Abstract
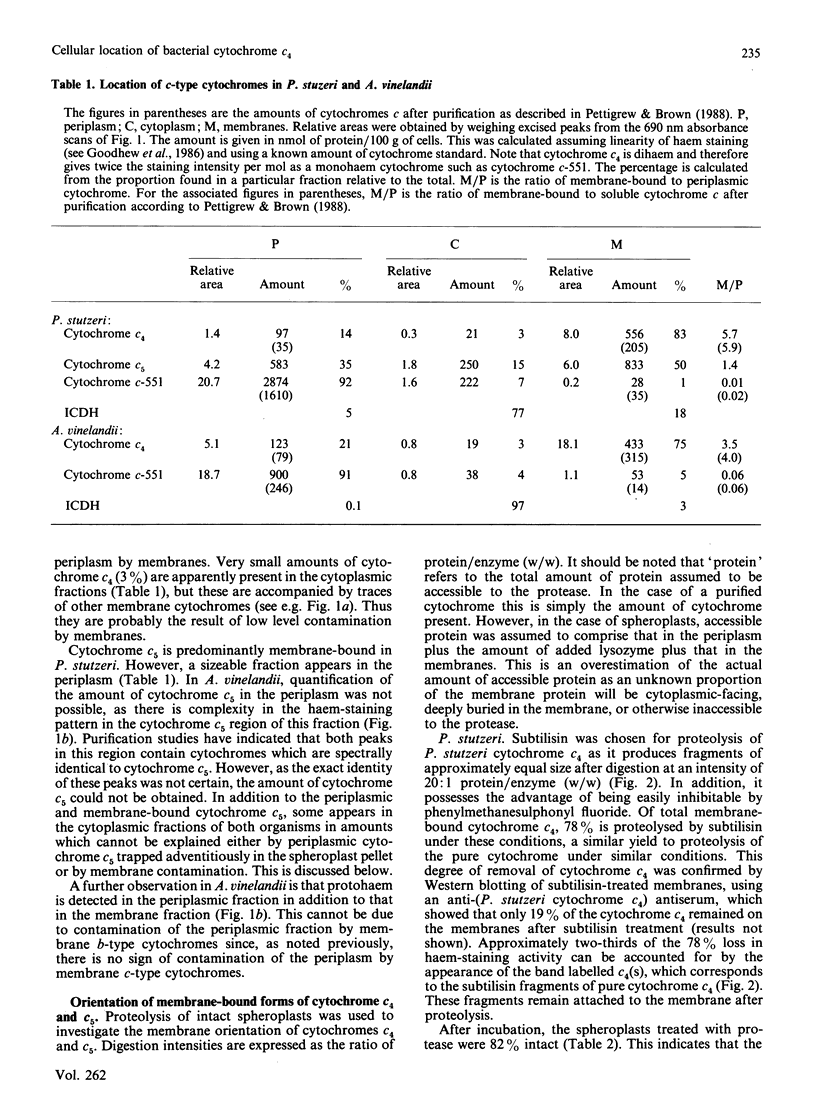
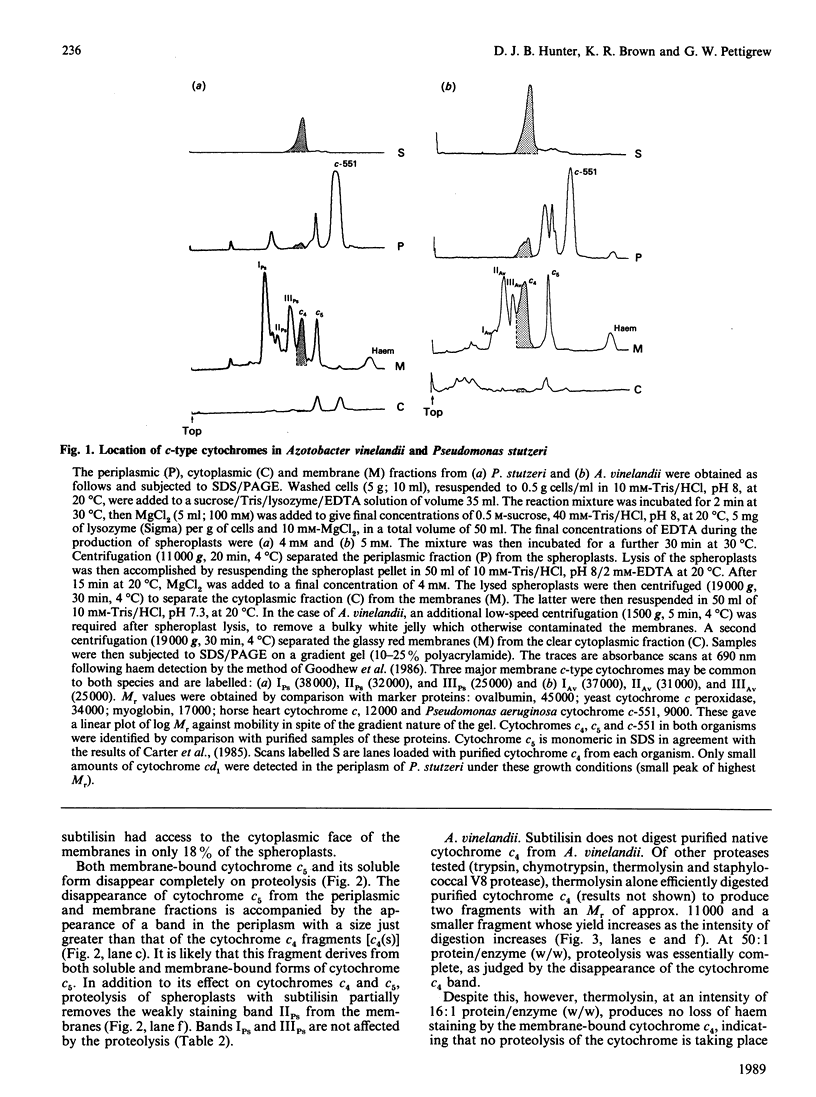
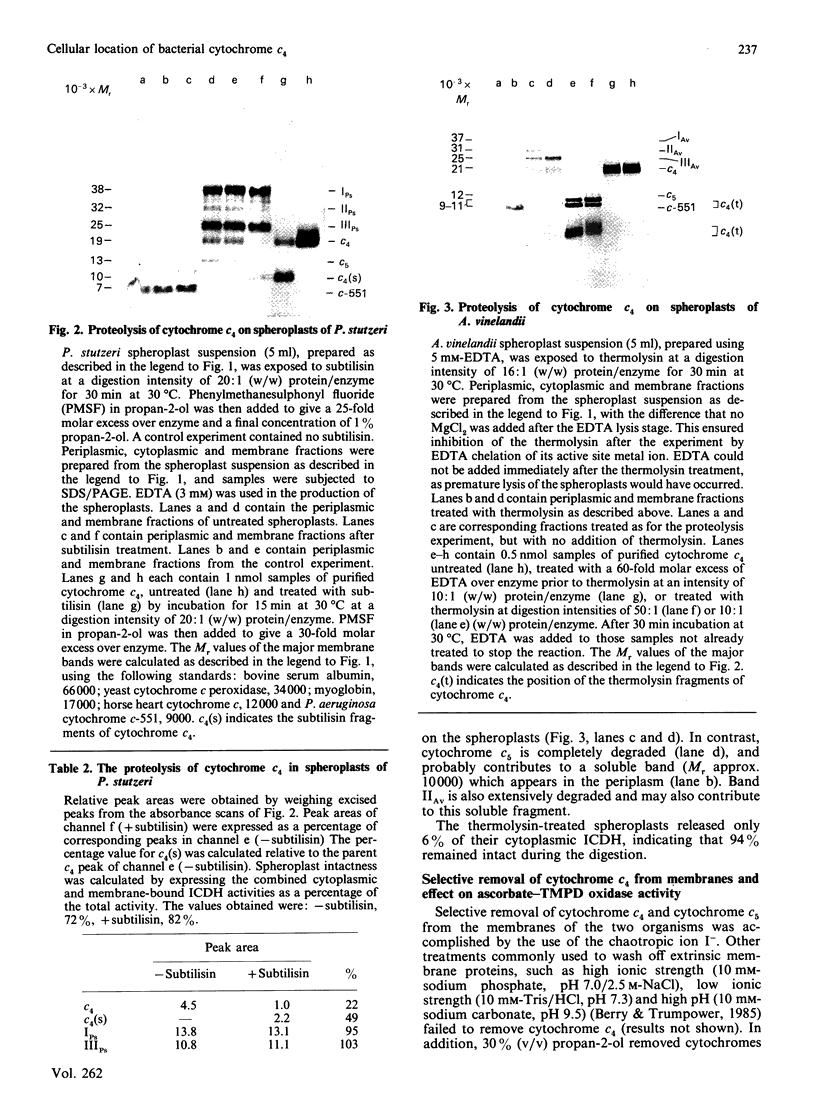
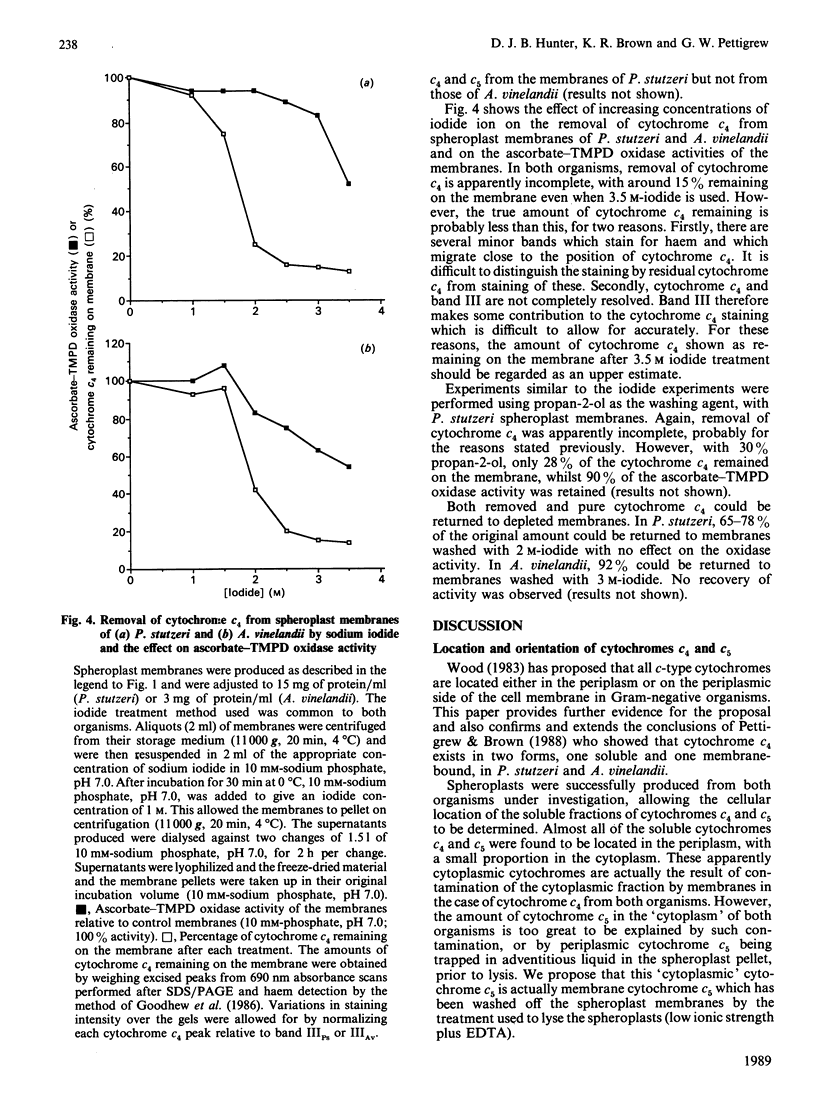
The cellular location of cytochrome c4 in Pseudomonas stutzeri and Azotobacter vinelandii was investigated by the production of spheroplasts. Soluble cytochrome c4 was found to be located in the periplasm in both organisms. The remaining cytochrome c4 was membrane-bound. The orientation of this membrane-bound cytochrome c4 fraction was investigated by proteolysis of the cytochrome on intact spheroplasts. In P. stutzeri, 78% of the membrane-bound cytochrome c4 could be proteolysed, whilst 82% of the spheroplasts remained intact, suggesting that the membrane-bound cytochrome c4 is on the periplasmic face of the membrane in this organism. Cytochrome c4 was not susceptible to proteolysis on A. vinelandii spheroplasts, in spite of being digestible in the purified state. Cytochrome c5 was shown to have a similar cellular distribution to cytochrome c4. Selective removal of cytochrome c4 from membranes of P. stutzeri was accomplished by the use of sodium iodide and propan-2-ol, with the retention of most of the ascorbate-TMPD (NNN'N'-tetramethylbenzene-1,4-diamine) oxidase activity associated with the membrane. Sodium iodide