265 (54%) | 254 (57%) | 11 (24%) | |
| >70 | 174 (35%) | 143 (32%) | 31 (67%) | |
| Metastatic site | ||||
| Visceral | 319 (65%) | 291 (65%) | 28 (61%) | 0.60 |
| CNS-metastases | 36 (7.3%) | 34 (7.6%) | 2 (4.3%) | 0.60 |
| Number of sites | 0.06 | |||
| 1–2 | 270 (55%) | 251 (56%) | 19 (41%) | |
| ≥3 | 224 (45%) | 197 (44%) | 27 (59%) | |
| Adjuvant treatment | ||||
| Chemotherapy2 | 260 (58%) | |||
| Combination anthracycline and taxane | 218 (84%)3 | |||
| Anthracycline alone | 14 (5%)3 | |||
| Taxane alone | 19 (7%)3 | |||
| Endocrine therapy2 | 184 (78%)4 |
Median (IQR); n (%)
No chemotherapy: n = 112 (25%), unknown chemotherapy: n = 76 (17%). No endocrine therapy: n = 16 (7%)5, unknown endocrine therapy: n = 37 (16%)5.
Percentage of patients who received chemotherapy (N = 260).
Percentage of patients who are ER-positive (N = 237).
ER: estrogen receptor; CNS: central nervous system.
Patients with ER-positive disease constituted 51% of the cohort. Patients with ER-negative disease were more likely to be older (p = 0.01) and present with de novo metastatic breast cancer (p = 0.009). Visceral metastases were more prevalent in patients with ER-positive disease (69% vs. 60%) (p = 0.02). However, patients with ER-positive disease had lesser tumour burden with 40% having three or more metastatic sites at baseline compared to 51% of patients with ER-negative disease (p = 0.02). No significant differences were found according to presence of CNS-metastases (p = 0.40) (Table 1).
A total of 448 (91%) patients presented with recurrent disease while 46 (9%) patients had de novo metastatic breast cancer. Patients with de novo metastatic breast cancer were significantly older than patients with recurrent breast cancer (median age 74 vs. 62, p < 0.001) (Table 2).
Of the 448 patient who presented with recurrent breast cancer, 260 (58%) had received (neo)adjuvant treatment with chemotherapy. Of these, 251 (97%) patients received anthracycline and/or a taxane. In total, 218 (84%) patients received a combination of anthracycline and taxane, 14 (5%) patients received anthracycline alone, and 19 (7%) received taxane alone. Seventy-eight percent of the patients with recurrent ER-positive disease had received adjuvant endocrine therapy (Table 2).
Outcomes
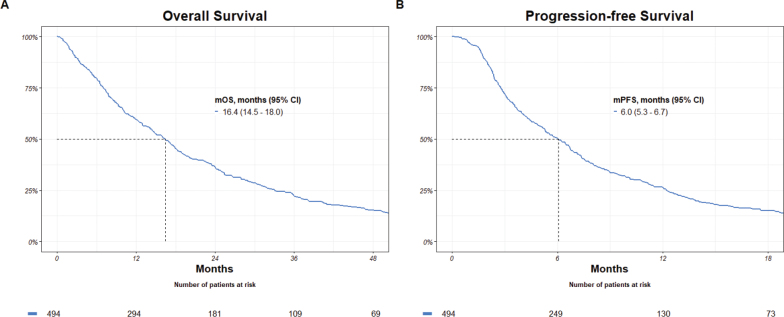
During an estimated median potential follow-up for OS of 91.4 months, 458 events were observed. Median OS was 16.4 months (95% CI: 14.5 – 18.0). The estimated median potential follow-up for PFS was 80.7 months and 479 events were observed. Median PFS was 6.0 months (95% CI: 5.3 – 6.7) (Figure 2).
Figure 2.
Overall and progression-free survival.
mOS: median overall survival; mPFS: median progression-free survival; CI: confidence interval.
