Abstract
Background
In the two European Union (EU)-funded projects, PCM4EU (Personalized Cancer Medicine for all EU citizens) and PRIME-ROSE (Precision Cancer Medicine Repurposing System Using Pragmatic Clinical Trials), we aim to facilitate implementation of precision cancer medicine (PCM) in Europe by leveraging the experience from ongoing national initiatives that have already been particularly successful.
Patients and methods
PCM4EU and PRIME-ROSE gather 17 and 24 partners, respectively, from 19 European countries. The projects are based on a network of Drug Rediscovery Protocol (DRUP)-like clinical trials that are currently ongoing or soon to start in 11 different countries, and with more trials expected to be established soon. The main aims of both the projects are to improve implementation pathways from molecular diagnostics to treatment, and reimbursement of diagnostics and tumour-tailored therapies to provide examples of best practices for PCM in Europe.
Results
PCM4EU and PRIME-ROSE were launched in January and July 2023, respectively. Educational materials, including a podcast series, are already available from the PCM4EU website (http://www.pcm4eu.eu). The first reports, including an overview of requirements for the reimbursement systems in participating countries and a guide on patient involvement, are expected to be published in 2024.
Conclusion
European collaboration can facilitate the implementation of PCM and thereby provide affordable and equitable access to precision diagnostics and matched therapies for more patients.
KEYWORDS: DRUP-like clinical trials, targeted drugs, reimbursement, public-private collaboration, synthetic control arms, molecular tumour boards, implementation
Introduction
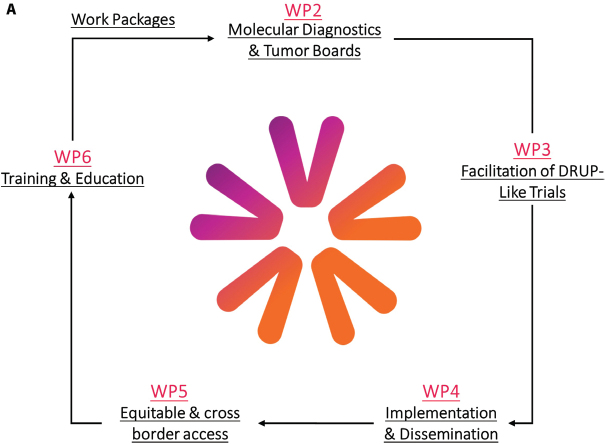
In recent years, evidence on the role of multigene sequencing in improving outcomes in patients with metastatic cancer has been widened, reinforcing the importance of precision medicine [1–3]. Several precision cancer medicine (PCM) initiatives have demonstrated the feasibility and benefits of implementing PCM within individual countries. PCM4EU (Personalized Cancer Medicine for all EU citizens) (http://www.pcm4eu.eu/) and PRIME-ROSE (Precision Cancer Medicine Repurposing System Using Pragmatic Clinical Trials) (http://www.prime-rose.eu/), are two projects funded through the EU4Health and Horizon Europe’s EU Mission on Cancer programmes. The projects are built on the successes of national initiatives centred around Drug Rediscovery Protocol (DRUP)-like clinical trials (DLCTs), intending to expand equitable and sustainable access to PCM for more patients by addressing key challenges related to implementation (see Figure 1).
Figure 1.
Overview of the planned work-packages. (A) Work packages in PCM4EU. H. Gelderblom (PCM4EU coordinator) is leading WP1, A. Edsjö and H. Russnes are leading WP2, U. Lassen and Å. Helland are leading WP3, E. Hult and K. Taskén are leading WP4, B. Ryll and U. Lassen are leading WP5, and I. Lugowska and J.Y. Blay are leading WP6. (B) Work packages in PRIME-ROSE. H. Gelderblom is leading WP1, R. Falk is leading WP2, Å. Helland is leading WP3, S.B van Waalwijk van Doorn-Khosrovani is leading WP4, K. Carlsson is leading WP5, B. Ryll is leading WP6, K. Taskén (PRIME-ROSE coordinator) is leading WP7, K. Jalkanen is leading WP8 whereas Charlotte J. Haug is the independent ethics review in WP9.
