Abstract
Combined large-cell neuroendocrine carcinoma (LCNEC) and small-cell lung cancer (SCLC) is extremely rare, with only a few reports available in the literature. An accurate diagnosis is difficult to make due to the overlapping clinical features between LCNEC and SCLC, and a standardized treatment option is lacking. A 53-year-old female patient was admitted to Xiaoshan Affiliated Hospital of Wenzhou Medical University (Hangzhou, China) due to symptoms of dyspnea and phlegm, with blood in the sputum. Computed tomography revealed a 52×32×26-mm irregular soft-tissue mass in the left upper lung. Pathological examination of the biopsy specimen showed a poorly differentiated neuroendocrine carcinoma with compression injury, consistent with a mixed type of large and small cell carcinoma. The patient was administered chemotherapy, radiotherapy and targeted therapy, and as of October 2023, the patient had a survival period of 29 months. LCNEC combined with SCLC is a sporadic tumor with a high potential for malignancy. Multidisciplinary treatment and close follow-up are recommended. The multidisciplinary treatment strategy used in the present study is expected to help inform future therapeutic decisions.
Keywords: large cell neuroendocrine carcinoma, small cell lung cancer, case report, multidisciplinary treatment, immunotherapy, chemotherapy, local radiotherapy, pathology
Introduction
Pulmonary high-grade neuroendocrine carcinoma is comprised of two distinct subtypes, namely, large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC) (1). The incidence rates of LCNEC and SCLC are low, at 3 and 15%, respectively, among all lung malignant tumors. The coexistence of these two subtypes is even rarer, occurring in <1% of lung cancer cases (2). SCLC and LCNEC are both characterized by their aggressive growth pattern, a high propensity for metastasis and a poor prognosis. The median survival times for LCNEC and SCLC are 9 and 7 months, respectively (3). LCNEC and SCLC are classified as neuroendocrine carcinomas and exhibit similar molecular expression patterns. Diagnosis poses considerable challenges and primarily relies on pathological examination to evaluate cellular morphology, such as the abundance of the cytoplasm, presence of nucleoli and the size of the nuclei (4). Due to the limited availability of relevant cases, a consensus has not yet been established regarding the clinical management of combined LCNEC and SCLC.
The current study presents an uncommon case of combined LCNEC and SCLC in which the patient achieved a survival time of more than two years following a series of treatments. We expect the diagnosis and treatment approach employed in this case to serve as a valuable reference for subsequent patients with similar conditions and their treating physicians.
Case report
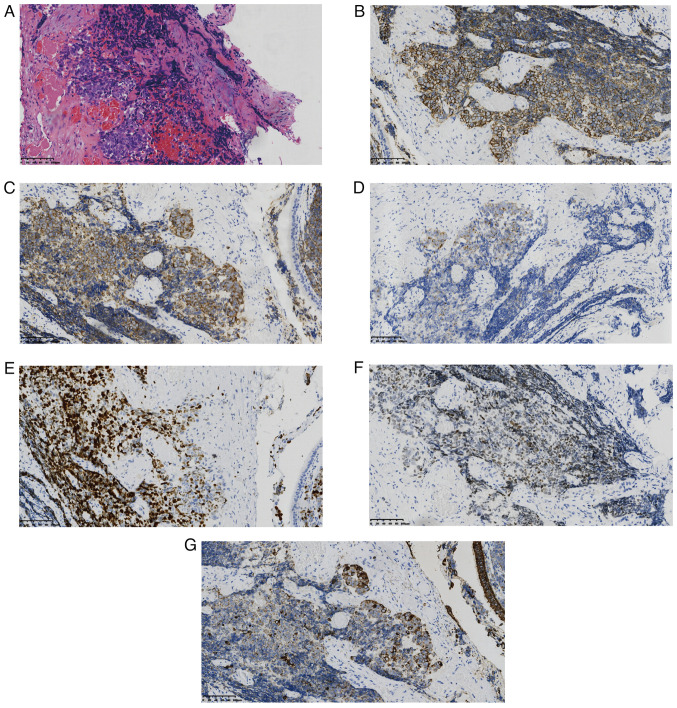
In June 2021, a 53-year-old female patient with no history of smoking was admitted to Xiaoshan Affiliated Hospital of Wenzhou Medical University (Hangzhou, China) due to symptoms of dyspnea and phlegm, with blood in the sputum. Computed tomography (CT) revealed a 52×32×26-mm irregular soft-tissue mass in the left upper lung, with a shallow lobulated edge (Fig. 1A). Blood tests showed elevated levels of carcinoembryonic antigen (6.01 µg/ml; normal range, 0–6 µg/ml) and carbohydrate antigen 19–9 (196.42 kU/l; normal value, <37 kU/l) (5). Blood biochemistry, cardiac enzyme levels and coagulation function were not clinically significant. A whole-body bone scan did not reveal other abnormalities. Subsequently, a transbronchial lung biopsy was performed on the mass in the left upper lung. Assessment of the tumor pathology using hematoxylin and eosin (HE) staining revealed a poorly