Abstract
Background
Whether sex is an independent prognostic factor in lung cancer survival is the subject of ongoing debate. Both large national registries and single hospital studies have shown conflicting findings. In this study, we explore the impact of sex on lung-cancer-specific survival in an unselected population that is well-characterized with respect to stage and other covariates.
Material and methods
All patients diagnosed with lung cancer at a single hospital serving a whole and defined region in Southern Norway during the 10 years 2007–2016 were included. Follow-up data were available for at least 56 months for all patients. Analyses were adjusted for stage, treatment, performance status, smoking, age, histology, epidermal growth factor receptor/anaplastic lymphoma kinase/immunotherapy treatment and period. Differences in lung-cancer-specific survival by sex were explored using restricted mean survival times (RMST).
Results
Of the 1,261 patients diagnosed with lung cancer, 596 (47%) were females and 665 (53%) males, with mean ages of 68.5 and 69.5 years, respectively. The observed 5-year lung-cancer-specific survival rate was 27.4% (95% CI 23.7, 31.2) in females and 21.4% (95% CI 18.2, 24.8) in males. However, after adjustment for covariates, no significant differences by sex were observed. The 5-year RMST was 0.9 months shorter (95% CI −2.1, 0.31, p = 0.26) in males compared to females.
Interpretation
In this cohort, sex was not associated with a difference in lung-cancer-specific survival after adjusting for clinical and biological factors. Imbalance in stage at diagnosis was the main contributor to the observed difference in lung-cancer-specific survival by sex.
KEYWORDS: Lung-cancer-specific survival, sex, curative treatment, palliative treatment
Introduction
In the last decade, the 5-year survival rate for lung cancer patients has nearly doubled in Scandinavia and other countries [1–4]. In Norway, the increase has been from a modest 15% in 2010 to 29.2% in 2021 and applies to both sexes [2]. However, a difference in lung cancer relative survival by sex is evident, with an expected 5-year survival of 25.7% among men versus 32.8% among women in the diagnostic period 2017–2021 [2].
Several epidemiological studies have reported that sex appears to be associated with lung-cancer-specific survival after adjusting for other possible prognostic factors [5–7], leading to suggestions that sex should be considered in treatment decisions [8]. However, the subject remains debatable since other studies have observed no survival difference [9–11]. Several former studies regarding sex-related survival have reported either superior female survival [12–14] or no significant difference [9, 15]. Moreover, most previous studies have been restricted to specific treatments (e.g. surgery [9, 13, 16, 17]), histologies (e.g. adenocarcinoma [7, 18]) or single stages (e.g. stage IV [8]). These former studies may suffer from limitations in reporting survival in only selected groups of patients. There is a lack of studies examining unselected and complete populations across all treatments, histologies and stages, mirroring a real-life population.
To further explore differences in lung-cancer-specific survival by sex, we report results based on data from a single hospital representing a complete, unselected population of lung cancer patients over a 10-year period. Firstly, we aimed to assess whether there is a significant difference in lung-cancer-specific survival between females and males after adjusting for multiple, relevant covariates. Secondly, we determined if sex-related differences in survival were observed in subgroups of patients treated with curative or palliative intent or for patients with adenocarcinoma histology.
Material and methods
Study population and inclusion criteria
Virtually all patients with lung cancer in the western part of the administrative region Agder in Norway are diagnosed at Sørlandet Hospital Kristiansand, a community hospital serving a population of 200,000 inhabitants. Fifteen per cent of the population are immigrants with 10% of the total population being non-Caucasian and 5% of Asian descent. Sørlandet Hospital offers free, up-to-date diagnostic investigation and treatment to everyone. There are no other competing options for the initial diagnosis and treatment of lung cancer in the region. During the 10-year period from 2007 to 2016, all patients given a lung cancer diagnosis according to code C34 in the 10th revision of the International Classification of Disease (ICD-10) were registered and added to hospital’s clinical lung cancer database by a single physician and a trained nurse. Patients without tissue-verified diagnosis of lung cancer were included.
Clinical data
Clinical parameters included sex, age at diagnosis, year of diagnosis (2007–2012 or 2013–2016), smoking status (present, former or never), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0, 1, 2, 3 or 4), histology (adenocarcinoma, squamous cell carcinoma, small-cell lung carcinoma, no biopsy or other), stage (7th Tumor-Node-Metastasis (TNM) classification was used for all patients, including SCLC) and treatment. Tyrosine-kinase inhibitors (epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutation) or immune-oncology therapy were treated as separate binary variables.
