Abstract
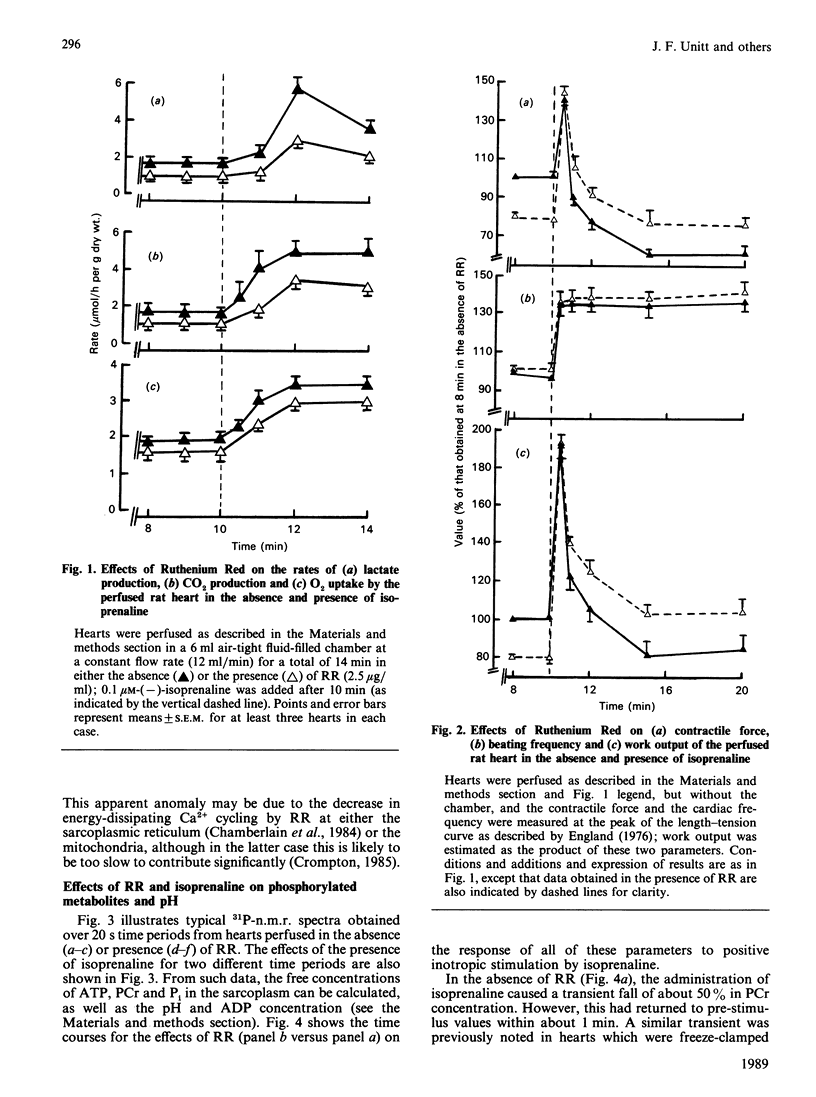
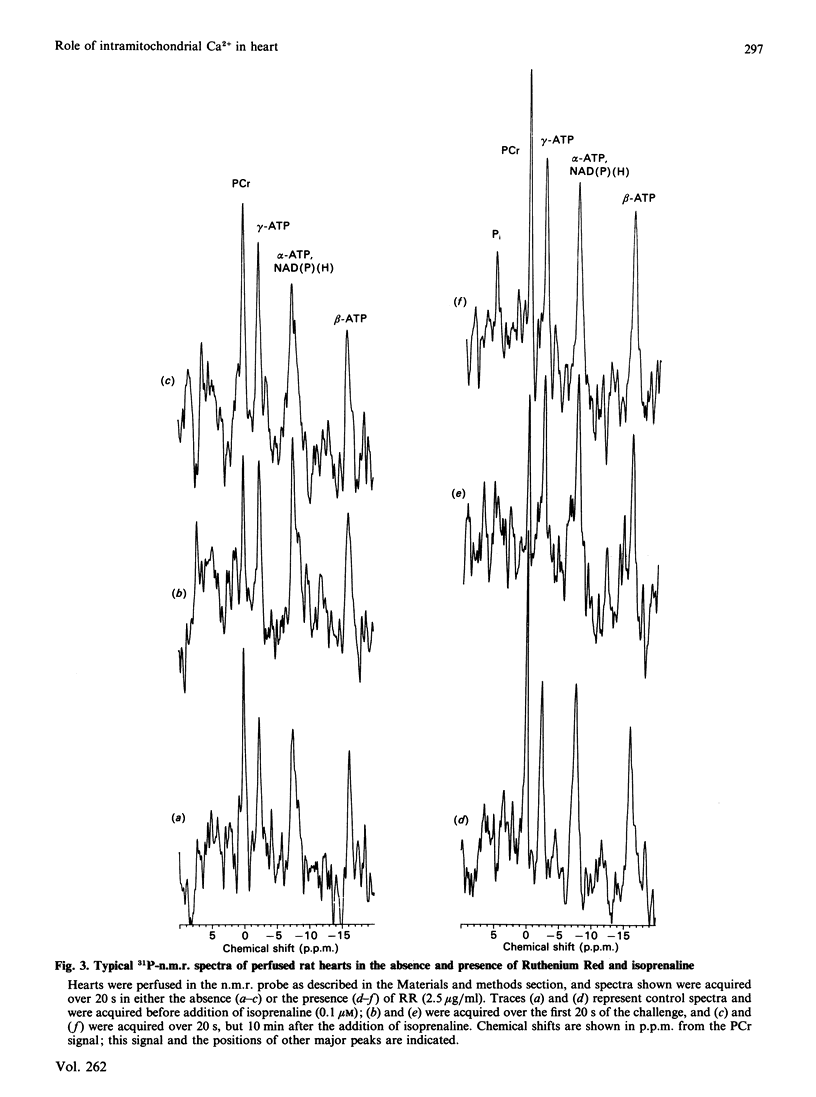
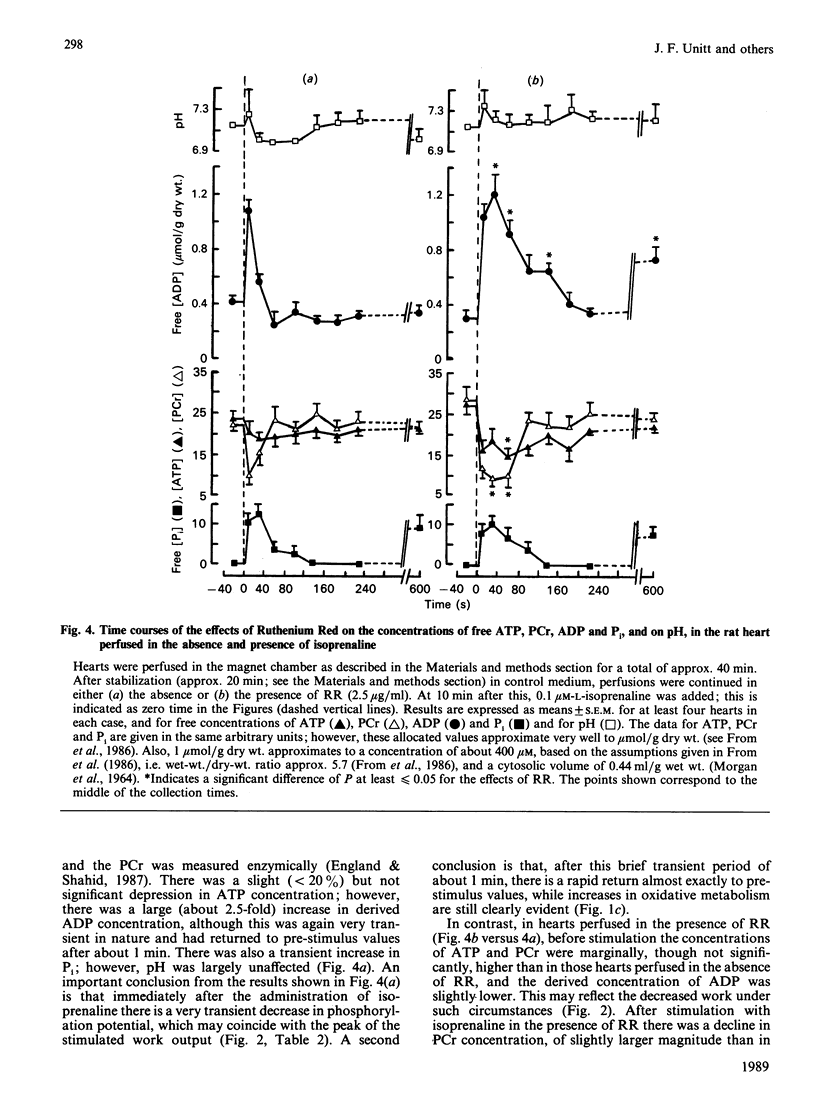
1. The concentrations of free ATP, phosphocreatine (PCr), Pi, H+ and ADP (calculated) were monitored in perfused rat hearts by 31P n.m.r. before and during positive inotropic stimulation. Data were accumulated in 20 s blocks. 2. Administration of 0.1 microM-(-)-isoprenaline resulted in no significant changes in ATP, transient decreases in PCr, and transient increases in ADP and Pi. However, the concentrations of all of these metabolites returned to pre-stimulated values within 1 min, whereas cardiac work and O2 uptake remained elevated. 3. In contrast, in hearts perfused continuously with Ruthenium Red (2.5 micrograms/ml), a potent inhibitor of mitochondrial Ca2+ uptake, administration of isoprenaline caused significant decreases in ATP, and also much larger and more prolonged changes in the concentrations of ADP, PCr and Pi. In this instance values did not fully return to pre-stimulated concentrations. Administration of Ruthenium Red alone to unstimulated hearts had minor effects. 4. It is proposed that, in the absence of Ruthenium Red, the transmission of changes in cytoplasmic Ca2+ across the mitochondrial inner membrane is able to maintain the phosphorylation potential of the heart during positive inotropic stimulation, through activation of the Ca2+-sensitive intramitochondrial dehydrogenases (pyruvate, NAD+-isocitrate and 2-oxoglutarate dehydrogenases) leading to enhanced NADH production. 5. This mechanism is unavailable in the presence of Ruthenium Red, and oxidative phosphorylation must be stimulated primarily by a fall in phosphorylation potential, in accordance with the classical concept of respiratory control. However, the full oxidative response of the heart to stimulation may not be achievable under such circumstances.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Morris P. G., Pirolo J. S., Smith G. L. Metabolic consequences of increasing intracellular calcium and force production in perfused ferret hearts. J Physiol. 1986 Jul;376:121–141. doi: 10.1113/jphysiol.1986.sp016145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987 May;62(2):141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Camacho S. A., Wikman-Coffelt J., Wu S. T., Watters T. A., Botvinick E. H., Sievers R., James T. L., Jasmin G., Parmley W. W. Improvement in myocardial performance without a decrease in high-energy phosphate metabolites after isoproterenol in Syrian cardiomyopathic hamsters. Circulation. 1988 Mar;77(3):712–719. doi: 10.1161/01.cir.77.3.712. [DOI] [PubMed] [Google Scholar]