Subgroup analyses: Overall survival
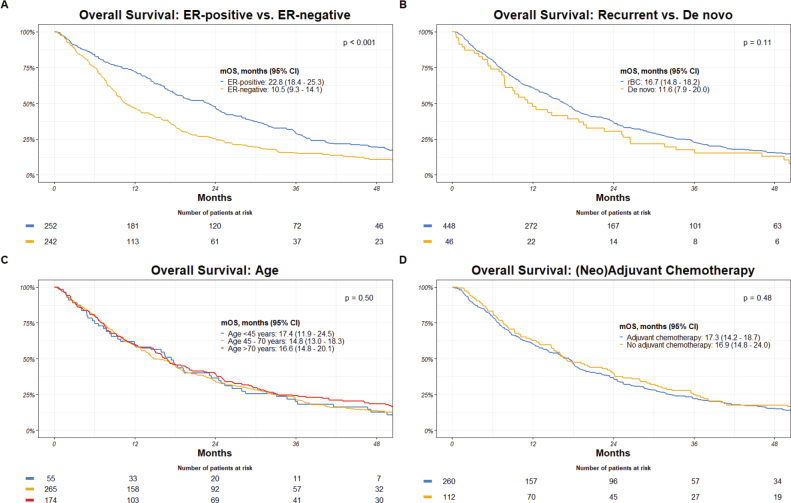
Patients with ER-positive breast cancer displayed a significantly extended OS with a median of 22.8 months, compared to 10.5 months for those with ER-negative disease (p < 0.001). Differences, though not statistically significant, also emerged in the context of disease presentation, with recurrent breast cancer patients demonstrating a median OS of 16.7 months and de novo metastatic breast cancer patients showing a median OS of 11.6 months (p = 0.11). Age-stratified analyses revealed little variation, as individuals under 45 years displayed a median OS of 17.4 months, while those aged 45–70 years and over 70 years had median OS of 14.8 and 16.6 months, respectively (p = 0.50). Furthermore, the impact of (neo)adjuvant chemotherapy on OS was explored. Patients who received (neo)adjuvant chemotherapy had a median OS of 17.3 months, similar to the median OS of 16.9 months for those who did not receive (neo)adjuvant chemotherapy (p = 0.48) (Figure 3).
Figure 3.
Overall survival by: (A) ER-receptor-status, (B) disease presentation, (C) age, and (D) (neo)adjuvant chemotherapy.
mOS: median overall survival; CI: confidence interval; ER: estrogen receptor; rBC: recurrent breast cancer.
Subgroup analyses: Progression free survival
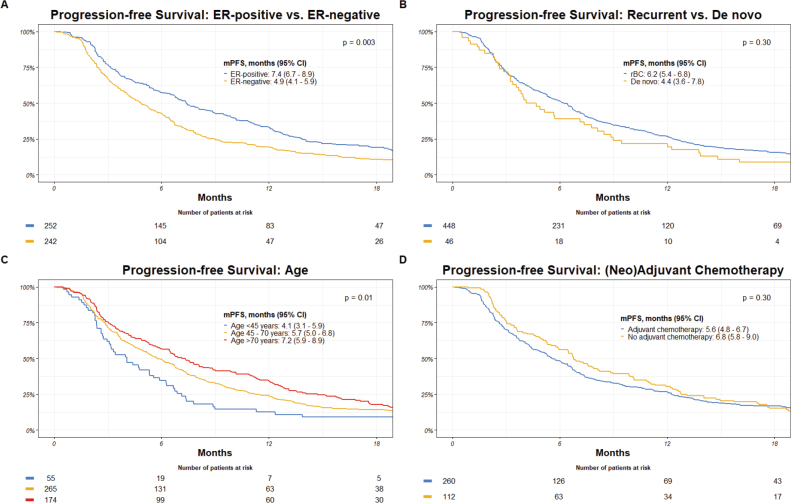
Patients with ER-positive disease demonstrated a median PFS of 7.4 months, which was significantly longer than the median PFS of 4.9 months observed in those with ER-negative breast cancer (p = 0.003). Again, a non-significant difference was present concerning disease presentation, with recurrent breast cancer patients exhibiting a median PFS of 6.2 months and de novo metastatic breast cancer patients having a median PFS of 4.4 months (p = 0.30). Stratifying patients by age, individuals under 45 years displayed a median PFS of 4.1 months, while those aged 45–70 years and over 70 years had median PFS of 5.7 and 7.2 months, respectively (p = 0.01). Patients who underwent (neo)adjuvant chemotherapy exhibited a median PFS of 5.6 months, which was not significantly different from the median PFS of 6.8 months observed in those who did not receive (neo)adjuvant chemotherapy (p = 0.30) (Figure 4).
Figure 4.