differentiated neuroendocrine carcinoma with crush injury (Fig. 2A). This finding was consistent with the mixed characteristics of large and small cell carcinoma. Immunohistochemical analyses further demonstrated positive staining for CD56, synaptophysin (Syn) and thyroid transcription factor-1 (TTF-1), and punctate staining for cytokeratin (CK). The chromogranin A (CgA) proliferation index (PI) was 30% and the Ki-67 PI was 65% (Fig. 2B-G). Conversely, negative staining results were observed for napsin A, tumor protein 40 (p40) and p63. Based on these findings, the patient was diagnosed with stage IIb (cT3N0M0) left pulmonary neuroendocrine carcinoma, specifically of the mixed type comprising large and small cell components. Multidisciplinary treatment was therefore initiated. The patient was diagnosed with an advanced-stage local lung cancer (mainly SCLC), for which National Comprehensive Cancer Network guidelines recommend curative radiotherapy and chemotherapy rather than immunotherapy (6). Therefore, a decision was made to administer concurrent etoposide and cisplatin (EP) chemotherapy and radiotherapy. The first cycle consisted of 100 mg etoposide on days 1–3 and 110 mg cisplatin on day 1. After the first cycle of EP chemotherapy, severe bone marrow suppression and liver function impairment were observed. Consequently, the treatment was changed to tumor radiotherapy. The specific plan was as follows: The gross tumor volume was identified as a left upper lung mass; the total dose was 6,020 cGy over 28 fractions; the clinical target volume (CTV) was a 0.8-cm external expansion of the mass and left hilar lymphatic drainage area; the planning target volume was a 0.5-cm external expansion of the CTV, and the total dose was 5,040 cGy over 28 fractions, at 1 fraction/day and 5 fractions/week. The radiotherapy intervention proved efficacious, as evidenced by a subsequent chest CT scan, which revealed a notable decrease in the size of the left upper lung mass (Fig. 1B). The patient underwent three more cycles of chemotherapy with a modified EP regimen. The second cycle consisted of 100 mg etoposide on days 1–3 and 75 mg cisplatin on day 1. The third and fourth cycle consisted of 100 mg etoposide on days 1–3 and 30 mg cisplatin on day 1. A month after chemotherapy, CT re-examination indicated increased consolidation in the left upper lung and enlargement of the mediastinal lymph nodes (Fig. 1C). The abdominal ultrasound revealed a space-occupying lesion in the liver parenchyma. Single-photon emission CT indicated numerous high metabolic changes in the entire skeletal system. The patient underwent five cycles of irinotecan (90 mg per day on days 1 and 8; 21 days per cycle) chemotherapy in conjunction with anlotinib (12 mg per day on days 1–14; 21 days per cycle) maintenance targeted therapy. Subsequently, there was no apparent progression or reduction of the tumor lesions.
Figure 1.
CT images of the patient. (A) A soft-tissue mass in the left lung was shown in a chest CT scan performed in June 2021. (B) The mass had decreased after radiotherapy, as detected in September 2021. (C) The mass showed progression after chemotherapy, as detected in December 2021. (D) There was no progression of the mass in October 2023. CT, computed tomography.
Figure 2.
Lung pathology of the patient. (A) Left lung tissues were positive for small and large malignant cells (hematoxylin and eosin staining; ×200 magnification). The left side of the diagram shows the large cells (~10%) and the right side shows the small cells (~90%) with crush injury. Immunohistochemical staining of (B) CD56, (C) synaptophysin, (D) chromogranin A, (E) Ki-67, (F) thyroid transcription factor-1 and (G) cytokeratin (all ×200 magnification).
