Abstract
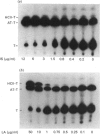
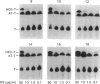
The interactions of two proteinase inhibitors, heparin cofactor II and antithrombin, with thrombin are potentiated by heparin. Using two methods, we have studied the potentiating effects of a series of heparin (poly)saccharides with high affinity for antithrombin and mean Mr ranging from approx. 1700 to 18,800. First, catalytic amounts of heparin (poly)saccharide were added to purified systems containing thrombin and either heparin cofactor II or antithrombin. Residual thrombin activity was determined with a chromogenic substrate. It was found that only the higher-Mr polysaccharides (Mr greater than 8000) efficiently catalysed thrombin inhibition by heparin cofactor II, there being a progressive catalytic effect with increasing Mr of the polysaccharide. Weak accelerating effects were noted with low-Mr saccharides (Mr less than 8000). This contrasted with the well-characterized interaction of heparin with antithrombin and thrombin, where heparin oligosaccharides of Mr less than 5400 had absolutely no ability to accelerate the reaction, while (poly)saccharides of Mr exceeding 5400 showed rapidly increasing catalytic activity with increasing Mr. Secondly, these and other heparin preparations were added in a wide concentration range to plasma with which 125I-labelled thrombin was then incubated for 30 s. Inhibited thrombin was determined from the distribution of labelled thrombin amongst inhibitor-thrombin complexes, predominantly antithrombin-thrombin and heparin cofactor II-thrombin complexes. In this situation, where the inhibitors competed for thrombin and for the (poly)saccharides, it was found that, provided the latter were of high affinity for antithrombin and exceeded a Mr of 5400, thrombin inhibition in plasma was mediated largely through antithrombin. Polysaccharides of Mr exceeding 8000 that were of low affinity for antithrombin accelerated thrombin inhibition in plasma through their interaction with heparin cofactor II. High concentrations of saccharides of Mr 1700-5400 exhibited a size-dependent acceleration of thrombin inhibition, not through their interaction with antithrombin, but through their interaction with heparin cofactor II.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U., Larsen M. L. Assay of dermatan sulfate cofactor (heparin cofactor II) activity in human plasma. Thromb Res. 1984 Aug 1;35(3):257–266. doi: 10.1016/0049-3848(84)90357-8. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Lormeau J. C., Petitou M., Rosenberg R. D., Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985 Nov 5;24(23):6723–6729. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Stephens A. W., Rimon A., Rosenberg R. D. Sequence variation in heparin octasaccharides with high affinity for antithrombin III. Biochemistry. 1984 Nov 20;23(24):5801–5812. doi: 10.1021/bi00319a020. [DOI] [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Béguin S., Lindhout T., Hemker H. C. The mode of action of heparin in plasma. Thromb Haemost. 1988 Dec 22;60(3):457–462. [PubMed] [Google Scholar]
- Casu B., Oreste P., Torri G., Zoppetti G., Choay J., Lormeau J. C., Petitou M., Sinäy P. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981 Sep 1;197(3):599–609. doi: 10.1042/bj1970599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Raub E., Lindahl U., Björk I. Role of ternary complexes, in which heparin binds both antithrombin and proteinase, in the acceleration of the reactions between antithrombin and thrombin or factor Xa. J Biol Chem. 1986 Nov 25;261(33):15467–15473. [PubMed] [Google Scholar]
- Denton J., Lane D. A., Thunberg L., Slater A. M., Lindahl U. Binding of platelet factor 4 to heparin oligosaccharides. Biochem J. 1983 Feb 1;209(2):455–460. doi: 10.1042/bj2090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fernandez F. A., Buchanan M. R., Hirsh J., Fenton J. W., 2nd, Ofosu F. A. Catalysis of thrombin inhibition provides an index for estimating the antithrombotic potential of glycosaminoglycans in rabbits. Thromb Haemost. 1987 Jun 3;57(3):286–293. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Griffith M. J. Heparin-catalyzed inhibitor/protease reactions: kinetic evidence for a common mechanism of action of heparin. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5460–5464. doi: 10.1073/pnas.80.18.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M. J., Marbet G. A. Dermatan sulfate and heparin can be fractionated by affinity for heparin cofactor II. Biochem Biophys Res Commun. 1983 Apr 29;112(2):663–670. doi: 10.1016/0006-291x(83)91514-0. [DOI] [PubMed] [Google Scholar]
- Holmer E., Kurachi K., Söderström G. The molecular-weight dependence of the rate-enhancing effect of heparin on the inhibition of thrombin, factor Xa, factor IXa, factor XIa, factor XIIa and kallikrein by antithrombin. Biochem J. 1981 Feb 1;193(2):395–400. doi: 10.1042/bj1930395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Ireland H., Lane D. A., Curtis J. R. Objective assessment of heparin requirements for hemodialysis in humans. J Lab Clin Med. 1984 Apr;103(4):643–652. [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980 Nov 10;255(21):10081–10090. [PubMed] [Google Scholar]