Progression-free survival by: (A) ER-receptor-status, (B) disease presentation, (C) age, and (D) (neo)adjuvant chemotherapy.
mPFS: median progression free survival; CI: confidence interval; ER: estrogen receptor; rBC: recurrent breast cancer
Discussion
Information on real-world efficacy of capecitabine as a first-line treatment in advanced breast cancer is sparse. To our knowledge, this is the first nationwide study evaluating the real-world efficacy of capecitabine monotherapy as a first-line treatment for advanced breast cancer. Our study found a median OS of 16.4 months (95% CI, 14.5 – 18.0), and a median PFS of 6.0 months (95% CI, 5.3 – 6.7).
Previous studies on capecitabine as monotherapy have shown median PFS from 3.1 to 7.9 months [8, 12, 13, 22–24]. Differences in treatment lines may explain shorter median PFS in some studies. Comparable real-world data are scarce in regard to first-line capecitabine and are mainly retrospective in nature [23, 24]. Some effort has been made to produce comparable evidence in reviews as well [8, 12, 13, 22]. Table 3 presents a comparison between the current study and the other six referenced studies across type of study, line(s) of treatment and percentage of patient previously treated with anthracyclines and/or taxanes.
Table 3.
Overview of studies evaluating treatment with capecitabine in treatment of HER-non-amplified advanced breast cancer.
| Study (year) | Type of study | Number of patients | Line(s) of treatment | Median PFS/TTP | Median OS | Percentage pre-treated with anthracyclines and/or taxanes |
|---|---|---|---|---|---|---|
| Blum et al. (2012) | Pooled analysis of RCTs | n = 268 | 1st (2nd line data not used) | 4.9 months | 21.9 months | 57% |
| Alsaloumi et al. (2020) | Meta-analysis (RCTs) | n = 3,257 | Multiple (not specified) | 3.1 – 5.4 months | 13.1 months | 100% |
| Oostendorp et al. (2011) | Systematic review | n = 1,174 | Multiple (not specified) | 4.2 / 3.9 months | 13.5 months | 100% |
| Thijssen et al. (2021) | Real-world study, retrospective, observational, single centre | n = 506 | 1st to 5th | 6.4 months | 13.3 months | 98.8% |
| Babacan et al. (2015) | Real-world study, retrospective, observational, single centre | n = 109 | 1st | 7.0 months | 30.0 months | 69% |
| O’Shaughnessy et al. (2012) | Review of RCTs | n = 958 | 1st (primarily) | 6.0 – 7.9 months | 18.6 – 29.4 months | Variable |
| This study | Real-world study, retrospective, observational, nationwide | n = 494 | 1 st | 6.1 months | 16.4 months | 51% |
PFS: progression-free survival; OS: overall survival; TTP: time to progression; RCT: randomized controlled trials.
A significant difference in OS and PFS was demonstrated for patients with ER-positive and ER-negative advanced breast cancer. In line with this, other studies evaluating capecitabine treatment in advanced breast cancer display a significant difference in outcomes between HER2-normal/ER-positive and triple-negative patients [8, 22, 23, 25].
When solely assessing the ER-positive patient group, the median PFS and median OS from our study compared significantly unfavourably, especially in studies evaluating CDK4/6-inhibitors and/or endocrine therapy as first-line treatment for ER-positive advanced breast cancer [3, 17, 26–34]. In comparison, a recent Danish real-world study evaluating the CDK4/6-inhibitor palbociclib in a first-line setting showed a median PFS of 24.3 months and median OS of 51.7 months [34]. Despite several alternative treatment modalities, such as CDK4/6-inhibitors (introduced in 2017 in Danish practice), having surfaced over the years for ER-positive advanced breast cancer patients, there is still a use of capecitabine as first-line therapy. An average of approximately 20 ER-positive patients were included in our study annually from 2017. This indicates a lack of shift to newer, and maybe more effective treatments, in Danish clinical practice. A possible explanation for the inferior PFS and OS found in our study may be attributed to the nature of real-world studies versus randomized clinical trials where patients must meet strict inclusion criteria for meaningful participation and often present with few, if any, comorbidities. When comparing results from our study with first-line chemotherapy for advanced breast cancer, clinical reasoning behind choosing capecitabine as the first-line treatment must be considered. Thus, patients treated with capecitabine in the first-line may have experienced visceral crisis or have been unable to tolerate treatment with other, intravenous, chemotherapies [5, 15]. It is fair to assume such a population would have a worse prognosis when compared to a general population of ER-positive advanced breast cancer patients. During the study period, chemotherapy has primarily been recommended for patients with rapidly progressing disease, particularly in cases of visceral crisis in Denmark [35]. However, recent studies suggest that patients with performance status scores of 0–2 should be recommended antihormonal therapy with a CDK4/6-inhibitor instead of chemotherapy in cases of visceral crisis [36, 37]. Supplementary survival analyses were conducted for subgroups with and without visceral metastases, which showed significantly lower PFS and OS for the subgroup with visceral metastases (p < 0.001) (Supplementary Table 1).