removed most of the cytochrome c4 from A. vinelandii membranes with retention of 62% of the ascorbate-TMPD oxidase activity. Cytochrome c4 could be returned to the washed membranes, but with no recovery of this enzyme activity. We conclude that cytochrome c4 is not involved in the ascorbate-TMPD oxidase activity associated with the membranes of these two organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Ferguson S. J. The location of dissimilatory nitrite reductase and the control of dissimilatory nitrate reductase by oxygen in Paracoccus denitrificans. Biochem J. 1980 Oct 15;192(1):231–240. doi: 10.1042/bj1920231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Melis K., Stout C. D. The amino acid sequence of the dihaem cytochrome c4 from the bacterium Azotobacter vinelandii. Biochem J. 1984 Aug 15;222(1):217–227. doi: 10.1042/bj2220217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem. 1985 Feb 25;260(4):2458–2467. [PubMed] [Google Scholar]
- Carter D. C., Melis K. A., O'Donnell S. E., Burgess B. K., Furey W. R., Jr, Wang B. C., Stout C. D. Crystal structure of Azotobacter cytochrome c5 at 2.5 A resolution. J Mol Biol. 1985 Jul 20;184(2):279–295. doi: 10.1016/0022-2836(85)90380-8. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Davis K. A., Baltscheffsky H., Baltscheffsky M., Johansson B. C. Isolation and properties of succinate dehydrogenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1972 Oct;152(2):613–618. doi: 10.1016/0003-9861(72)90257-3. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Wong T. Y. Isolation and purification of the cytochrome oxidase of Azotobacter vinelandii. Biochim Biophys Acta. 1981 Sep 14;637(2):374–382. doi: 10.1016/0005-2728(81)90176-6. [DOI] [PubMed] [Google Scholar]
- Leitch F. A., Brown K. R., Pettigrew G. W. Complexity in the redox titration of the dihaem cytochrome c4. Biochim Biophys Acta. 1985 Jul 17;808(2):213–218. doi: 10.1016/0005-2728(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Leonard K., Wingfield P., Arad T., Weiss H. Three-dimensional structure of ubiquinol:cytochrome c reductase from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1981 Jun 25;149(2):259–274. doi: 10.1016/0022-2836(81)90301-6. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Yoshida T., Findling K. L., Fee J. A. Observations on the c-type cytochromes of the extreme thermophile, Thermus thermophilus HB8: cytochrome c552 is located in the periplasmic space. Biochem Biophys Res Commun. 