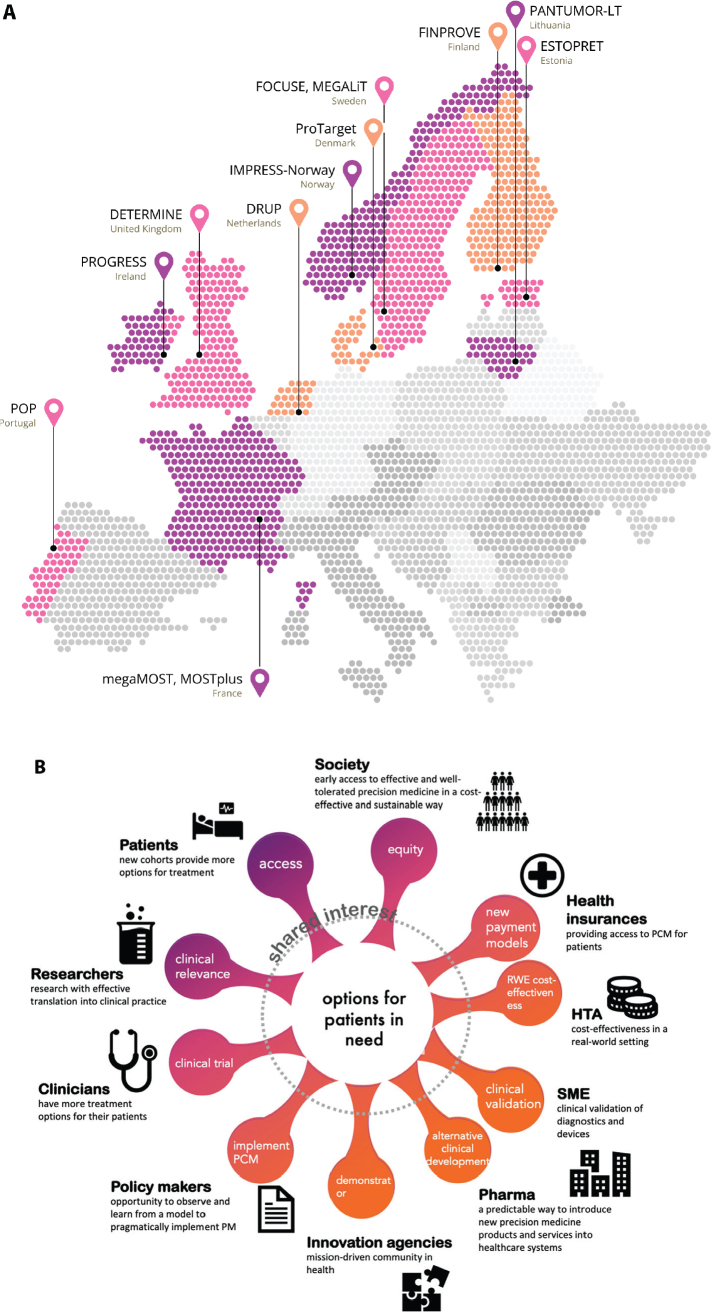
The success of the original DRUP-trial from the Netherlands, showing high inclusion rate and clinical benefit (CB), inspired similar trials in other countries [4]. All involved national trials are based on or aligned with DRUP (PIs Emile Voest, Hans Gelderblom, Henk Verheul) [5, 6], while being independently organised, governed and financed (Table 1). Such trials are ongoing or soon to be initiated in 11 European countries (Figure 2A), with more countries preparing to launch DLCTs. DRUP is also collaborating with the American TAPUR Study and CAPTUR in Canada, that are built on the same principles as the European trials [7, 8], and has aggregated data with the Australian MoST trial [9].
Table 1.
Overview of overlapping primary and secondary endpoints in the DRUP-like clinical trials.
| Endpoint in DRUP-like clinical trials | DRUP | ProTarget | IMPRESS | FINPROVE | MOSTplus | DETERMINE | POP | FOCUSE | ESTOPRET |
|---|---|---|---|---|---|---|---|---|---|
| Disease control at 16 weeks after treatment initiation | X | X | X | X | X | X* | X | X | X |
| Progression-free and overall survival | X | X | X | X | X | X | X | X | X |
| Duration of treatment on study (time on drug) | X | X | X | X | X | X | X | X | X |
| Treatment related grade ≥3 and SAE | X | X | X | X | X | X | X | X | |
| Objective tumour response | X | X | X | X | X | X | X | ||
| % of patients treated based on their molecular profile | X | X | X | X | X | X | X |
An overview of overlapping main endpoints in the ongoing DRUP-like clinical trials.
Durable Clinical Benefit, defined as the absence of disease progression for at least 24 weeks from the start of trial treatment. Dark blue = 100% similarity across trials, blue = 90% similarity across trials, and light blue = 80% similarity across trials.