Due to a high degree of correlation between stage and treatment, we created a combined variable including combinations of stage and treatment (stage I/surgery, stage I/stereotactic body radiotherapy (SBRT), stage I/palliative treatments (PT) or supportive care only (SCO), stage II/surgery, stage II/SBRT and chemoradiotherapy of curative intent (CRT), stage II/PT or SCO, stage III/surgery, stage III/SBRT and CRT, stage III/PT, stage III/SCO, stage IV/PT or stage IV/SCO) to capture differences in stage and treatment distribution between males and females. In general, patients without biopsy confirmation were also included based on typical morphology on chest CT scanning in case of PT. In patients with curative intent treatment and no biopsy, growth on consecutive CT scans was required in addition to positive FDG-uptake on PET-CT scanning.
The diagnostic year was stratified into 2007–2012 and 2013–2016, due to a local initiative involving diagnostic work-up that was initiated in 2013.
To investigate the second aim, patients were divided into two major treatment groups, curative and palliative intent, defined by the initial treatment received. The former group included patients receiving either surgery, SBRT, CRT or radiation with curative intent (fractionated radiotherapy with cumulative dosage ≥60 Gy). To ensure group uniformity, we included only patients with biopsy-verified lung cancer and ECOG 0–2. The patients treated with palliative intent were divided into two groups. ‘Palliative treatments’ included patients who received palliative chemotherapy and/or radiotherapy. ‘Supportive care only’ included patients who did not receive tumor-specific treatments.
All patients were followed until the end of August 2021. The date of death was available for all patients from the National Population Register of Norway. The underlying cause of death was used to identify deaths related to lung cancer. Only one patient was lost to follow-up.
With the available sample of 1,261 patients, we estimated that we were well within the required sample size of a non-superiority study. This was based on a non-superiority limit of 3 months, i.e. the largest difference in 5-year survival that is clinically acceptable [14]. The standard deviation of the 5-year survival time was set to 9 months. Then, if there is truly no difference between the sexes, 224 patients (both sexes combined) are required to be 80% certain (i.e. power) so that the lower limit of a one-sided 95% confidence interval will be above the non-inferiority limit of 3 months. Thus, many covariates can be adjusted for the analysis that considers the observational design of the data. For the secondary endpoints, the study was also appropriately powered for the subgroups defined by treatment in curative or palliative intent and adenocarcinoma histology.
This study was approved by the Regional Committees for Medical Research Ethics South-East Norway.
Statistical analysis
Categorical variables are presented as frequencies and percentages and continuous variables as means with standard deviation (SD). Comparison between males and females was performed by students’ t-tests for continuous and chi-squared tests for categorical variables.
Lung-cancer-specific survival was defined as the time from diagnosis of lung cancer to date of death due to lung cancer. Patients who died of other causes than lung cancer were censored at the date of death, and patients alive at the end of the study were censored on August 31, 2021 (end of follow-up). Cumulative mortality by cause of death up to 5 years of follow-up was presented separately for males and females.
To explore differences in lung-cancer-specific survival between males and females, we performed restricted mean survival times (RMST) and Cox regression analyses. Because the proportional hazard assumption was unmet, we present RMST as the main analysis. RMST is the mean event-free cause-specific survival time to a prespecified time t [19]. It corresponds to the area under the Kaplan–Meier curve up to time t, and is a robust measure that is not dependent on proportional hazards. A flexible parametric Royston–Parmar model with adjustment for age, smoking status, period, combined stage and treatment variable, ECOG performance status, histology and EGFR/ALK/immunotherapy treatment was used [20]. The difference in RMST in men compared to women was calculated at 1, 3, and 5 years of follow-up.
To estimate the impact of the difference in stage distribution by sex, we calculated the change in estimate (CIE) of sex as CIE = (O – Z)/O. O (only sex) was the unadjusted estimate of sex using RMST, and Z (sex + stage) was the estimate of sex adjusted for stage. Similar calculations were performed with combined stage and treatment variable instead of only stage.
To investigate the second aim, where we explored sex-related differences between subgroups of patients, stratified analysis was performed with relevant adjustments based on treatment with curative and palliative intent and adenocarcinoma histology. Variable adjustments for the different subgroups are given in Table 3. Thereafter, two sensitivity analyses were performed: (1) A subgroup analysis of patients diagnosed after the quality initiative initiated in 2013 and (2) Cox regression analysis was performed, although the proportional hazard assumption was not met.
Table 3.
Lung-cancer-specific RMST in males compared to females treated with curative or palliative intent and for different histologies.
| Stratification | N | Follow-up | Difference in RMST (months) | 95% confidence interval |
|---|---|---|---|---|
| Treatment intent | ||||
| Curative treatmenta,c | 409 | 5 years | -0.07 | -3.4, 3.2 |
| Palliative treatmentb,c | 804 | 2 years | -0.88 | -1.9, 0.16 |
| Histology | ||||
| Adenocarcinomad | 545 | 5 years | -1.0 | -2.9, 0.88 |
Surgery, stereotactic beam radiation therapy or chemoradiation with curative intent, ECOG 0–2 and histology confirmed lung cancer. RMST: restricted mean survival time.
Palliative chemotherapy, radiotherapy, chemoradiotherapy or supportive care only.