1981 Mar 31;99(2):591–599. doi: 10.1016/0006-291x(81)91786-1. [DOI] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- Pettigrew G. W., Brown K. R. Free and membrane-bound forms of bacterial cytochrome c4. Biochem J. 1988 Jun 1;252(2):427–435. doi: 10.1042/bj2520427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., McLain G., Smith L. Purification and properties of a c-type cytochrome from Micrococcus denitrificans. Biochemistry. 1971 May 25;10(11):2072–2076. doi: 10.1021/bi00787a017. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Burris R. H. Purification and properties of cytochromes c of Azotobacter vinelandii. Biochim Biophys Acta. 1969 Aug 5;180(3):473–489. doi: 10.1016/0005-2728(69)90026-7. [DOI] [PubMed] [Google Scholar]
- TISSIERES A. Purification, some properties and the specific biological activity of cytochromes c4 and c5 from Azotobacter vinelandii. Biochem J. 1956 Nov;64(3):582–589. doi: 10.1042/bj0640582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 1978 Aug 15;92(2):214–218. doi: 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Why do c-type cytochromes exist? FEBS Lett. 1983 Dec 12;164(2):223–226. doi: 10.1016/0014-5793(83)80289-0. [DOI] [PubMed] [Google Scholar]
- Yang T. Biochemical and biophysical properties of cytochrome o of Azotobacter vinelandii. Biochim Biophys Acta. 1986 Mar 12;848(3):342–351. doi: 10.1016/0005-2728(86)90209-4. [DOI] [PubMed] [Google Scholar]