- Ceconi C., Curello S., Cargnoni A., Ferrari R., Albertini A., Visioli O. The role of glutathione status in the protection against ischaemic and reperfusion damage: effects of N-acetyl cysteine. J Mol Cell Cardiol. 1988 Jan;20(1):5–13. doi: 10.1016/s0022-2828(88)80174-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Kent J., McCully K., Nioka S., Clark B. J., Maris J. M., Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Kessar P., Al-Nasser I. The alpha-adrenergic-mediated activation of the cardiac mitochondrial Ca2+ uniporter and its role in the control of intramitochondrial Ca2+ in vivo. Biochem J. 1983 Nov 15;216(2):333–342. doi: 10.1042/bj2160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J., Shahid M. Effects of forskolin on contractile responses and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987 Sep 15;246(3):687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R., di Lisa F., Raddino R., Visioli O. The effects of ruthenium red on mitochondrial function during post-ischaemic reperfusion. J Mol Cell Cardiol. 1982 Dec;14(12):737–740. doi: 10.1016/0022-2828(82)90186-9. [DOI] [PubMed] [Google Scholar]
- From A. H., Petein M. A., Michurski S. P., Zimmer S. D., Uğurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986 Oct 6;206(2):257–261. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol. 1985 Aug;17(8):727–731. doi: 10.1016/s0022-2828(85)80034-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between cytosolic free Ca2+ concentration and the control of pyruvate dehydrogenase in isolated cardiac myocytes. Biochem J. 1987 Jan 1;241(1):145–151. doi: 10.1042/bj2410145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- He M. X., Wangler R. D., Dillon P. F., Romig G. D., Sparks H. V. Phosphorylation potential and adenosine release during norepinephrine infusion in guinea pig heart. Am J Physiol. 1987 Nov;253(5 Pt 2):H1184–H1191. doi: 10.1152/ajpheart.1987.253.5.H1184. [DOI] [PubMed] [Google Scholar]
- Hess D. S., Bache R. J. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976 Jan;38(1):5–15. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- Hess D. S., Bache R. J. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976 Jan;38(1):5–15. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- Hiraoka T., DeBuysere M., Olson M. S. Studies of the effects of beta-adrenergic agonists on the regulation of pyruvate dehydrogenase in the perfused rat heart. J Biol Chem. 1980 Aug 25;255(16):7604–7609. [PubMed] [Google Scholar]