In April 2023, the patient presented with dizziness, headaches and left-sided facial numbness. Subsequent magnetic resonance imaging revealed thickening of the left parietal pillow, left skull base meninges, and the top and left walls of the nasopharynx. Additionally, patchy shadows with blurred edges were observed in the left maxillary sinus, ethmoid sinus, nasal cavity and bilateral sphenoid sinuses (Fig. 3). A further biopsy of the mass at the top and lateral wall of the left nasal cavity was performed, and the pathology suggested mixed characteristics of large and small cell carcinoma (Fig. 4A). Immunohistochemistry was positive for CK, TTF-1, CK7, CgA, Syn, CD56, neuron-specific enolase and Ki-67 (PI, 95%) (Fig. 4B-I). The pathological and immunohistochemical results revealed that the tumor was a poorly differentiated neuroendocrine carcinoma. As a palliative measure, the patient underwent nasal radiotherapy. After radiotherapy, the local pain and bleeding symptoms improved, but the breath shortness worsened. Based on this evaluation, the patient was administered four cycles of albumin paclitaxel (100 mg per day on days 1 and 8; 21 days per cycle) single-agent injectable chemotherapy, while anlotinib (12 mg per day on days 1–14; 21 days per cycle) maintenance treatment was also continued. As of October 2023, the patient's monthly outpatient follow-up showed no significant progress in lung lesions (Fig. 1D). However, the patient gave up treatment after being discharged from the hospital and died in November 2023.
Figure 3.
MRI of the patient. The left parietal occipital, left skull base meninges, and the top and left walls of the nasopharynx showed thickening. (A) T1WI, (B) T2WI and (C) contrast-enhanced MRI. WI, weighted imaging; MRI, magnetic resonance imaging.
Figure 4.
(A) Nasopharyngeal mass tissues were positive for small and large malignant cells (hematoxylin and eosin staining; ×200 magnification). The left side of the diagram shows the large cells and the right side shows the small cells with crush injury. Immunohistochemical staining of (B) CD56, (C) synaptophysin, (D) Ki-67, (E) chromogranin A, (F) cytokeratin 7, (G) cytokeratin (all ×200 magnification), (H) neuron-specific enolase (×100 magnification) and (I) thyroid transcription factor-1 (×200 magnification).
Tissue analysis
The tissue was fixed with 4% neutral formalin (24 h at 25°C) and embedded in paraffin, and then 3-µm serial sections were prepared and subjected to H&E staining (Beijing Jinqiao Zhongshan Biological Co. Ltd; OriGene Technologies, Inc.) (8 h at 25°C). Observations were made using a Leica DM2000 light microscope (Leica Microsystems GmbH).
The undyed tissue sections (3-µm) were dewaxed and washed, and then placed in EDTA (pH9.0±0.2) buffer (1:50; cat. no. ZLI9069; Beijing Zhongshan Jinqiao Biological Co. Ltd.; OriGene Technologies, Inc.) and the repair solution was used for antigen repair for 20 min (hot repair at 100°C in EDTA 1:50, 2,500 ml liquid for 20 min). After sealing, the tissue sections were incubated with primary antibody at room temperature for 40 min, and then the tissue was incubated with 3,3′-diaminobenzidine chromogenic solution (1:50; cat. no. PV-8000D; Beijing Zhongshan Jinqiao Biological Co. Ltd.; OriGene Technologies, Inc.) at 25°C for 5–10 min under an optical microscope (Leica DM2000; Leica Microsystems GmbH).
IHC was performed using an EnVision IHC kit (polymer method; cat. no. KIT-0014; Beijing Zhongshan Jinqiao Biotechnology Co. Ltd.; Origene Technologies, Inc.) using primary antibodies obtained from Beijing Zhongshan Jinqiao Biological Co., Ltd., (TTF-1, CK and Ki-67) and Fuzhou Maixin Biotechnology development Co., Ltd., (CD56, Syn, CgA, napsin A, CK7, NSE, p40 and p30) to target the following proteins (pre-diluted working solutions unless otherwise indicated): CD56 (cat. no. MX039), Syn (cat. no. MX038), TTF-1 (1:200; cat. no. SPT24), CgA (cat. no. MX018), Ki-67 (1:200; cat. no. UMAB107), napsin A (cat. no. MX015), CK (1:200; cat. no. AE1/AE3), CK7 (cat. no. OV-T212/30), NSE (cat. no. 3-3-C), p40 (cat. no. MXR010) and p63 (cat. no. MXR013).
