Abstract
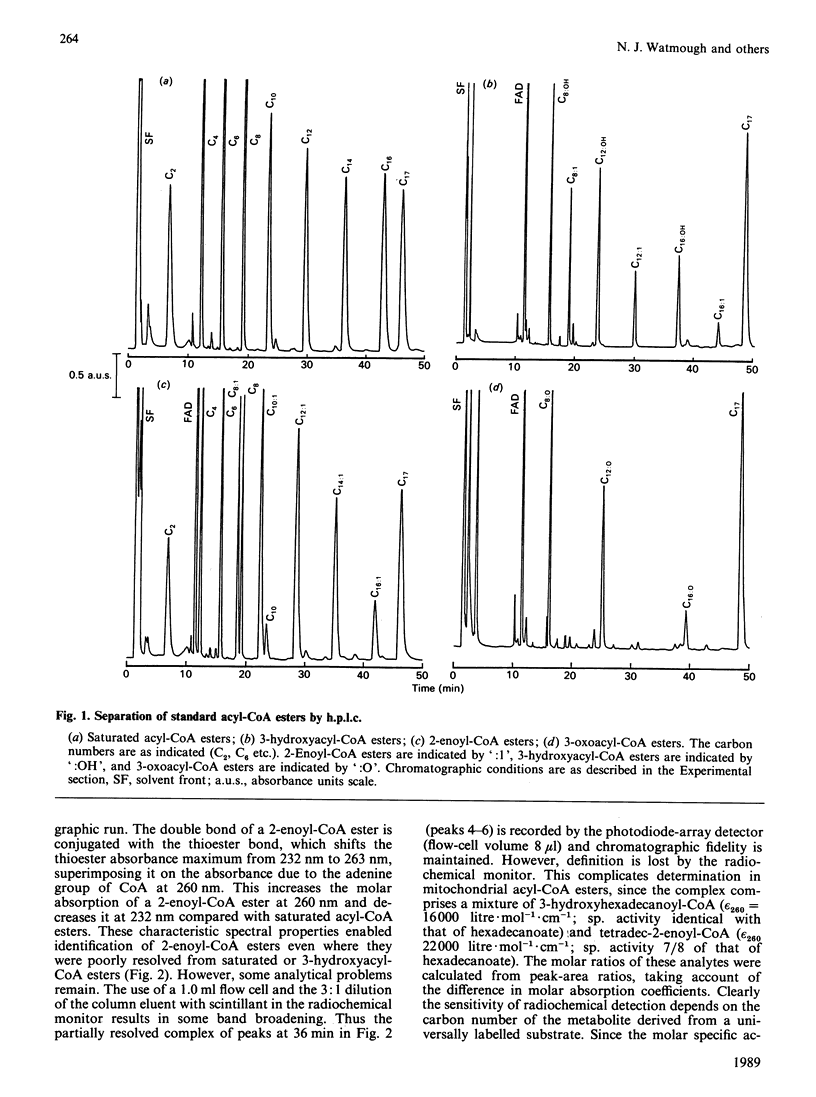
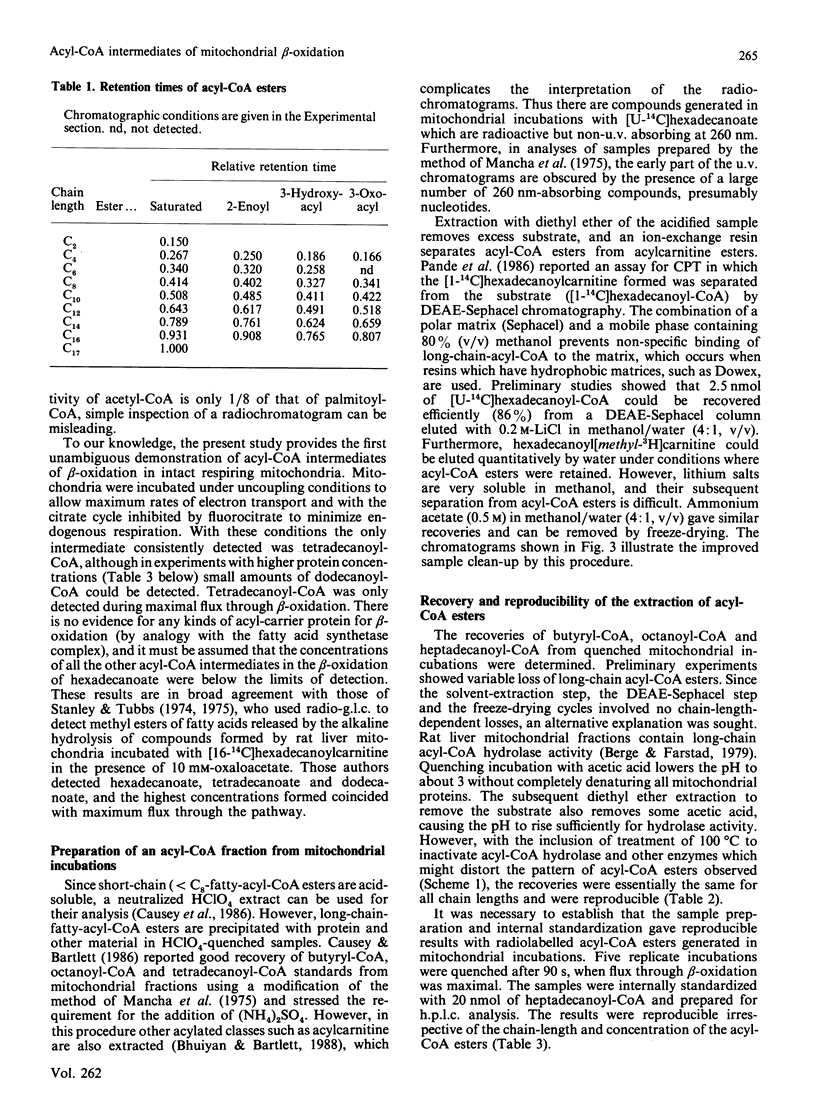
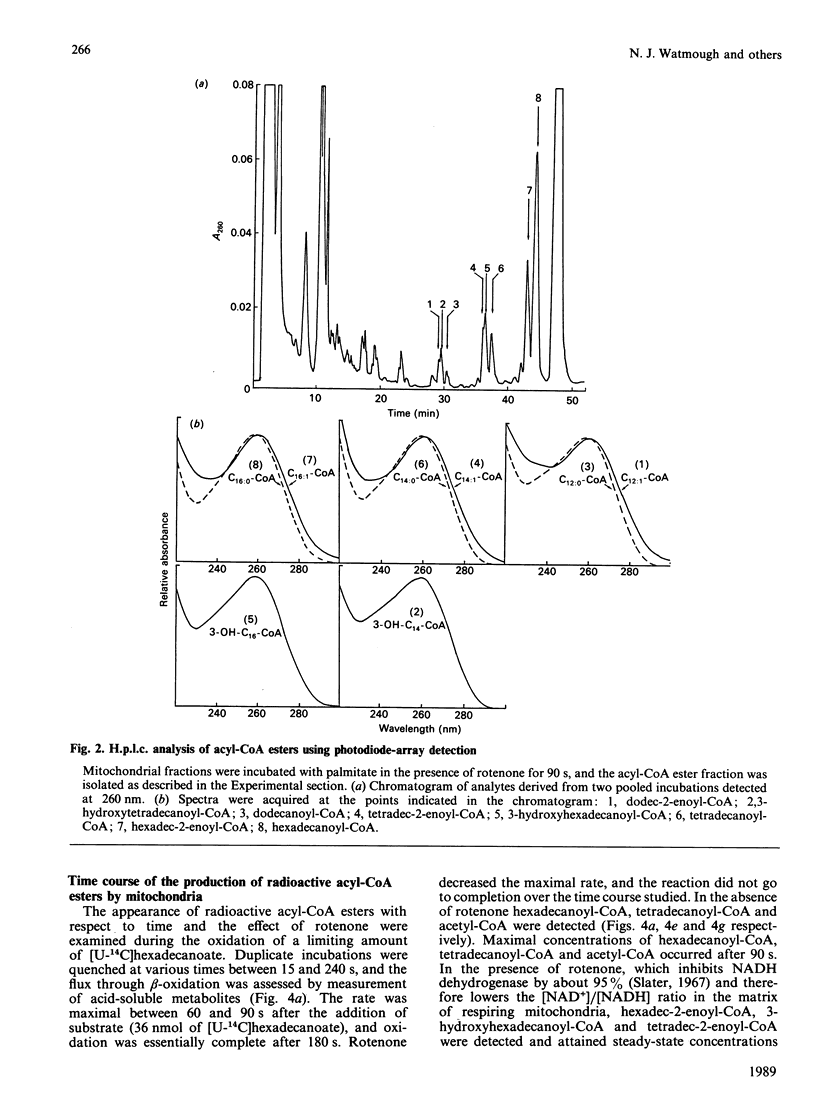
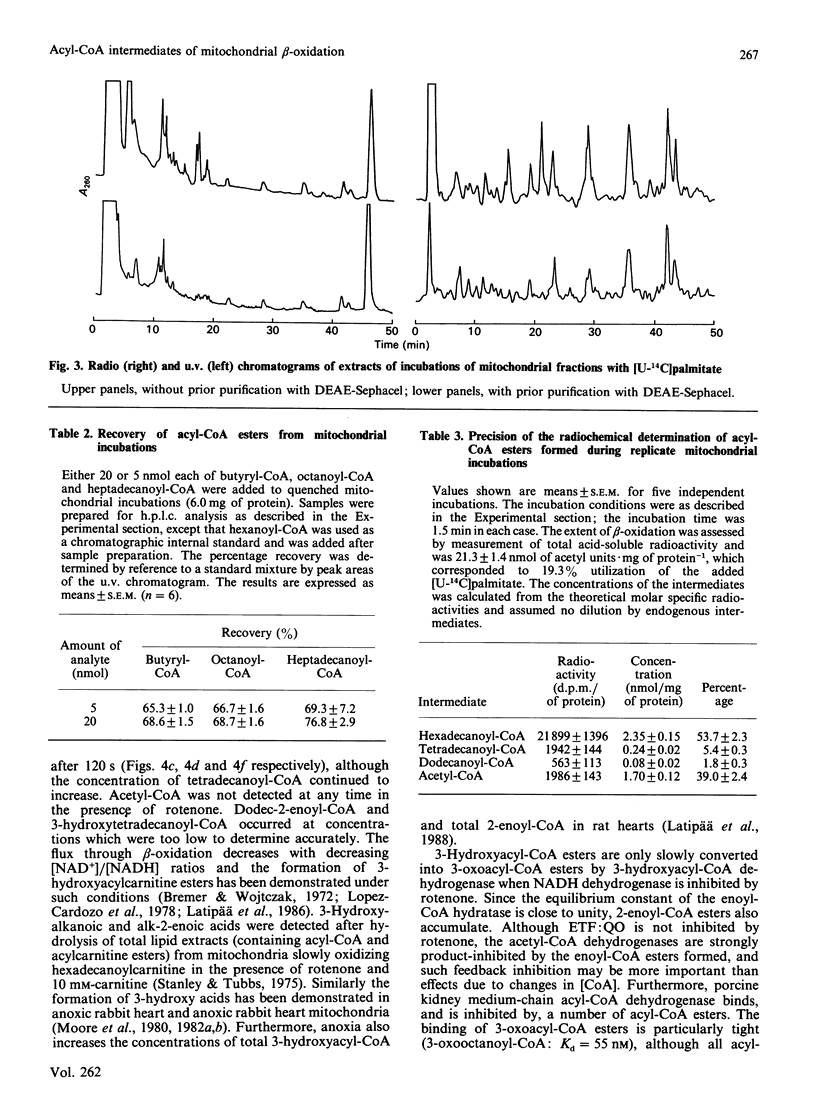
The quantitative isolation of acyl-CoA esters of chain length C2-C17 from mitochondrial incubations and their analysis by reverse-phase radio-h.p.l.c. is described. Photodiode-array detection was used to characterize 2-enoyl-CoA esters. The chromatographic behaviour of all 27 intermediates of the beta-oxidation of hexadecanoyl-CoA is documented. Only C16, C14 and C12 intermediates were detected in uncoupled mitochondria oxidizing [U-14C]hexadecanoyl-CoA in the presence of fluorocitrate and carnitine, providing evidence for some organization of the enzymes of beta-oxidation [Garland, Shepherd & Yates (1965) Biochem. J. 97, 587-594; Sumegi & Srere (1984) J. Biol. Chem. 259, 8748-8752]. Rotenone increased concentrations of 3-hydroxyacyl-CoA and 2-enoyl-CoA esters and inhibited flux. These experiments provide the first direct unambiguous measurements of acyl-CoA esters in intact respiring rat liver mitochondrial fractions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Arif A., Blecher M. Synthesis of fatty acyl CoA and other thiol esters using N-hydroxysuccinimide esters of fatty acids. J Lipid Res. 1969 May;10(3):344–345. [PubMed] [Google Scholar]
- Amendt B. A., Rhead W. J. The multiple acyl-coenzyme A dehydrogenation disorders, glutaric aciduria type II and ethylmalonic-adipic aciduria. Mitochondrial fatty acid oxidation, acyl-coenzyme A dehydrogenase, and electron transfer flavoprotein activities in fibroblasts. J Clin Invest. 1986 Jul;78(1):205–213. doi: 10.1172/JCI112553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F. C., Schooley D. A. Analysis and purification of acyl coenzyme A thioesters by reversed-phase ion-pair liquid chromatography. Anal Biochem. 1979 Apr 15;94(2):417–424. doi: 10.1016/0003-2697(79)90384-1. [DOI] [PubMed] [Google Scholar]
- Baker F. C., Schooley D. A. Separation of S-acyl-CoA thioesters and related compounds by reversed-phase ion-pair chromatography. Methods Enzymol. 1981;72:41–52. doi: 10.1016/s0076-6879(81)72007-x. [DOI] [PubMed] [Google Scholar]