Adjusted for combined stage and treatment group, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking, age, histology, EGFR/ALK/immunotherapy treatment and period.
Adjusted for the same as c except histology.
Power calculations ensured adequate power in main RMST calculation as well as in included subgroups.
All statistical significance tests were 2-sided, at the 0.05 level. Statistical analyses were performed using Stata, version 16 (StataCorp LLC). The STPM2 function was used for the RMST analysis.
Results
Of the 1,261 patients diagnosed with lung cancer in the period 2007–2016, 596 (47%) were females and 665 (53%) males (Table 1). Compared to males, females were more frequently diagnosed with stage I disease (25% vs. 17%) and less frequently with stage IV (53% vs. 45%). There was no difference in the use of PET-CT scanning in stages I to III in females (63%) and males (67%). Women received more often treatment with curative intent (40%) compared to men (33%) (p = 0.007). Present smokers and never smokers were more common in the female compared to the male populations (54% and 9% vs. 47% and 2%, respectively). In the patients receiving PT, squamous cell carcinoma was more predominant in males, while small cell lung cancer (SCLC) was more common in females. However, significant histological differences were not found in patients receiving curative treatment.
Table 1.
Characteristics by sex in overall lung cancer population and in patients with curative and palliative intent treatment from 2007 to 2016.
| Characteristics | All | Curative | Palliative | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women n = 596 | Men n = 665 | P | Women n = 239 | Men n = 218 | P | Women n = 357 | Men n = 447 | P | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Age in years, mean (SD) | 68.5 | 10.9 | 69.5 | 10.0 | 0.09 | 66.8 | 8.7 | 67.1 | 9.3 | 0.73 | 69.7 | 12.0 | 70.7 | 10.1 | 0.19 |
| Period | 0.09 | 0.46 | 0.21 | ||||||||||||
| 2007–2012 | 333 | 55.9 | 403 | 60.6 | 120 | 50.2 | 117 | 53.7 | 213 | 59.7 | 286 | 64.0 | |||
| 2013–2016 | 263 | 44.1 | 262 | 39.4 | 119 | 49.8 | 101 | 46.3 | 144 | 40.3 | 161 | 36.0 | |||
| Smoking | < 0.001 | 0.02 | < 0.001 | ||||||||||||
| Present | 323 | 54.2 | 312 | 46.9 | 128 | 53.6 | 100 | 45.9 | 195 | 54.6 | 212 | 47.4 | |||
| Former | 222 | 37.2 | 338 | 50.8 | 97 | 40.6 | 113 | 51.8 | 125 | 35.0 | 225 | 50.3 | |||
| Never | 51 | 8.6 | 15 | 2.3 | 14 | 5.9 | 5 | 2.3 | 37 | 10.4 | 10 | 2.2 | |||
| ECOG PS | 0.81 | 0.57 | 0.20 | ||||||||||||
| 0 | 164 | 27.5 | 197 | 29.6 | 117 | 49.0 | 125 | 57.3 | 47 | 13.2 | 72 | 16.1 | |||
| 1 | 200 | 33.6 | 230 | 34.6 | 93 | 38.9 | 71 | 32.6 | 107 | 30.0 | 159 | 35.6 | |||
| 2 | 107 | 18.0 | 111 | 16.7 | 23 | 9.6 | 18 | 8.3 | 84 | 23.5 | 93 | 20.8 | |||
| 3 | 100 | 16.7 | 99 | 14.9 | 6 | 2.5 | 4 | 2.8 | 95 | 26.6 | 96 | 21.5 | |||
| 4 | 25 | 4.2 | 28 | 4.2 | 0 | 0 | 0 | 24 | 6.7 | 27 | 6.0 | ||||
| Histology a | 0.03 | 0.36 | 0.01 | ||||||||||||
| Adenoc. | 254 | 42.6 | 291 | 43.8 | 111 | 46.4 | 102 | 46.8 | 143 | 40.1 | 189 | 42.3 | |||