Discussion
Pulmonary neuroendocrine tumors encompass a spectrum of morphological entities, including low-grade typical carcinoids, intermediate-grade atypical carcinoids and high-grade neuroendocrine carcinomas, which comprise LCNECs and SCLCs (1). Both LCNEC and SCLC exhibit neuroendocrine differentiation, as evidenced by positive immunohistochemical staining for Syn, chromogranin, CD56, TTF-1 and Ki-67, and a high proliferation rate that exceeds 10 mitoses per 2 mm2 (2). Additionally, LCNEC and SCLC exhibit similar histological characteristics, such as the formation of rosettes, nuclear molding, and a lack of prominent glandular formation and keratinization (7). Consequently, the accurate differentiation between LCNEC and SCLC poses considerable difficulties. Pathologically, the distinction between these two entities primarily relies on assessments of cellular morphology. Small cell carcinoma is characterized by a high nuclear-cytoplasmic ratio, limited cytoplasm, fine chromatin, and an abundance of crush artifacts and apoptotic debris when observed under a microscope. Conversely, large cell carcinoma exhibits a variable amount of cytoplasm, irregular nuclei, small nucleoli, coarse chromatin and palisading/rosette necrosis (8). In the present case report, HE staining of the patient tissue sample indicated the coexistence of large and small cells. The diagnosis of neuroendocrine involvement was further supported by the immunohistochemical results. Therefore, it was concluded that the patient had a mixed neuroendocrine carcinoma.
LCNEC and SCLC have similarities in terms of clinical presentation. Both types are associated with smoking and can cause symptoms such as coughing, difficulty in breathing, hemoptysis, chest pain and weight loss (2). SCLC tends to be predominantly observed in advanced stages, whereas LCNEC is frequently diagnosed in the early stages. SCLC, but not LCNEC, is commonly associated with diverse paraneoplastic syndromes. LCNEC typically manifests as peripheral lobulated masses, occasionally accompanied by spiculated nodules, whereas SCLC frequently presents as a large central mass (8). In a case reported by Ai et al (4), the patient had a survival period of only 4 months after the diagnosis of LCNEC combined with SCLC. It is reasonable to hypothesize that the coexistence of LCNEC and SCLC is associated with a higher degree of malignancy, accelerated progression and an unfavorable prognosis. In the present case, the patient displayed features of both LCNEC and SCLC, and experienced rapid progression. Despite these findings, the patient in this case had a survival period exceeding two years following a series of treatments.
SCLC is typically managed using a comprehensive approach that encompasses chemotherapy, radiation therapy and surgery (6). By contrast, the treatment strategy for LCNEC remains uncertain owing to the rarity of the condition, and the limited number and retrospective nature of available studies (9–11). Notably, chemotherapy regimens for SCLC have demonstrated favorable outcomes in patients with early stage LCNEC, whether employed as first-line treatment or adjuvant therapy following surgery (12–14). Primary tumor radiation therapy has been found to have a positive effect on LCNEC, leading to improvements in both median progression-free survival time and overall survival time (15). Furthermore, some promising results have been observed with immunotherapy and targeted therapy, although the most effective treatment strategy has yet to be definitively established (11). In the specific case of the present patient, EP chemotherapy was initially administered. However, the patient exhibited poor tolerance and experienced adverse effects, such as bone marrow suppression and liver function damage, after completing only one cycle of chemotherapy. Due to these events, local radiation therapy was administered to the primary tumor, resulting in size reduction. This observation suggests that when substantial adverse effects of chemotherapy occur, prompt implementation of local radiotherapy is a viable alternative. Nevertheless, despite the continuation of the EP chemotherapy regimen, there was no substantial disease control. At 1 month after the conclusion of chemotherapy, the cancer had disseminated throughout the entire body and the patient underwent second-line chemotherapy, immunotherapy and targeted therapy to manage tumor progression. This finding suggests that the efficacy of the initial EP regimen is constrained, highlighting the need for supplementary therapeutic alternatives, including second-line chemotherapy. In addition, comprehensive strategies, such as immunotherapy and targeted interventions, should be considered to achieve optimal tumor management.