Regarding TNBC, median PFS and OS for this study corroborated with results from a Danish real-world study as well as two reviews on TNBC treatments [38–40]. When outcomes for the ER-negative population are compared to studies evaluating PD-1/PD-L1 inhibitors, such as KEYNOTE-522, IMpassion130 and IMpassion131 with median PFS ranging from 5.7 to 7.5 months and median OS ranging from 17.2 to 22.1 months, the outcomes of the present study fall short [41–43].
Our study showed no significant difference in OS and PFS concerning disease presentation. Prior research indicates that patients with HER2-positive de novo disease exhibit significantly improved PFS and OS compared to those with recurrent disease, as evidenced by findings from a real-world Danish population [44]. Furthermore, among ER-positive patients treated with CDK4/6 inhibitors, superior PFS and OS outcomes are observed in patients with de novo metastatic disease [45]. Conversely, for TNBC, outcomes vary. A Danish real-world study highlights the fact that TNBC patients with de novo disease experience worse PFS and OS compared to those with recurrent disease [38]. However, when chemotherapy is combined with immunotherapy for the same TNBC patient group, those with de novo disease demonstrate extended PFS and OS [46]. Nevertheless, it is important to interpret our current cohort’s OS and PFS differences cautiously due to the limited sample size of the de novo group (n = 46).
Patients who had received (neo)adjuvant chemotherapy (218 patients received a combination of anthracycline and taxane, 14 patients received an anthracycline alone and 19 received taxane alone) did not have a significantly different PFS or OS when compared to patients being chemotherapy-naïve. This lack of significant difference in outcomes is unexpected when considering previous literature that examines the impact of exposure to adjuvant chemotherapy on the efficacy of first-line chemotherapy for advanced breast cancer [47, 48]. Patients differed in terms of PFS between age group, with the youngest patients having the shortest PFS (p = 0.01). No significant difference was found in OS (p = 0.50). The underlying reasons for this discrepancy in PFS could stem from several factors. One plausible explanation is that younger patients may present with more aggressive disease profiles as also seen in early breast cancer populations [49–53].
This study holds certain strengths and limitations. This study utilized the DBCG national database, and all patients known to initiate capecitabine as the first-line treatment for their advanced disease were included, minimizing biases related to socioeconomic factors and geographic variations. Furthermore, the study had a long inclusion and follow-up period, which enhances the robustness of outcome assessments. The study lacks information regarding performance status, comorbidities, objective response rates, DPYD genetic testing, and data concerning the safety of treatment. Furthermore, the study had a restricted sample size within the de novo subgroup (n = 46), rendering the establishment of robust scientific evidence challenging.
Conclusion
Capecitabine monotherapy treatment is often preferred due to relatively manageable side effects and possibility for peroral administration. However, the outcomes assessed in terms of PFS and OS are not impressive, which should be stated when involving the patient in shared decision. When outcomes were compared with contemporary and newer treatments, the efficacy as a first-line treatment, in both ER-positive and ER-negative populations might be limited.
Author contributions
A.C.: Conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review & editing.
T.B.: Conceptualization, data curation, investigation, methodology, supervision, visualization, writing – review & editing.
M.G.: Data curation, formal analysis, writing – original draft.
M.J.: Conceptualization, data curation, methodology, supervision, writing – review & editing.
I.K.: Investigation, writing – review & editing.
S.E.: Investigation, writing – review & editing.
E.J.: Investigation, writing – review & editing.
A.K.: Conceptualization, investigation, methodology, project administration, supervision, validation, visualization, writing – review & editing.
D.N.: Conceptualization, investigation, methodology, project administration, supervision, validation, visualization, writing – review & editing.
Disclosure of interest
A.C.: Unrestricted research grant from Danish Cancer Society.