| Squamous cc. | 94 | 15.8 | 145 | 21.7 | 55 | 23.1 | 65 | 29.8 | 39 | 10.9 | 80 | 17.9 | |||
| Small-cell c. | 96 | 16.1 | 87 | 13.1 | 19 | 7.9 | 14 | 6.4 | 78 | 21.8 | 73 | 16.3 | |||
| No-biopsy | 99 | 16.6 | 91 | 13.7 | 24 | 10.0 | 18 | 8.3 | 75 | 21.0 | 73 | 16.3 | |||
| Other | 53 | 8.9 | 51 | 7.7 | 30 | 12.6 | 19 | 8.7 | 22 | 6.2 | 32 | 7.2 | |||
| TNM stage b | 0.002 | 0.03 | 0.72 | ||||||||||||
| I | 151 | 25.3 | 115 | 17.3 | 137 | 57.3 | 98 | 45.0 | 14 | 3.9 | 17 | 3.8 | |||
| II | 49 | 8.2 | 63 | 9.5 | 41 | 17.2 | 55 | 25.2 | 8 | 2.2 | 8 | 1.8 | |||
| III | 128 | 21.5 | 134 | 20.1 | 60 | 25.1 | 61 | 28.0 | 68 | 19.1 | 73 | 16.3 | |||
| IV | 268 | 45.0 | 353 | 53.1 | 1 | 0.4 | 4 | 1.8 | 267 | 74.8 | 349 | 78.1 | |||
| Treatment | 0.06 | 0.69 | 0.33 | ||||||||||||
| Surgery | 130 | 21.8 | 124 | 18.6 | 130 | 54.4 | 124 | 56.9 | |||||||
| SBRT | 48 | 8.1 | 37 | 5.6 | 48 | 20.1 | 37 | 17.0 | |||||||
| CRT | 61 | 10.2 | 57 | 8.6 | 61 | 25.5 | 57 | 26.1 | |||||||
| Palliative treatmentc | 223 | 37.4 | 294 | 44.2 | 223 | 62.5 | 294 | 65.8 | |||||||
| Supportive care only | 134 | 22.5 | 153 | 23.0 | 134 | 37.5 | 153 | 34.2 | |||||||
| Treatment EGFR, ALK or immuno | 0.27 | 0.84 | 0.25 | ||||||||||||
| No | 574 | 96.3 | 632 | 95.0 | 230 | 96.2 | 209 | 95.9 | 344 | 96.4 | 423 | 94.6 | |||
| Yes | 22 | 3.7 | 33 | 5.0 | 9 | 3.8 | 9 | 4.1 | 13 | 3.6 | 24 | 5.4 | |||
Data are shown as n (%) except when otherwise specified.
ECOG PS: Eastern Cooperative Oncology Group performance status; SBRT: stereotactic beam radiation therapy; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase, SD: standard deviation; adenoc.: adenocarcinoma; squamous cc.: squamous-cell cancer; CRT: chemoradiation therapy.
Other histology includes NSCLC NOS and carcinoids.
TNM version 7.
cPalliative chemotherapy, radiotherapy or chemoradiotherapy.
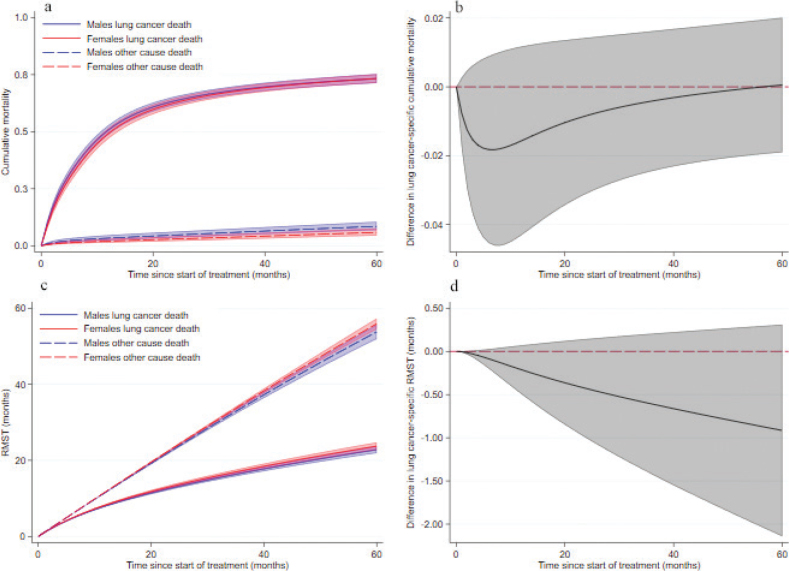
During follow-up, a total of 916 lung-cancer-specific deaths (498 in males and 418 in females) and 105 deaths due to other causes than lung cancer (58 in males and 47 in females) occurred. Unadjusted 1-year lung cancer-specific survival was 47.7% (95% confidence interval [CI] 43.8, 51.5) in males and 54.5% (95% CI 50.4, 58.4) in females. The corresponding 5-year survival was 21.4% (95% CI 18.2, 24.8) and 27.4% (95% CI 23.7, 31.2), respectively. The cause-specific cumulative mortality by sex is presented in Figure 1a. No difference between sexes was observed in lung-cancer-specific cumulative mortality (Figure 1b).
Figure 1.
Cumulative mortality and RMST with 95% confidence interval by sex in cohort of lung cancer patients during 5 years of follow-up after start of treatment. (a) Cumulative mortality of lung cancer and other deaths for females and males. (b) Difference in lung-cancer-specific cumulative mortality by sex. (c) RMST of lung cancer death and other death for females and males. (d) Difference in RMST of lung cancer death by sex. RMST: restricted mean survival time.