In summary, SCLC and LCNEC have similarities and differences regarding diagnostic methods, clinical manifestations and treatment options. An accurate diagnosis and reasonable treatment of combined LCNEC and SCLC are important for improving the survival rate and quality of life of affected patients. The present case suggests that a combination of chemotherapy, radiotherapy and targeted therapy may be effective in achieving a sustained stable disease status in patients with this rare and aggressive form of lung cancer. It is hoped that the treatment strategy in this case will be helpful for the clinical management of future cases of combined LCNEC and SCLC.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
XK and YJ performed case data collection and drafting of the manuscript, and conceived the study. JY was in charge of the literature search and review, acquiring the patient's pathological images and interpreting the reports. YJ revised the manuscript and interpreted the patient's data. In addition, all authors agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript. XK, YJ and JY confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written consent for the case to be published.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, et al. A common classification framework for neuroendocrine neoplasms: An international agency for research on cancer (IARC) and world health organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–1786. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisch D, Christopoulos P. Double trouble: Combined large-cell neuroendocrine and small-cell lung carcinoma. Transl Cancer Res. 2022;11:3006–3011. doi: 10.21037/tcr-22-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinslow CJ, May MS, Saqi A, et al. Large-Cell Neuroendocrine Carcinoma of the Lung: A Population-Based Study. Clin Lung Cancer. 2020;21:e99–e113. doi: 10.1016/j.cllc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Ai L, Li J, Ye T, Wang W, Li Y. Progressive neuroendocrine tumor of lung with combined categories in metastatic site: A case report. Transl Cancer Res. 2022;11:2438–2442. doi: 10.21037/tcr-21-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatabe Y, Dacic S, Borczuk AC, Warth A, Russell PA, Lantuejoul S, Beasley MB, Thunnissen E, Pelosi G, Rekhtman N, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol. 2019;14:377–407. doi: 10.1016/j.jtho.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:1441–1464. doi: 10.6004/jnccn.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JT, Li Y, Yan LX, Zhu ZF, Dong XR, Chu Q, Wu L, Zhang HM, Xu CW, Lin G, et al. Disparity in clinical outcomes between pure and combined pulmonary large-cell neuroendocrine carcinoma: A multi-center retrospective study. Lung Cancer. 2020;139:118–123. doi: 10.1016/j.lungcan.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Borczuk AC. Pulmonary neuroendocrine tumors. Surg Pathol Clin. 2020;13:35–55. doi: 10.1016/j.path.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Raman V, Jawitz OK, Yang CJ, Voigt SL, Tong BC, D'Amico TA, Harpole DH. Outcomes for surgery in large cell lung neuroendocrine cancer. J Thorac Oncol. 2019;14:2143–2151. doi: 10.1016/j.jtho.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Yang L, Lu H. Pulmonary combined large cell neuroendocrine carcinoma. Pathol Oncol Res. 2022;28:1610747. doi: 10.3389/pore.2022.1610747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buium C, Negru S, Ionescu DN, Dediu M. The unmet diagnostic and treatment needs in large cell neuroendocrine carcinoma of the lung. Curr Oncol. 2023;30:7218–7228. doi: 10.3390/curroncol30080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujtan L, Muthukumar V, Kennedy KF, Davis JR, Masood A, Subramanian J. The role of systemic therapy in the management of stage I large cell neuroendocrine carcinoma of the lung. J Thorac Oncol. 2018;13:707–714. doi: 10.1016/j.jtho.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Niho S, Kenmotsu H, Sekine I, Ishii G, Ishikawa Y, Noguchi M, Oshita F, Watanabe S, Nakajima R, Tada H, Nagai K. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: A multicenter phase II study. J Thorac Oncol. 2013;8:980–984. doi: 10.1097/JTO.0b013e31828f6989. [DOI] [PubMed] [Google Scholar]
- 14.Le Treut J, Sault MC, Lena H, Souquet PJ, Vergnenegre A, Le Caer H, Berard H, Boffa S, Monnet I, Damotte D, Chouaid C. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: The GFPC 0302 study. Ann Oncol. 2013;24:1548–1552. doi: 10.1093/annonc/mdt009. [DOI] [PubMed] [Google Scholar]
- 15.Prelaj A, Rebuzzi SE, Del Bene G, Giròn Berrìos JR, Emiliani A, De Filippis L, Prete AA, Pecorari S, Manna G, Ferrara C, et al. Evaluation of the efficacy of cisplatin-etoposide and the role of thoracic radiotherapy and prophylactic cranial irradiation in LCNEC. ERJ Open Res. 2017;3:00128–2016. doi: 10.1183/23120541.00128-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.