Figure 2.
Overview of all ongoing and soon-to-start DRUP-like trials in Europe. (A) DRUP in the Netherlands, PIs E.E Voest, H. Gelderblom & H.M.W. Verheul, ProTarget in Denmark, PIs K.S. Rohrberg & U. Lassen. FINPROVE in Finland, PI K. Jalkanen, IMPRESS-Norway in Norway, PI Å. Helland, MOSTplus and megaMOST in France, PIs J.Y. Blay & L. Verlingue, DETERMINE in United Kingdom, PI M.G. Krebs, POP in Portugal, PI J. Oliveira, ESTOPRET in Estonia, PI K. Ojamaa, and PANTUMOR-LT in Lithuania, PI E. Baltruškevičienė. (B) Stakeholder involvement in PRIME-ROSE.
DLCTs are prospective phase II combined umbrella-basket trials in which patients with advanced cancers receive targeted therapies matched to genomic alterations in their tumour. Each trial enrols participants into defined cohorts, based on the combination of tumour type, molecular alteration, and targeted therapy, with CB as the primary endpoint. Common for the DLCTs is that they combine accessibility with affordability by providing broad access to PCM for patients who have exhausted all other standard treatment options. The trials continuously generate evidence that can be linked to pragmatic outcome-based reimbursement schemes, thereby enabling reimbursement when the necessary evidence (e.g. the PASKWIL criteria in the Netherlands) has been gathered [10, 11]. An overview of on-label and off-label reimbursement systems in Europe is also part of the deliverables in PRIME-ROSE.
The projects will provide recommendations regarding the use of next-generation sequencing (NGS)-panels and clinical decision support system (CDSS)-tools, together with detailed guidelines for molecular diagnostics in the cancer care pathway to facilitate implementation of PCM in additional countries. Furthermore, novel methods for establishing relative effectiveness, as well as strategies for evaluation of cost-effectiveness of PCM, will be developed. This will be used to facilitate access to new treatment options.
Advancing Molecular Diagnostics in PCM
PCM4EU aims to facilitate implementation of adequate molecular diagnostics into standard-of-care for all patients in Europe (Figure 1A). Clinical molecular diagnostic assays must detect all relevant genetic variants for adequate therapy decisions, while simultaneously identifying patients for clinical trials. The rapid development of new classes of targeted therapies and the promising results of immunotherapy, both necessitating use of more complex biomarkers, has resulted in a growing need for tools to guide in choosing NGS-based assays and CDSS tools.
To address this challenge, PCM4EU is gathering data on NGS-based assays used by the participating centres to create a curated database containing information on gene panel content in relation to targeted therapy and clinical trial inclusion criteria. Several of the DLCTs use both NGS and whole genome sequencing (WGS) as part of the diagnostic work-up. Based on available data, in-silico comparisons will be performed on commonly used NGS-panels versus WGS, and the added value of introducing WGS will be evaluated through health technology assessment. Real-life and synthetic large-scale datasets will be developed to harmonise the interpretation of key complex biomarkers such as microsatellite instability (MSI), tumour mutational burden (TMB), and homologous recombination deficiency (HRD).
PCM4EU will map out currently available CDSS tools for clinical decision-making in oncology. Evaluation will include feature mapping, tool requirements, integration options, compatibility with requirements for patient security, and In Vitro Diagnostic Regulation status. To expand and structure knowledge on available CDSS tools, including artificial intelligence-based tools, these tools and their knowledge management strategies will be charted. Moreover, the project will develop a conceptual framework for performance testing across multiple cancer-relevant features beyond the core variant characterisation, like single nucleotide variants and insertions and deletions, including copy-number variations, selected complex biomarkers (i.e., TMB, HRD, MSI), neoantigens, transcription profiles and specific gene signatures (e.g. immune signatures), and gene fusions. Finally, we plan to evaluate the performance of clinical trial matching across CDSS platforms to improve harmonisation between different recommendations by enhancing the accuracy of available clinical trial data and mapping the identification and definition of actionable targets [12].
Initiation of DLCTs in Europe
The PCM4EU and PRIME-ROSE projects will support countries without DLCTs in setting up trials in countries where they are not yet available by sharing current protocols, standard operating procedures, electronic case report forms (eCRFs), and ways for harmonised data collection (Figure 1). Each DRUP-founder country will co-create efficient knowledge transfer to centres in the start-up phase.