Unadjusted lung-cancer-specific RMST was significantly shorter in males compared to females at 5 years (−3.6 months, 95% CI −6.2, −1.0) (Table 2).
Table 2.
Lung-cancer-specific RMST in males compared to females.
| Adjustments | Follow-up | Difference in RMST (months) | 95% Confidence Interval | P |
|---|---|---|---|---|
| Unadjusted | 1 year | −0.52 | −0.89, −0.15 | 0.03 |
| 3 years | −2.1 | −3.6, −0.61 | 0.01 | |
| 5 years | −3.6 | −6.2, −1.0 | 0.006 | |
| Adjusteda | 1 year | −0.21 | −0.50, 0.07 | 0.19 |
| 3 years | −0.61 | −1.4, 0.21 | 0.35 | |
| 5 years | −0.92 | −2.1, 0.31 | 0.26 |
Difference in lung-cancer-specific RMST in males and females at 1, 3 and 5 years. Negative numbers indicating shorter survival in males. RMST: restricted mean survival time.
Adjusted for combined stage and treatment group, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking, age, histology, EGFR/ALK/immunotherapy treatment and period.
There was no difference in lung-cancer-specific RMST by sex after adjusting for covariates (Table 2, Figures 1c and 1d). At 1, 3 and 5 years, males had 0.2 months (95% CI −0.5, 0.1), 0.6 months (95% CI −1.4, 0.2) and 0.9 months (95% CI −2.1, 0.3) shorter lifetime expectancy than females, although not statistically significant.
The estimated impact of adding information about stage to the unadjusted model, including only sex, showed a change from −3.6 to −0.1 months in the 5-year RMST, corresponding to a CIE of 98%. Similar calculations for the combined stage and treatment variable showed a CIE of 83%.
Stratified analysis by treatment intent showed no statistical differences by sex. In a well-defined population of patients treated with curative intent (surgery, SBRT or CRT) with tissue-verified lung cancer and ECOG 0–2, no difference in survival was found (−0.1 months, 95% CI −3.4, 3.2) at 5 years (Table 3). Similarly, in patients receiving PT or SCO, no difference was found at 2 years (−0.9 months, 95% CI −1.9, 0.2).
Stratification according to the histological subgroup adenocarcinoma also revealed no significant differences by sex.
In sensitivity analyses, results of univariate Cox regression analysis suggested higher mortality among males compared to females (hazard ratio [HR] 1.2, 95% CI 1.1, 1.4) (Table 4). However, after adjusting for covariates, no significant difference was observed (HR 1.1, 95% CI, 0.96, 1.25).
Discussion
In this study, with complete data from an unselected and defined population of lung cancer patients over a time span of 10 years, we have studied the impact of sex as an independent factor affecting survival. Although unadjusted lung-cancer-specific survival at 1-year and 5-year survival was superior in women compared to men, this difference disappeared after adjustments. It seemed to be explained by selected clinical variables, in particular stage at presentation. A recent Australian study [21], supports our findings by showing a large reduction in male excess dying after adjusting for several prognostic factors.
In our study, the unequal stage distribution with more early-stage cancer in females and late-stage cancer in males was the major factor accounting for differences in lung-cancer-specific survival. Previous studies have suggested that sex inequity could be explained by differences in health-seeking behavior [22, 23]. The great majority of stage I tumors are asymptomatic and discovered due to imaging procedures performed for other reasons [24]. Other studies have indicated that females are generally more prone than men to utilize medical care [25, 26]. However, there are to our knowledge no studies linking sex-related differences in health awareness or healthcare utilization specifically to stage at presentation in lung cancer.
Several previous single hospital studies [8, 10, 27] or larger population-based cohort studies [5, 7, 28] conclude that there is an independent survival benefit in females.
While single hospital studies could be limited by selection bias, larger registry studies may often include less detailed clinical data and thus have limited ability to adjust for important factors affecting survival in males and females. This study combines the detail level of single-hospital series with the benefit of an unselected cohort as population-based studies could offer. In a large-scale national study (>40,000 lung cancer cases) based on data from the Cancer Registry of Norway, Sagerup et al. [5] found a higher relative risk of death in males (HR 1.14). This study including patients from 1988 to 2007 had an uneven distribution of sexes with approximately 1/3 female and 2/3 male, corresponding to different exposure to cigarette smoking. Diagnostic and staging modalities were scarcer and unevenly distributed in Norway in that time period. Furthermore, the stage distribution used by Sagerup was different and more unrefined, not using the TNM-system, but recorded at the cancer registry as either ‘localized’, ‘regional’ or ‘distant’, based on the best available clinical information they received. Neither performance status nor treatment information was available, both of which are possible factors affecting survival in lung cancer. A similar large-scale study based on data from the neighboring Swedish Lung Cancer Registry (2002–2016) also found poorer prognosis in males, particularly those with adenocarcinoma [7]. A wide range of prognostic factors were adjusted for. Still, only patients with verified adenocarcinoma and squamous cell cancer were included, excluding other histologies and non-biopsied patients with clinical lung cancer diagnosis. Patients were staged according to different TNM versions, TNM 6th in the first part of the cohort and 7th edition in the latter, and treatment was not included in the final analysis. Both the Norwegian and the Swedish data sets are large national registry-based studies that have some limitations with respect to prognostic factors that might affect adjusted analyses.