- Bartlett K., Causey A. G. Radiochemical high-performance liquid chromatography methods for the study of branched-chain amino acid metabolism. Methods Enzymol. 1988;166:79–92. doi: 10.1016/s0076-6879(88)66013-7. [DOI] [PubMed] [Google Scholar]
- Bartlett K., Watmough N. J., Causey A. G. Intermediates of beta-oxidation. Biochem Soc Trans. 1988 Jun;16(3):410–416. doi: 10.1042/bst0160410a. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Frerman F. E., McKean M. C. Inhibition of general acyl CoA dehydrogenase by electron transfer flavoprotein semiquinone. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1290–1294. doi: 10.1016/s0006-291x(81)80151-9. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Farstad M. Dual localization of long-chain acyl-CoA hydrolase in rat liver: one in the microsomes and one in the mitochondrial matrix. Eur J Biochem. 1979 Mar 15;95(1):89–97. doi: 10.1111/j.1432-1033.1979.tb12942.x. [DOI] [PubMed] [Google Scholar]
- Bernert J. T., Jr, Sprecher H. An analysis of partial reactions in the overall chain elongation of saturated and unsaturated fatty acids by rat liver microsomes. J Biol Chem. 1977 Oct 10;252(19):6736–6744. [PubMed] [Google Scholar]
- Billington D., Osmundsen H., Sherratt H. S. Mechanisms of the metabolic disturbances caused by hypoglycin and by pent-4-enoic acid. In vitro studies. Biochem Pharmacol. 1978;27(24):2879–2890. doi: 10.1016/0006-2952(78)90204-6. [DOI] [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Causey A. G., Middleton B., Bartlett K. A study of the metabolism of [U-14C]3-methyl-2-oxopentanoate by rat liver mitochondria using h.p.l.c. with continuous on-line monitoring of radioactive intact acyl-coenzyme A intermediates. Biochem J. 1986 Apr 15;235(2):343–350. doi: 10.1042/bj2350343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey B. E., Martin-Requero A., Walajtys-Rode E., Williams R. J., Williamson J. R. Regulation of the branched chain alpha-ketoacid pathway in liver. J Biol Chem. 1982 Aug 25;257(16):9668–9676. [PubMed] [Google Scholar]