T.B.: Institutional grants from: Pfizer, Astra Zeneca, Novartis, Samsung Bioepis, Seattle Genetics, Merck, Eli Lilly and Daiichi Sankyo. Advisory board: Novartis. Travel: Daiichi Sankyo.
M.G.: Unrestricted research grant from Danish Cancer Society.
M.J.: Advisory board, Novartis.
I.K.: None.
S.E.: Institutional grants from Novartis og Merck.
E.J.: None.
A.K.: Institutional grants from Pfizer, AstraZeneca, Merck, Eli Lilly, Seattle Genetics, Roche, Novartis. Personal grants from Astra Zeneca (travel + advisory board), MSD (travel), Daiichi Sankyo (advisory Board), Novartis (advisory Board), Seagen (advisory Board), Gilead (advisory Board).
D.N.: None
Data availability statement
All data are stored in the DBCG database. The dataset can be made available to qualified researchers through application to the Danish Breast Cancer Group. Please contact dbcg.rigshospitalet@regionh.dk.
Ethics declarations & trial registry information
The study was approved by the Danish Breast Cancer Group’s oncological committee. The study was also registered and approved by the Capital Regions research overview (P-2021-656).
Supplementary Material
Funding Statement
Funding No funding was given for this study.
References
- [1].Jensen MB, Ejlertsen B, Mouridsen HT, Christiansen P. Improvements in breast cancer survival between 1995 and 2012 in Denmark: the importance of earlier diagnosis and adjuvant treatment. Acta Oncol. 2016. Jun;55 Suppl 2:24–35. 10.3109/0284186X.2015.1128119 [DOI] [PubMed] [Google Scholar]
- [2].Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017. Nov;377(19):1836–46. 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020. Volume 138, Pages S4-S4. 10.1016/S0959-8049(20)30540-2 [DOI] [PubMed] [Google Scholar]
- [4].Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021. Dec;39(35):3959–77. 10.1200/JCO.21.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49. 10.1016/j.annonc.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].European Medicines Association . Mabthera, Scientific Discussion. 2005. [cited 2023 Oct 6]. p. 1–54. Xeloda EPAR – scientific discussion. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion/xeloda-epar-scientific-discussion_en.pdf
- [7].European Medicines Association . Xeloda EPAR – procedural steps taken before authorisation [Internet]. 2005. [cited 2023 Oct 6]. p. 1. Available from: https://www.ema.europa.eu/en/documents/procedural-steps/xeloda-epar-procedural-steps-taken-authorisation_en.pdf
- [8].O’Shaughnessy JA, Kaufmann M, Siedentopf F, Dalivoust P, Debled M, Robert NJ, et al. Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer. Oncologist. 2012;17(4):476–84. 10.1634/theoncologist.2011-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaufmann M, Maass N, Costa SD, Schneeweiss A, Loibl S, Sütterlin MW, et al. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur J Cancer. 2010. Dec;46(18):3184–91. 10.1016/j.ejca.2010.07.009 [DOI] [PubMed] [Google Scholar]
- [10].Harbeck N, Saupe S, Jäger E, Schmidt M, Kreienberg R, Müller L, et al. A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat. 2017. Jan;161(1):63–72. 10.1007/s10549-016-4033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001. Sep;12(9):1247–54. 10.1023/A:1012281104865 [DOI] [PubMed] [Google Scholar]
- [12].Oostendorp LJM, Stalmeier PFM, Donders ART, van der Graaf WTA, Ottevanger PB. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. Lancet Oncol. 2011. Oct;12(11): 1053–61. 10.1016/S1470-2045(11)70045-6 [DOI] [PubMed] [Google Scholar]
- [13].Alsaloumi L, Shawagfeh S, Abdi A, Basgut B. Efficacy and safety of Capecitabine alone or in combination in advanced metastatic breast cancer patients previously treated with Anthracycline and Taxane: a systematic review and meta-analysis. Oncol Res Treat. 2020;43: 694–702. 10.1159/000510356 [DOI] [PubMed] [Google Scholar]
- [14].Moy B, Rumble RB, Come SE, Davidson NE, Di Leo A, Gralow JR, et al. Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline update. J Clin Oncol. 2021. Dec;39(35):3938–58. [DOI] [PubMed] [Google Scholar]
- [15].Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–95. 10.1016/j.annonc.2021.09.019 [DOI] [PubMed] [Google Scholar]