We present a complete cohort, including every patient diagnosed with lung cancer in our region. Our cohort is a real-life population of lung cancer patients where as much prognostic information as possible is retained to minimize confounding in interpreting sex and its influence on survival [29]. Heterogeneity in histology, treatment and stage was properly adjusted for in the calculations. The population also includes similar numbers of males (53%) and females (47%), from a country where the percentage of daily smokers has been similar in males and females since the mid-1990s. Furthermore, in Norway, a universal healthcare insurance system financed by taxation ensures coverage of all citizens. This allows equal access to health services, including diagnostics and lung cancer treatment, independent of social status, age or address. Norway is ranked 3rd in the world in gender equality, limiting differences by sex that are not detected by clinical parameters [30].
To further reflect real-world conditions, unbiopsied patients with a clinical lung cancer diagnosis were included. This latter group of the cohort, constituting 15% of the total, consists of patients of older age, complex comorbidity and poor performance status. It also includes 40 patients of older age and/or poor lung function who, based on morphological diagnosis of lung cancer, received curative intent treatment with SBRT for their stage I disease. Virtually all patients in Norway are registered in the Cancer Registry of Norway, including patients without tissue biopsy-proven diagnosis. This latter group of patients were included in the analysis as we intended to examine differences in sex-related survival in a complete lung cancer population. One-fifth of our cohort had poor performance status, ECOG 3–4, a population that is often lacking in other studies. Performance status is an independent predictor of lung-cancer-specific survival and should be included in order to avoid misinterpretation of apparent differences in sex-related survival [31]. It has been shown that ECOG may predict outcome of lung cancer better than comorbidity [32].
Both stage and treatment modalities are important factors influencing survival in lung cancer. In multivariate analysis, multicollinearity arises between these two factors, forcing one of the two to be removed from the analysis. To avoid losing prognostic information, we created combined stage and treatment groups. This distinguishes our study from other similar studies and might be an explanatory factor for our finding of similar lung-cancer-related mortality in males and females.
In Norway, the incidence of lung cancer in females and males is now equal, and at the same time, smoking prevalence over the last decades has changed. In 1975 there were 20 percentage points more daily male smokers compared to the female population aged 16–74 years (50% vs. 30%) [33]. The gap diminished gradually and was eliminated in the mid-1990s, with 31% daily smokers in both populations, followed by a gradual reduction to 9% for both sexes in 2020. In our cohort, 54% of women and 47% of men were active smokers at diagnosis. Although this is a small numerical difference, it reverses the historical distribution of male excess smoking. In 2010 in Norway, less than 20% of daily and occasional smokers used non-cigarette types of tobacco, including mainly cigars and less pipe tobacco. Duration and intensity of cigarette smoking show a dose-dependent risk in the development of lung cancer [34].
Analysis of survival in subgroups (Table 3) also did not reveal sex-related differences. We were not able to find other reports examining sex-specific survival in the group of curative intent treatment taking into account the three separate treatments: surgery, SBRT or curative intent CRT. In our cohort, curative intent treatment was initiated among 40% of females, which was more frequent than in males (33%). This is as expected from the observed stage differences. However, there was no difference in lung-cancer-specific survival between males and females treated with curative intent. Other studies report adenocarcinoma being more common in females [13, 14]. Superior survival of adenocarcinoma compared to other histologies has been suggested as one explanatory factor of survival benefit in females [12, 13]. We observed an equal incidence of adenocarcinoma in males and females, and after adjusting for relevant covariates, there was no survival benefit in adenocarcinoma compared to other histologies.
The results were robust to the statistical method used. Both RMST and Cox-regression analysis did not reveal any statistically significant difference in female and male lung-cancer-specific survival after adjusting for relevant clinical factors. Just as important, our observed difference of 0.9 months between females and males at 5 years, a 1% difference, is far from what would be considered a clinically meaningful difference [35].
Among the limitations of this study is the retrospective nature of the first period of the cohort, which may introduce information bias. We do not expect this to have distorted interpretations of sex-related differences. Our data were not detailed enough to allow differentiating according to type and total consumption of tobacco. There was also incomplete data regarding workplace exposure. Including these parameters would have added additional background information. If the effects of these data would have been strong enough to influence our survival findings is thus an open question. The study population comes from a single hospital, which may limit the validity of our findings. Preferably, to confirm our findings, the study should be repeated in another population. Furthermore, the population in our region is mainly Caucasian (>90%), whereas 5% are of Asian origin. Due to differences in genetic susceptibility, exposure, molecular profiles, and clinical outcomes in East-Asian versus Caucasian populations, the study has lower external validity with respect to Asian populations [36]. Finally, analyses were not adjusted for comorbidity and social status as these data were lacking.