- DeBuysere M. S., Olson M. S. The analysis of acyl-coenzyme A derivatives by reverse-phase high-performance liquid chromatography. Anal Biochem. 1983 Sep;133(2):373–379. doi: 10.1016/0003-2697(83)90097-0. [DOI] [PubMed] [Google Scholar]
- El-Fakhri M., Middleton B. The existence of an inner-membrane-bound, long acyl-chain-specific 3-hydroxyacyl-CoA dehydrogenase in mammalian mitochondria. Biochim Biophys Acta. 1982 Nov 12;713(2):270–279. doi: 10.1016/0005-2760(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Frerman F. E., Goodman S. I. Deficiency of electron transfer flavoprotein or electron transfer flavoprotein:ubiquinone oxidoreductase in glutaric acidemia type II fibroblasts. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4517–4520. doi: 10.1073/pnas.82.13.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerman F. E. Reaction of electron-transfer flavoprotein ubiquinone oxidoreductase with the mitochondrial respiratory chain. Biochim Biophys Acta. 1987 Sep 10;893(2):161–169. doi: 10.1016/0005-2728(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Lane M. D. Liver acetyl coenzyme A carboxylase. I. Isolation and cat- alytic properties. J Biol Chem. 1968 Aug 25;243(16):4227–4235. [PubMed] [Google Scholar]
- Hale D. E., Batshaw M. L., Coates P. M., Frerman F. E., Goodman S. I., Singh I., Stanley C. A. Long-chain acyl coenzyme A dehydrogenase deficiency: an inherited cause of nonketotic hypoglycemia. Pediatr Res. 1985 Jul;19(7):666–671. doi: 10.1203/00006450-198507000-00006. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y., Shimomura Y., Harris R. A., Ozawa T. Determination of short-chain acyl-coenzyme A esters by high-performance liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):45–49. doi: 10.1016/0003-2697(86)90058-8. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O. C., Bakken A. M., Farstad M. The content of coenzyme A, acetyl-CoA and long-chain acyl-CoA in human blood platelets. Clin Chim Acta. 1982 Dec 23;126(3):307–313. doi: 10.1016/0009-8981(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O. C., Farstad M. Direct measurement of free coenzyme A in biological extracts by reversed-phase high-performance liquid chromatography. J Chromatogr. 