- [16].Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016. Sep;34(25):3069–103. 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- [17].Martin M, Zielinski C, Ruiz-Borrego M, Carrasco E, Turner N, Ciruelos EM, et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021. Apr;32(4):488–99. 10.1016/j.annonc.2020.12.013 [DOI] [PubMed] [Google Scholar]
- [18].Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015. Feb;33(6):594–601. 10.1200/JCO.2013.52.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ejlertsen B, Offersen BV, Overgaard J, Christiansen P, Jensen MB, Kroman N, et al. Forty years of landmark trials undertaken by the Danish Breast Cancer Cooperative Group (DBCG) nationwide or in international collaboration. Acta Oncol. 2018. Jan;57(1):3–12. 10.1080/0284186X.2017.1408962 [DOI] [PubMed] [Google Scholar]
- [20].Jensen MB, Laenkholm AV, Offersen B V, Christiansen P, Kroman N, Mouridsen HT, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007–2016. Acta Oncol. 2018. Jan;57(1):13–8. 10.1080/0284186X.2017.1404638 [DOI] [PubMed] [Google Scholar]
- [21].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. Jan;45(2):228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- [22].Blum JL, Barrios CH, Feldman N, Verma S, McKenna EF, Lee LF, et al. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2012. Dec;136(3):777–88. [DOI] [PubMed] [Google Scholar]
- [23].Thijssen S, Wildiers H, Punie K, Beuselinck B, Clement P, Remmerie C, et al. Features of durable response and treatment efficacy for capecitabine monotherapy in advanced breast cancer: real-world evidence from a large single-centre cohort. J Cancer Res Clin Oncol. 2021. Apr;147(4):1041–8. 10.1007/s00432-020-03487-1 [DOI] [PubMed] [Google Scholar]
- [24].Babacan T, Efe O, Hasirci AS, Demirci F, Buyukhatipoglu H, Balakan O, et al. Efficacy of capecitabine monotherapy as the first-line treatment of metastatic HER2-negative breast cancer. Tumori. 2015;101(4):418–23. 10.5301/tj.5000332 [DOI] [PubMed] [Google Scholar]
- [25].Hoon SN, Lau PK, White AM, Bulsara MK, Banks PD, Redfern AD. Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer. Cochrane Database Syst Rev. 2021. May;5(5):CD011220. 10.1002/14651858.CD011220.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park YH, Kim TY, Kim GM, Kang SY, Park IH, Kim JH, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019. Dec;20(12):1750–9. [DOI] [PubMed] [Google Scholar]
- [27].Jerusalem G, de Boer RH, Hurvitz S, Yardley DA, Kovalenko E, Ejlertsen B, et al. Everolimus plus exemestane vs everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-negative advanced breast cancer: the BOLERO-6 randomized clinical trial. JAMA Oncol. 2018. Oct;4(10):1367–74. 10.1001/jamaoncol.2018.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019. Oct;20(10):1360–9. 10.1016/S1470-2045(19)30420-6 [DOI] [PubMed] [Google Scholar]
- [29].Lobbezoo DJA, van Kampen RJW, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol. 2016. Feb;27(2):256–62. 10.1093/annonc/mdv544 [DOI] [PubMed] [Google Scholar]
- [30].Bonotto M, Gerratana L, Di Maio M, De Angelis C, Cinausero M, Moroso S, et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. 2017. Feb;31:114–20. 10.1016/j.breast.2016.10.021 [DOI] [PubMed] [Google Scholar]
- [31].Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in advanced breast cancer. N Engl J Med. 2016. Nov;375(20):1925–36. [DOI] [PubMed] [Google Scholar]
- [32].Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017. Nov;35(32):3638–46. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- [33].Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with Ribociclib plus Letrozole in advanced breast cancer. N Engl J Med. 2022. Mar;386(10):942–50. [DOI] [PubMed] [Google Scholar]
- [34].Garly R, Berg T, Jensen MB, Knoop A, Volmer L, Glavicic V, et al. A retrospective, non-interventional study of breast cancer patients diagnosed with ER+/HER2 negative, locally advanced or metastatic breast cancer treated with palbociclib in Denmark. Acta Oncol. 2023. Mar;62(3):290–7. 10.1080/0284186X.2023.2194030 [DOI] [PubMed] [Google Scholar]
- [35].DMCG . Systemisk behandling af brystkræft III – palliativ og systemisk behandling af metastaserende brystkræft (MBC), VERSION 1.3 [Internet]. 2021. [cited 2024 Apr 5]. Available from: https://www.dmcg.dk/Kliniske-retningslinjer/kliniske-retningslinjer-opdelt-paa-dmcg/brystcancer/systemisk-behandling-af-brystkraft-iii--palliativ-og-systemisk-behandling-af-metastaserende-brystkraft-mbc/