Conclusion
In this complete cohort of 1,261 lung cancer patients, the superior unadjusted survival seen in females does not persist after adjusting for biological and clinical factors. We conclude that the apparent survival advantage in females is not due to the difference in sex-related biological behavior of lung cancer, but can be explained by differences in other clinical parameters where stage at presentation remains most important.
Declarations
Ethics approval and consent to participate
This study was approved by the regional Committees for Medical Research Ethics South-East Norway (reference number 405718-2022). Informed consent was deemed unnecessary by the regional Committees for Medical Research Ethics according to Norwegian legislation by authority of the Norwegian Health Personnel Act § 29 and the Health Research act § 10.
All methods were carried out in accordance with relevant guidelines and regulations in the declaration – Ethics approval and consent to participate section.
Consent for publication
Not applicable.
Data availability statement
The datasets generated and/or analyzed during this study are not publicly available due to national regulations. Access to the dataset was restricted to the authors of the article, and sharing of the data would require new applications to the regional Committees for Medical Research Ethics South-East Norway. Further details on the data are available from the corresponding author on reasonable request.
Competing interests
The authors report there are no competing interests to declare.
Author contributions
CRediT author statement.
D.L.: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization. O.T.B.: Writing – review and editing, Conceptualization F.G.: Writing – review and editing and Conceptualization. R.F.: Writing – review and editing, Validation. T.-E.S.: Writing – review and editing. M.T.D.: Writing – review and editing. L.F.: Writing – original draft, review and editing, Conceptualization, Supervision.
Acknowledgments
No other persons need to be acknowledged for participating in the preparation of the article.
Funding Statement
Funding The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interests
All authors declare that they have no conflicts of interest.
Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- [1].Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–53. 10.2147/CMAR.S187317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cancer Registry of Norway . Cancer in Norway 2021 – cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2022. [Google Scholar]
- [3].Cancer Research UK [Internet]. 2021. [cited 23-12-2023]. Available from: https://www.cancerresearchuk.org/sites/default/files/cstream-node/surv_5yr_lung.pdf
- [4].DLCR . Yearly report lung cancer 2019–20 [Internet]. 2021. [cited 23-12-2023]. Available from: https://www.lungecancer.dk/wp-content/uploads/2021/10/Årsrapport-20192020_netudgave.pdf
- [5].Sagerup CM, Småstuen M, Johannesen TB, et al. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66(4):301–7. 10.1136/thx.2010.151621 [DOI] [PubMed] [Google Scholar]
- [6].Pitz MW, Musto G, Navaratnam S. Sex as an independent prognostic factor in a population-based non-small cell lung cancer cohort. Can Respir J. 2013;20(1):30–4. 10.1155/2013/618691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Radkiewicz C, Dickman PW, Johansson ALV, et al. Sex and survival in non-small cell lung cancer: a nationwide cohort study. PLoS One. 2019;14(6):e0219206. 10.1371/journal.pone.0219206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barquín M, Calvo V, García-García F, et al. Sex is a strong prognostic factor in stage IV non-small-cell lung cancer patients and should be considered in survival rate estimation. Cancer Epidemiol. 2020;67:101737. 10.1016/j.canep.2020.101737 [DOI] [PubMed] [Google Scholar]
- [9].Bugge A, Kongerud J, Brunborg C, et al. Gender-specific survival after surgical resection for early stage non-small cell lung cancer. Acta Oncol. 2017;56(3):448–54. 10.1080/0284186X.2016.1253862 [DOI] [PubMed] [Google Scholar]
- [10].Franceschini JP, Jamnik S, Santoro IL. Survival in a cohort of patients with lung cancer: the role of age and gender in prognosis. J Bras Pneumol. 2017;43(6):431–6. 10.1590/s1806-37562016000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foeglé J, Hédelin G, Lebitasy MP, et al. Specific features of non-small cell lung cancer in women: a retrospective study of 1738 cases diagnosed in Bas-Rhin between 1982 and 1997. J Thorac Oncol. 2007;2(6): 466–74. 10.1097/01.JTO.0000275340.39960.25 [DOI] [PubMed] [Google Scholar]
- [12].Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705–12. 10.1200/JCO.2006.08.1455 [DOI] [PubMed] [Google Scholar]
- [13].Chang JW, Asamura H, Kawachi R, et al. Gender difference in survival of resected non-small cell lung cancer: histology-related phenomenon? J Thorac Cardiovasc Surg. 2009;137(4):807–12. 10.1016/j.jtcvs.2008.09.026 [DOI] [PubMed] [Google Scholar]