1980 Dec 26;202(3):439–445. doi: 10.1016/s0021-9673(00)91829-6. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O. C., Normann P. T., Flatmark T. Determination of CoASH by high-performance liquid chromatography and its application in the assay of long-chain acyl-CoA derivatives. Anal Biochem. 1979 Jul 1;96(1):181–188. doi: 10.1016/0003-2697(79)90571-2. [DOI] [PubMed] [Google Scholar]
- King M. T., Reiss P. D. Separation and measurement of short-chain coenzyme-A compounds in rat liver by reversed-phase high-performance liquid chromatography. Anal Biochem. 1985 Apr;146(1):173–179. doi: 10.1016/0003-2697(85)90412-9. [DOI] [PubMed] [Google Scholar]
- Kunz W. S. Evaluation of electron-transfer flavoprotein and alpha-lipoamide dehydrogenase redox states by two-channel fluorimetry and its application to the investigation of beta-oxidation. Biochim Biophys Acta. 1988 Jan 20;932(1):8–16. doi: 10.1016/0005-2728(88)90134-x. [DOI] [PubMed] [Google Scholar]
- Latipä P. M., Hassinen I. E., Hiltunen J. K. Enzymatic assay for 3-hydroxyacyl-CoA and 2-trans-enoyl-CoA intermediates of beta-oxidation. Anal Biochem. 1988 May 15;171(1):67–72. doi: 10.1016/0003-2697(88)90125-x. [DOI] [PubMed] [Google Scholar]
- Latipä P. M., Kärki T. T., Hiltunen J. K., Hassinen I. E. Regulation of palmitoylcarnitine oxidation in isolated rat liver mitochondria. Role of the redox state of NAD(H). Biochim Biophys Acta. 1986 Feb 12;875(2):293–300. doi: 10.1016/0005-2760(86)90179-7. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., Klazinga W., van den Bergh S. G. Accumulation of carnitine esters of beta-oxidation intermediates during palmitate oxidation by rat-liver mitochondria. Eur J Biochem. 1978 Feb;83(2):629–634. doi: 10.1111/j.1432-1033.1978.tb12132.x. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat liver mitochondria. I. Relationships with the citric acid cycle and with the mitochondrial energy state. Biochim Biophys Acta. 1972;283(1):1–15. doi: 10.1016/0005-2728(72)90092-8. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat liver mitochondria. II. Factors affecting the rate of beta-oxidation. Biochim Biophys Acta. 1974 Jul 25;357(1):43–52. doi: 10.1016/0005-2728(74)90110-8. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat liver mitochondria. III. Relationship with the rate of beta-oxidation. Biochim Biophys Acta. 1974 Jul 25;357(1):53–62. doi: 10.1016/0005-2728(74)90111-x. [DOI] [PubMed] [Google Scholar]
- Mancha M., Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The determination of acyl-acyl carrier protein and acyl coenzyme A in a complex lipid mixture 1,2. Anal Biochem. 1975 Oct;68(2):600–608. doi: 10.1016/0003-2697(75)90655-7. [DOI] [PubMed] [Google Scholar]
- Middleton B., Bartlett K. The synthesis and characterisation of 2-methylacetoacetyl coenzyme A and its use in the identification of the site of the defect in 2-methylacetoacetic and 2-methyl-3-hydroxybutyric aciduria. Clin Chim Acta. 1983 Mar 14;128(2-3):291–305. doi: 10.1016/0009-8981(83)90329-7. [DOI] [PubMed] [Google Scholar]
- Molaparast-Saless F., Shrago E., Spennetta T. L., Donatello S., Kneeland L. M., Nellis S. H., Liedtke A. J. Determination of individual long-chain fatty acyl-CoA esters in heart and skeletal muscle. Lipids. 1988 May;23(5):490–492. doi: 10.1007/BF02535525. [DOI] [PubMed] [Google Scholar]
- Moore K. H., Koen A. E., Hull F. E. beta-Hydroxy fatty acid production by ischemic rabbit heart. J Clin Invest. 1982 Feb;69(2):377–383. doi: 10.1172/JCI110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. H., Radloff J. F., Hull F. E., Sweeley C. C. Incomplete fatty acid oxidation by ischemic heart: beta-hydroxy fatty acid production. Am J Physiol. 1980 Aug;239(2):H257–H265. doi: 10.1152/ajpheart.1980.239.2.H257. [DOI] [PubMed] [Google Scholar]