- Hoerter J. A., Miceli M. V., Renlund D. G., Jacobus W. E., Gerstenblith G., Lakatta E. G. A phosphorus-31 nuclear magnetic resonance study of the metabolic, contractile, and ionic consequences of induced calcium alterations in the isovolumic rat heart. Circ Res. 1986 Apr;58(4):539–551. doi: 10.1161/01.res.58.4.539. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Activation of dehydrogenase activity and cardiac respiration: a 31P-NMR study. Am J Physiol. 1988 Jul;255(1 Pt 2):H185–H188. doi: 10.1152/ajpheart.1988.255.1.H185. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Intracellular pH and inorganic phosphate content of heart in vivo: a 31P-NMR study. Am J Physiol. 1988 Jul;255(1 Pt 2):H189–H196. doi: 10.1152/ajpheart.1988.255.1.H189. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- Koretsky A. P., Balaban R. S. Changes in pyridine nucleotide levels alter oxygen consumption and extra-mitochondrial phosphates in isolated mitochondria: a 31P-NMR and NAD(P)H fluorescence study. Biochim Biophys Acta. 1987 Oct 7;893(3):398–408. doi: 10.1016/0005-2728(87)90092-2. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Jacobus W. E., Marban E. Calcium oscillations in digitalis-induced ventricular fibrillation: pathogenetic role and metabolic consequences in isolated ferret hearts. Circ Res. 1988 Mar;62(3):609–619. doi: 10.1161/01.res.62.3.609. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- MORGAN H. E., REGEN D. M., PARK C. R. IDENTIFICATION OF A MOBILE CARRIER-MEDIATED SUGAR TRANSPORT SYSTEM IN MUSCLE. J Biol Chem. 1964 Feb;239:369–374. [PubMed] [Google Scholar]
- Matthews P. M., Williams S. R., Seymour A. M., Schwartz A., Dube G., Gadian D. G., Radda G. K. A 31P-NMR study of some metabolic and functional effects of the inotropic agents epinephrine and ouabain, and the ionophore R02-2985 (X537A) in the isolated, perfused rat heart. Biochim Biophys Acta. 1982 Apr 29;720(2):163–171. doi: 10.1016/0167-4889(82)90008-8. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Browne H. M., Dawes N. J. Studies on mitochondrial Ca2+-transport and matrix Ca2+ using fura-2-loaded rat heart mitochondria. Biochim Biophys Acta. 1989 Mar 23;973(3):420–427. doi: 10.1016/s0005-2728(89)80384-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem J. 1985 Nov 1;231(3):581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984 Feb 15;218(1):235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The activation of pyruvate dehydrogenase in the perfused rat heart by adrenaline and other inotropic agents. Biochem J. 1981 Feb 15;194(2):639–643. doi: 10.1042/bj1940639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Edgell N. J., Denton R. M. Studies on the interactions of Ca2+ and pyruvate in the regulation of rat heart pyruvate dehydrogenase activity. Effects of starvation and diabetes. Biochem J. 1982 Feb 15;202(2):419–427. doi: 10.1042/bj2020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin J. B., Pauly D. F. Control of mitochondrial respiration in muscle. Mol Cell Biochem. 1988 Jun;81(2):121–129. doi: 10.1007/BF00219314. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R. G. Relation between cytosolic free calcium and respiratory rates in cardiac myocytes. Am J Physiol. 1988 Aug;255(2 Pt 2):H347–H357. doi: 10.1152/ajpheart.1988.255.2.H347. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. F., Kane J. J., Straub K. D., Murphy M. L. Improvement of mitochondrial energy production in ischemic myocardium by in vivo infusion of ruthenium red. J Cardiovasc Pharmacol. 1980 Jan-Feb;2(1):45–54. doi: 10.1097/00005344-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Sellevold O. F., Jynge P., Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J Mol Cell Cardiol. 1986 May;18(5):517–527. doi: 10.1016/s0022-2828(86)80917-8. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Smith H. J. Depressed contractile function in reperfused canine myocardium: metabolism and response to pharmacological agents. Cardiovasc Res. 1980 Aug;14(8):458–468. doi: 10.1093/cvr/14.8.458. [DOI] [PubMed] [Google Scholar]
- Sparks H. V., Jr, Bardenheuer H. Regulation of adenosine formation by the heart. Circ Res. 1986 Feb;58(2):193–201. doi: 10.1161/01.res.58.2.193. [DOI] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F. Ca-pools involved in the regulation of cardiac contraction under positive inotropy. X-ray microanalysis on rapidly-frozen ventricular muscles of guinea-pig. Basic Res Cardiol. 1986;81 (Suppl 1):25–32. doi: 10.1007/978-3-662-11374-5_3. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Possible role of citrate in the control of epinephrine-stimulated glycogenolysis in rat heart. Nature. 1965 May 1;206(983):473–475. doi: 10.1038/206473a0. [DOI] [PubMed] [Google Scholar]