- Lane D. A., Denton J., Flynn A. M., Thunberg L., Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725–732. doi: 10.1042/bj2180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. A., Flynn A. M., Pejler G., Lindahl U., Choay J., Preissner K. Structural requirements for the neutralization of heparin-like saccharides by complement S protein/vitronectin. J Biol Chem. 1987 Dec 5;262(34):16343–16348. [PubMed] [Google Scholar]
- Lane D. A., Flynn A., Ireland H., Erdjument H., Samson D., Howarth D., Thompson E. Antithrombin III Northwick Park: demonstration of an inactive high MW complex with increased affinity for heparin. Br J Haematol. 1987 Apr;65(4):451–456. doi: 10.1111/j.1365-2141.1987.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Lane D. A., Pejler G., Flynn A. M., Thompson E. A., Lindahl U. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor 4. J Biol Chem. 1986 Mar 25;261(9):3980–3986. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L., Leder I. G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt R. J., Rice K. G., Merchant Z. M., Kim Y. S., Lohse D. L. Structure and activity of a unique heparin-derived hexasaccharide. J Biol Chem. 1986 Nov 5;261(31):14448–14454. [PubMed] [Google Scholar]
- Maimone M. M., Tollefsen D. M. Activation of heparin cofactor II by heparin oligosaccharides. Biochem Biophys Res Commun. 1988 May 16;152(3):1056–1061. doi: 10.1016/s0006-291x(88)80391-7. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G. J., Smith L. M., Buchanan M. R., Hirsh J. The importance of thrombin inhibition for the expression of the anticoagulant activities of heparin, dermatan sulphate, low molecular weight heparin and pentosan polysulphate. Br J Haematol. 1985 Aug;60(4):695–704. doi: 10.1111/j.1365-2141.1985.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Sie P., Modi G. J., Fernandez F., Buchanan M. R., Blajchman M. A., Boneu B., Hirsh J. The inhibition of thrombin-dependent positive-feedback reactions is critical to the expression of the anticoagulant effect of heparin. Biochem J. 1987 Apr 15;243(2):579–588. doi: 10.1042/bj2430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. A., Tollefsen D. M. The protease specificity of heparin cofactor II. Inhibition of thrombin generated during coagulation. J Biol Chem. 1985 Mar 25;260(6):3501–3505. [PubMed] [Google Scholar]
- Petitou M., Lormeau J. C., Perly B., Berthault P., Bossennec V., Sié P., Choay J. Is there a unique sequence in heparin for interaction with heparin cofactor II? Structural and biological studies of heparin-derived oligosaccharides. J Biol Chem. 1988 Jun 25;263(18):8685–8690. [PubMed] [Google Scholar]
- Pomerantz M. W., Owen W. G. A catalytic role for heparin. Evidence for a ternary complex of heparin cofactor thrombin and heparin. Biochim Biophys Acta. 1978 Jul 21;535(1):66–77. doi: 10.1016/0005-2795(78)90033-8. [DOI] [PubMed] [Google Scholar]
- Ragg H. A new member of the plasma protease inhibitor gene family. Nucleic Acids Res. 1986 Jan 24;14(2):1073–1088. doi: 10.1093/nar/14.2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Kakkar V. V. Comparison of the molecular mass dependency of heparin stimulation of heparin cofactor II:thrombin interaction to antithrombin III:thrombin interaction. Thromb Res. 1987 May 1;46(3):491–502. doi: 10.1016/0049-3848(87)90136-8. [DOI] [PubMed] [Google Scholar]
- Sie P., Dupouy D., Pichon J., Boneu B. Constitutional heparin co-factor II deficiency associated with recurrent thrombosis. Lancet. 1985 Aug 24;2(8452):414–416. doi: 10.1016/s0140-6736(85)92737-0. [DOI] [PubMed] [Google Scholar]
- Sié P., Petitou M., Lormeau J. C., Dupouy D., Boneu B., Choay J. Studies on the structural requirements of heparin for the catalysis of thrombin inhibition by heparin cofactor II. Biochim Biophys Acta. 1988 Aug 11;966(2):188–195. doi: 10.1016/0304-4165(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Tew C. J., Lane D. A., Thompson E., Ireland H., Curtis J. R. Relationship between ex vivo anti-proteinase (factor Xa and thrombin) assays and in vivo anticoagulant effect of very low molecular weight heparin, CY222. Br J Haematol. 1988 Nov;70(3):335–340. doi: 10.1111/j.1365-2141.1988.tb02491.x. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Blank M. K. Detection of a new heparin-dependent inhibitor of thrombin in human plasma. J Clin Invest. 1981 Sep;68(3):589–596. doi: 10.1172/JCI110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tollefsen D. M., Peacock M. E., Monafo W. J. Molecular size of dermatan sulfate oligosaccharides required to bind and activate heparin cofactor II. J Biol Chem. 1986 Jul 5;261(19):8854–8858. [PubMed] [Google Scholar]
- Yamagishi R., Koide T., Sakuragawa N. Binding of heparin or dermatan sulfate to thrombin is essential for the sulfated polysaccharide-accelerated inhibition of thrombin by heparin cofactor II. FEBS Lett. 1987 Dec 10;225(1-2):109–112. doi: 10.1016/0014-5793(87)81140-7. [DOI] [PubMed] [Google Scholar]