For joint data analyses across DLCTs, the key primary endpoint for the combined analyses is uniformly implemented across the trials and defined as CB, meaning a confirmed stable disease, partial or complete response at 16 weeks after treatment initiation (Table 1). A formal data sharing protocol has been developed and agreed upon.
Access to New Therapies: Single Point Access to Multi-Trial Network
Data sharing and aggregation between the trials will allow for monitoring of cohort recruitment from each trial and cross-trial evaluation of cohorts when recruitment is complete, increasing the inclusion rate. Moreover, data sharing will facilitate collaboration with the pharmaceutical industry. Pharmaceutical companies can choose to access all trials simultaneously with their drugs through PRIME-ROSE, providing a single point of entry, where pharma partners provide free drugs and floating treatment slots to the consortium. This approach will significantly reduce time for initiation and rapidly increase inclusion rates while removing a major barrier for establishing a DLCT, since access to drugs is critical for trial initiation.
Establishment of Relative Effect: Innovative Models for Synthetic Control Cohorts
DLCTs are actively searching for patients with defined target mutations to treat them with the specified drugs. Randomisation for treatment allocation after progression on standard-of-care is not advised due to the rarity of driver genomic alterations and the lack of treatment alternatives. To establish relative efficacy, multiple synthetic control cohorts will be built to reduce bias and resembling a randomised controlled trial.
PRIME-ROSE will establish three types of control cohorts, based on available data from registries and sequenced cohorts that have received standard-of-care therapy: 1) Patients with a known genomic alteration that received standard treatment. However, as specific molecular alterations will have a low prevalence, these control cohorts might be small. 2) Patients without a targetable genomic alteration. The characteristics of patients with and without genomic alterations will be compared to find patterns associated with specific targets and calculate the expected probability of the alterations. 3) Extraction of larger patient control cohorts from registry data. For these cohorts, information about the presence or absence of a specific mutation will not necessarily be available, but the frequency of the mutation in the population may be known. Therefore, based on the probability of the target mutation, randomisation will be performed to synthetic control arms to reduce selection bias.
Implementation into Standard-of-Care
Whereas regulatory approval is centralised to the European Medicines Agency, reimbursement systems are country-specific, with varying requirements in terms of data. To facilitate implementation, PRIME-ROSE will identify requirements of reimbursement systems in participating countries, including systems for off-label reimbursement. A model for economic evaluation will be designed and constructed, including budget impact and cost-effectiveness analysis of PCM, integrating the chain of decision from molecular diagnostics to treatment. Information collected on local requirements for reimbursement assessment will be used to ensure that conducted analyses will meet the criteria for decision-making in different countries. Furthermore, collected data will provide a background to evaluate and compare how differences in reimbursement systems can impact access, timing and affordability.
National Guidelines for Precision Diagnostics
To facilitate the development of national guidelines for precision diagnostics, the PCM4EU consortium will develop a best practice on which cancer patients should be offered molecular diagnostics and what should be included in the molecular diagnostic work-up as part of standard-of-care. This includes recommendations on selecting the most appropriate molecular assays and how to process data to match results with anti-cancer therapies according to up-to-date evidence (in accordance with European Society for Medical Oncology (ESMO) clinical guidelines [13]). The guidelines will focus on the added value of using advanced diagnostic tests in terms of higher precision in choosing treatment strategies as well as costs and value of implementation. We aim to include costs from a wide societal perspective to demonstrate broader impact. However, results will be reported disaggregated to allow for interpretations from more narrow perspectives preferred in some countries.
Risk-Sharing Agreement and Access to Therapies
Participants in pivotal trials are usually not representative of patients encountered in standard clinical practice, especially since rare tumour types are often not represented. Here, we face the ragged edges of clinical practice, where off-label use is common since there are no alternative treatment options, the effectiveness and safety data are often not collected, the practice is not harmonise or regulated, and there are disparities and inequalities in access. A comprehensive approach to this clinical reality can stimulate repurposing drugs to offer safe, effective, and affordable treatment options for the diverse cancer patient population commonly seen in clinical practice.
To evaluate the effectiveness of a treatment in routine clinical practice, real-world data will be systematically collected. Such practice is carried out by the DRUG Access Protocol (DAP) in the Netherlands [14]. DAP is a pragmatic, non-randomised protocol that prospectively collects effectiveness and toxicity data on novel authorised or unauthorised anticancer therapies awaiting regulatory approval and reimbursement, but also authorised anticancer therapies that are not being reimbursed for an on-label or off-label indication due to data gaps.