- Moore K. H., Radloff J. F., Koen A. E., Hull F. E. Incomplete fatty acid oxidation by heart mitochondria: beta-hydroxy fatty acid production. J Mol Cell Cardiol. 1982 Aug;14(8):451–459. doi: 10.1016/0022-2828(82)90151-1. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Murthy M. S., Noël H. Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochim Biophys Acta. 1986 Jun 27;877(2):223–230. doi: 10.1016/0005-2760(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Powell P. J., Lau S. M., Killian D., Thorpe C. Interaction of acyl coenzyme A substrates and analogues with pig kidney medium-chain acyl-coA dehydrogenase. Biochemistry. 1987 Jun 16;26(12):3704–3710. doi: 10.1021/bi00386a066. [DOI] [PubMed] [Google Scholar]
- Pullman M. E. A convenient and versatile method for the purification of CoA thiol esters. Anal Biochem. 1973 Jul;54(1):188–198. doi: 10.1016/0003-2697(73)90262-5. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Derrick J. P., Friend A. S., Tubbs P. K. Purification and properties of the soluble carnitine palmitoyltransferase from bovine liver mitochondria. Biochem J. 1987 Jun 1;244(2):271–278. doi: 10.1042/bj2440271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt H. S., Watmough N. J., Johnson M. A., Turnbull D. M. Methods for study of normal and abnormal skeletal muscle mitochondria. Methods Biochem Anal. 1988;33:243–335. doi: 10.1002/9780470110546.ch6. [DOI] [PubMed] [Google Scholar]
- Stanley C. A., Hale D. E., Coates P. M., Hall C. L., Corkey B. E., Yang W., Kelley R. I., Gonzales E. L., Williamson J. R., Baker L. Medium-chain acyl-CoA dehydrogenase deficiency in children with non-ketotic hypoglycemia and low carnitine levels. Pediatr Res. 1983 Nov;17(11):877–884. doi: 10.1203/00006450-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The occurrence of intermediates in mitochondrial fatty acid oxidation. FEBS Lett. 1974 Mar 1;39(3):325–328. doi: 10.1016/0014-5793(74)80141-9. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The role of intermediates in mitochondrial fatty acid oxidation. Biochem J. 1975 Jul;150(1):77–88. doi: 10.1042/bj1500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi B., Srere P. A. Binding of the enzymes of fatty acid beta-oxidation and some related enzymes to pig heart inner mitochondrial membrane. J Biol Chem. 1984 Jul 25;259(14):8748–8752. [PubMed] [Google Scholar]