- [36].Behrouzi R, Armstrong AC, Howell SJ. CDK4/6 inhibitors versus weekly paclitaxel for treatment of ER+/HER2- advanced breast cancer with impending or established visceral crisis. Breast Cancer Res Treat. 2023. Nov;202(1):83–95. 10.1007/s10549-023-07035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu YS, Mahidin EIBM, Azim H, ERALP Y, Yap YS, Im SA, et al. Abstract GS1-10: primary results from the randomized Phase II RIGHT Choice trial of premenopausal patients with aggressive HR+/HER2− advanced breast cancer treated with ribociclib + endocrine therapy vs physician’s choice combination chemotherapy. Cancer Res. 2023. Mar 1;83(5_Supplement):GS1-10-GS1-10. 10.1158/1538-7445.SABCS22-GS1-10 [DOI] [Google Scholar]
- [38].Celik A, Berg T, Jensen MB, Jakobsen E, Nielsen HM, Kümler I, et al. Real-world survival and treatment regimens across first- to third-line treatment for advanced triple-negative breast cancer. Breast Cancer (Auckl). 2023;17:11782234231203292. 10.1177/11782234231203292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2016;10:25–36. 10.4137/BCBCR.S32783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li CH, Karantza V, Aktan G, Lala M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 2019. Dec 16;21(1):143. 10.1186/s13058-019-1210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022. Jul;387(3):217–26. 10.1056/NEJMoa2202809 [DOI] [PubMed] [Google Scholar]
- [42].Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020. Jan;21(1):44–59. 10.1016/S1470-2045(19)30689-8 [DOI] [PubMed] [Google Scholar]
- [43].Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021. Aug;32(8):994–1004. [DOI] [PubMed] [Google Scholar]
- [44].Christensen T, Berg T, Nielsen LB, Andersson M, Jensen MB, Knoop A. Dual HER2 blockade in the first-line treatment of metastatic breast cancer – a retrospective population-based observational study in Danish patients. Breast. 2020;51:34–9. 10.1016/j.breast.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Prat A, Solovieff N, André F, O’Shaughnessy J, Cameron DA, Janni W, et al. Intrinsic subtype and overall survival of patients with advanced HR+/HER2- breast cancer treated with Ribociclib and ET: correlative analysis of MONALEESA-2, -3, -7. Clin Cancer Res. 2024. Feb;30(4):793–802. 10.1158/1078-0432.CCR-23-0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020. Dec;396(10265):1817–28. [DOI] [PubMed] [Google Scholar]
- [47].Bonneterre J, Mercier M. Response to chemotherapy after relapse in patients with or without previous adjuvant chemotherapy for breast cancer. The French Epirubicin Study Group. Cancer Treat Rev. 1993. Apr;19 Suppl B:21–30. 10.1016/0305-7372(93)90004-B [DOI] [PubMed] [Google Scholar]
- [48].Park IH, Lee KS, Ro J. Effects of second and subsequent lines of chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2015. Feb;15(1):e55–62. 10.1016/j.clbc.2014.09.001 [DOI] [PubMed] [Google Scholar]
- [49].Azim HAJ, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014. Aug;16(4):427. 10.1186/s13058-014-0427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013. Jun;5 Suppl 1(Suppl 1):S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Johnson RH, Anders CK, Litton JK, Ruddy KJ, Bleyer A. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018. Dec;65(12):e27397. 10.1002/pbc.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fredholm H, Magnusson K, Lindström LS, Garmo H, Fält SE, Lindman H, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat. 2016. Nov;160(1):131–43. 10.1007/s10549-016-3983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kataoka A, Iwamoto T, Tokunaga E, Tomotaki A, Kumamaru H, Miyata H, et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese Breast Cancer Registry. Breast Cancer Res Treat. 2016. Nov;160(1):163–72. 10.1007/s10549-016-3984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are stored in the DBCG database. The dataset can be made available to qualified researchers through application to the Danish Breast Cancer Group. Please contact dbcg.rigshospitalet@regionh.dk.