In PRIME-ROSE, strategies for pragmatic outcome-based risk-sharing agreements will be explored by identifying factors that were critical for the successful establishment of such agreements in the Netherlands and Norway [15]. Based on a mapping of the varying requirements as regards to the implementation of new treatments and expansion to new treatment groups, PRIME-ROSE will assist stakeholders, including national healthcare providers, policymakers, and authorities in EU regions, Member States, and Associated Countries who desire to implement pragmatic reimbursement models for PCM in a real-world setting. The potential for a European framework to evaluate data effectively and efficiently to implement PCM will also be investigated, especially for rare cancers and those with high unmet medical needs. In addition, we will explore the potential of using DRUP-generated evidence to inform regulatory decision-makers at the European level.
PCM addressing Patient Needs
The patient relevance of the clinical trial endpoints in use will be confirmed to ensure that DLCTs adequately address patients’ needs. Currently, a multi-stakeholder consultation that will inform the selection of health-related quality-of-life endpoints for use across the joint cohorts is in preparation. Furthermore, the stability of patient preferences during and after trial participation will be investigated. Moreover, we are working with a growing community of European cancer patient advocates with expertise in PCM and iterate on existing patient involvement to ensure consistent and meaningful patient involvement throughout the work of the PCM community.
Successful Multistakeholder Collaborations for Change in Complex Systems
Changes in the healthcare system require participation and interaction between a multitude of stakeholders (Figure 2B). In PRIME-ROSE, we plan to map and describe the PCM ecosystem and develop a PCM theory of change for healthcare as a complex adaptive system with implications for governance, policy, and innovation. We will reflect on differences between the Member States. Building on the concept of Living Labs [16] and the already established practice of peer-to-peer support, the project will develop a ‘DRUP methodology’ to facilitate implementation.
Conclusion
The PCM4EU and PRIME-ROSE projects strive to reduce inequality in cancer treatment by promoting access to PCM for all European patients through collaborative efforts, patient engagement, and pragmatic outcomes-based approaches.
Acknowledgement
Key participants in the PCM4EU and PRIME-ROSE consortia are named as consortia authors. We are also grateful to a number of individuals, institutions, industry partners, and grant agencies that are not listed, but contribute to building national initiatives and DRUP-like clinical trials in each country.
This paper has been included in the Nordic Precision Cancer Medicine, NPCM, 2023 Symposia Collection
Funding Statement
Funding The European DRUP-like trial collaboration is supported by Precision Cancer Medicine for all EU Citizens (PCM4EU), co-funded by the EU4Health programme as part of Europe’s Beating Cancer Plan (grant: 101079984), Precision Cancer Medicine Repurposing System Using Pragmatic Clinical Trial (PRIME-ROSE), funded under the Horizon Europe programme (grant: 101104269), and The Nordic Precision Cancer Medicine Trial Network funded under the Nordic Trial Alliance programme.
Author contributions
Project design: KT, GLF, RSF, ÅH, SBWDK, KSC, BR, KJ, AE, HGR, UL, EHH, IL, JYB, LV, MAL, MGK, KSR, KO, JO, HV, EV, HG; draft manuscript: KT, GLF, SFHM, HG. All authors reviewed the results and approved the final version of the manuscript.
Disclaimer
The views and opinions expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of their respective organisations, those of the European Union or the Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
No new data were presented in this study. Data sharing is not applicable to this short report.
Ethics declarations & trial registry information
The projects reported in this manuscript adhere to high ethical standards and complies with all relevant legislation.