- Thorpe C. A method for the preparation of 3-ketoacyl-CoA derivatives. Anal Biochem. 1986 Jun;155(2):391–394. doi: 10.1016/0003-2697(86)90452-5. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Bartlett K., Stevens D. L., Alberti K. G., Gibson G. J., Johnson M. A., McCulloch A. J., Sherratt H. S. Short-chain acyl-CoA dehydrogenase deficiency associated with a lipid-storage myopathy and secondary carnitine deficiency. N Engl J Med. 1984 Nov 8;311(19):1232–1236. doi: 10.1056/NEJM198411083111906. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Bone A. J., Bartlett K., Koundakjian P. P., Sherratt H. S. The effects of valproate on intermediary metabolism in isolated rat hepatocytes and intact rats. Biochem Pharmacol. 1983 Jun 15;32(12):1887–1892. doi: 10.1016/0006-2952(83)90054-0. [DOI] [PubMed] [Google Scholar]
- Turnbull D. M., Shepherd I. M., Aynsley-Green A. Inherited defects of mitochondrial fatty acid oxidation. Biochem Soc Trans. 1988 Jun;16(3):424–427. doi: 10.1042/bst0160424. [DOI] [PubMed] [Google Scholar]
- Vianey-Liaud C., Divry P., Gregersen N., Mathieu M. The inborn errors of mitochondrial fatty acid oxidation. J Inherit Metab Dis. 1987;10 (Suppl 1):159–200. doi: 10.1007/BF01812855. [DOI] [PubMed] [Google Scholar]
- Watmough N. J., Bhuiyan A. K., Bartlett K., Sherratt H. S., Turnbull D. M. Skeletal muscle mitochondrial beta-oxidation. A study of the products of oxidation of [U-14C]hexadecanoate by h.p.l.c. using continuous on-line radiochemical detection. Biochem J. 1988 Jul 15;253(2):541–547. doi: 10.1042/bj2530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldegiorgis G., Spennetta T., Corkey B. E., Williamson J. R., Shrago E. Extraction of tissue long-chain acyl-CoA esters and measurement by reverse-phase high-performance liquid chromatography. Anal Biochem. 1985 Oct;150(1):8–12. doi: 10.1016/0003-2697(85)90434-8. [DOI] [PubMed] [Google Scholar]
- Wood R., Lee T. Metabolism of 2-hexadecynoate and inhibition of fatty acid elongation. J Biol Chem. 1981 Dec 10;256(23):12379–12386. [PubMed] [Google Scholar]