- [14].Wainer Z, Wright GM, Gough K, et al. Sex-dependent staging in non-small-cell lung cancer; analysis of the effect of sex differences in the eighth edition of the tumor, node, metastases staging system. Clin Lung Cancer. 2018;19(6):e933–44. 10.1016/j.cllc.2018.08.004 [DOI] [PubMed] [Google Scholar]
- [15].Jubelirer SJ, Varela NL, Welch CA, et al. Does sex make a difference in survival of patients undergoing resection for early stage non-small cell lung cancer (NSCLC)? W V Med J. 2009;105(4):18–22. [PubMed] [Google Scholar]
- [16].Båtevik R, Grong K, Segadal L, et al. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung Cancer. 2005;47(2):173–81. 10.1016/j.lungcan.2004.08.014 [DOI] [PubMed] [Google Scholar]
- [17].Sachs E, Sartipy U, Jackson V. Sex and survival after surgery for lung cancer: a Swedish nationwide cohort. Chest. 2021;159(5):2029–39. 10.1016/j.chest.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Z, Mo M, Zhou C, et al. Time-varying effect of sex on prognosis of lung adenocarcinoma surgical patients in China. Thorac Cancer. 2021;12(11):1699–707. 10.1111/1759-7714.13959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179–80. 10.1001/jamacardio.2017.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–90. 10.1177/1536867X0900900206 [DOI] [Google Scholar]
- [21].Yu XQ, Yap ML, Cheng ES, et al. Evaluating prognostic factors for sex differences in lung cancer survival: findings from a large Australian cohort. J Thorac Oncol. 2022;17(5):688–99. 10.1016/j.jtho.2022.01.016 [DOI] [PubMed] [Google Scholar]
- [22].Ek S. Gender differences in health information behaviour: a Finnish population-based survey. Health Promot Int. 2015;30(3):736–45. 10.1093/heapro/dat063 [DOI] [PubMed] [Google Scholar]
- [23].Rana RH, Alam F, Alam K, Gow J. Gender-specific differences in care-seeking behaviour among lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2020;146(5):1169–96. 10.1007/s00432-020-03197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hanagiri T, Sugio K, Mizukami M, et al. Postoperative prognosis in patients with non-small cell lung cancer according to the method of initial detection. J Thorac Oncol. 2007;2(10):907–11. 10.1097/JTO.0b013e318156079c [DOI] [PubMed] [Google Scholar]
- [25].Bertakis KD, Azari R, Helms LJ, et al. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147–52. [PubMed] [Google Scholar]
- [26].Hansen AH, Halvorsen PA, Ringberg U, et al. Socio-economic inequalities in health care utilisation in Norway: a population based cross-sectional survey. BMC Health Serv Res. 2012;12(1):336. 10.1186/1472-6963-12-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Costa GJ, Silva GTD, Ferreira CG, et al. Brazilian women with lung cancer have a higher overall survival than their male equivalents: a cohort study. Clin Lung Cancer. 2021;22(3):e313–19. 10.1016/j.cllc.2020.05.022 [DOI] [PubMed] [Google Scholar]
- [28].Fu JB, Kau TY, Severson RK, et al. Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database. Chest. 2005;127(3):768–77. 10.1378/chest.127.3.768 [DOI] [PubMed] [Google Scholar]
- [29].Miksad RA, Abernethy AP. Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clin Pharmacol Ther. 2018;103(2):202–5. 10.1002/cpt.946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gender equality by country [Internet]. 2022. [cited 23-12-2023]. Available from: https://worldpopulationreview.com/country-rankings/gender-equality-by-country
- [31].Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–30. 10.1097/JTO.0b013e3181d2dcd9 [DOI] [PubMed] [Google Scholar]
- [32].Ball D, Thursfield V, Irving L, et al. Evaluation of the simplified comorbidity score (colinet) as a prognostic indicator for patients with lung cancer: a cancer registry study. Lung Cancer. 2013;82(2):358–61. 10.1016/j.lungcan.2013.08.011 [DOI] [PubMed] [Google Scholar]
- [33].Statistics Norway . Percentage daily smokers 1973–2021 [Internet]. 2022. [cited 23-12-2023]. Available from: https://www.ssb.no/en/royk
- [34].Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. 10.5334/aogh.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fenchel K, Sellmann L, Dempke WC. Overall survival in non-small cell lung cancer-what is clinically meaningful? Transl Lung Cancer Res. 2016;5(1):115–19. 10.21037/tlcr.2016.07.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Izumi M, Suzumura T, Ogawa K, et al. Differences in molecular epidemiology of lung cancer among ethnicities (Asian vs. Caucasian). J Thorac Dis. 2020;12(7):3776–84. 10.21037/jtd.2019.08.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available due to national regulations. Access to the dataset was restricted to the authors of the article, and sharing of the data would require new applications to the regional Committees for Medical Research Ethics South-East Norway. Further details on the data are available from the corresponding author on reasonable request.