References
- [1].Hoes LR, van Berge Henegouwen JM, van der Wijngaart H, Zeverijn LJ, van der Velden DL, van de Haar Jet al. Patients with rare cancers in the Drug Rediscovery Protocol (DRUP) benefit from genomics-guided treatment. Clin Cancer Res. 2022. Apr 1;28(7):1402–1411. 10.1158/1078-0432.CCR-21-3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andre F, Filleron T, Kamal M, Mosele F, Arnedos M, Dalenc Fet al. Genomics to select treatment for patients with metastatic breast cancer. Nature. 2022. Oct;610(7931):343–348. 10.1038/s41586-022-05068-3 [DOI] [PubMed] [Google Scholar]
- [3].Leary A, Besse B, André F. The need for pragmatic, affordable, and practice-changing real-life clinical trials in oncology. Lancet. 2023;403(10424):406-408. 10.1016/S0140-6736(23)02199-2 [DOI] [PubMed] [Google Scholar]
- [4].van der Velden DL, Hoes LR, van der Wijngaart H, van Berge Henegouwen JM, van Werkhoven E, Roepman Pet al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature. 2019;574(7776):127–131. 10.1038/s41586-019-1600-x [DOI] [PubMed] [Google Scholar]
- [5].Helland Å, Russnes HG, Fagereng GL, Al-Shibli K, Andersson Y, Berg Tet al. Improving public cancer care by implementing precision medicine in Norway: IMPRESS-Norway. J Transl Med. 2022;20(1): 317. 10.1186/s12967-022-03432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kringelbach T, Højgaard M, Rohrberg K, Spanggaard I, Laursen BE, Ladekarl Met al. ProTarget: a Danish Nationwide Clinical Trial on Targeted Cancer Treatment based on genomic profiling – a national, phase 2, prospective, multi-drug, non-randomized, open-label basket trial. BMC Cancer. 2023. Feb 22;23(1):182. 10.1186/s12885-023-10632-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mangat PK, Halabi S, Bruinooge SS, Garrett-Mayer E, Alva A, Janeway KAet al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis Oncol. 2018;2018:10.1200. 10.1200/PO.18.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Skamene T, Siu L, Renouf D, Laskin J, Bedard P, Jones Set al. Canadian profiling and targeted agent utilisation trial (CAPTUR/PM.1): a phase II basket precision medicine trial. J Clin Oncol. 2018:36(15-suppl): TPS12127. 10.1200/JCO.2018.36.15_suppl.TPS12127 [DOI] [Google Scholar]
- [9].Zeverijn LJ, Looze EJ, Thavaneswaran S, van Berge Henegouwen JM, Simes RJ, Hoes LRet al. Limited clinical activity of palbociclib and ribociclib monotherapy in advanced cancers with cyclin D-CDK4/6 pathway alterations in the Dutch DRUP and Australian MoST trials. Int J Cancer. 2023. Oct 1;153(7):1413–1422. 10.1200/JCO.2023.41.16_suppl.3101 [DOI] [PubMed] [Google Scholar]
- [10].van der Wijngaart H, Hoes LR, van Berge Henegouwen JM, van der Velden DL, Zeverijn LJ, Roepman Pet al. Patients with Biallelic BRCA1/2 inactivation respond to olaparib treatment across histologic tumor types. Clin Cancer Res. 2021;27(22):6106–6114. 10.1158/1078-0432.CCR-21-1104 [DOI] [PubMed] [Google Scholar]
- [11].van Waalwijk van Doorn-Khosrovani SB, Pisters-van Roy A, van Saase L, van der Graaff M, Gijzen J, Sleijfer Set al. Personalised reimbursement: a risk-sharing model for biomarker-driven treatment of rare subgroups of cancer patients. Ann Oncol. 2019;30(5):663–665. 10.1093/annonc/mdz119 [DOI] [PubMed] [Google Scholar]
- [12].Rosenquist R, Edsjö A, Russnes H, Lethiö J, Tamborero D, Hovig Eet al. High-throughput molecular assays for inclusion in personalised oncology trials – state-of-the-art and beyond. J Intern Med. 2024. Jun;295(6):785-803. 10.1111/joim.13785 [DOI] [PubMed] [Google Scholar]
- [13].Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas Net al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018. Sep 1;29(9):1895–1902. 10.1093/annonc/mdy263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zeverijn LJ, van Waalwijk van Doorn-Khosrovani SB, van Roy AAMGP, Timmers L, Ly Tran TH, de Boer JEet al. Harmonising patient-access programmes: the Dutch DRUG Access Protocol platform. Lancet Oncol. 2022. Feb;23(2):198–201. 10.1016/S1470-2045(21)00707-5 [DOI] [PubMed] [Google Scholar]
- [15].Taskén K, Russnes HEG, Aas E, Bjørge L, Blix ES; CONNECT Public–Private Partnership Consortium. et al. A national precision cancer medicine implementation initiative for Norway. Nat Med. 2022. May;28(5):885–887. [DOI] [PubMed] [Google Scholar]
- [16].Steen K, van Bueren E. Urban living labs: a living lab way of working. 2017: pp1-96. (publ Amsterdam Institute for Advanced Metropolitan Solutions and Delft University of Technology, Amsterdam, The Netherlands: ) https://www.ams-institute.org/documents/28/AMS_Living_Lab_Way_of_Working-ed4.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were presented in this study. Data sharing is not applicable to this short report.