Abstract
Granular cell tumor (GCT) is a rare neoplasm. Its diagnosis is based on imaging and pathological findings. There are only a few reported cases of GCT of the breast (GCTB) in the literature. We present a case of a female patient diagnosed with GCTB and perform a review on the prevalence, diagnosis, histology, treatment, and prognosis.
Keywords: Breast, Granular cell tumor, Imaging, Case report
Introduction
Granular cell tumors (GCTs) can arise from Schwann cells and can be found in subcutaneous, intradermal, or submucosal tissues [1]. They can occur anywhere in the body and may be multifocal [1,2] with head, neck, chest wall and arms being the most common sites [1,3]. GCT of the breast (GCTB) arises in the intralobular breast stroma and occurs in the distribution of cutaneous branches of the supraclavicular nerve [1]. GCTs are rare and account for approximately 0.5% of all soft tissue tumors. However, GCTBs are even rarer. They account for 5% to 15% of all GCTs and are mostly benign [4].
Case history
A 50-year-old female patient presented for evaluation of a new palpable mass detected on self-examination 1 month ago in her right breast. A mammogram was performed (Fig. 1) demonstrating a subcentimetric irregular mass with spiculated margins in the upper outer quadrant of the right breast.
Fig. 1.
MLO (A) and CC (B) views demonstrate an irregular mass with spiculated margins in the upper outer quadrant of the right breast (arrow).
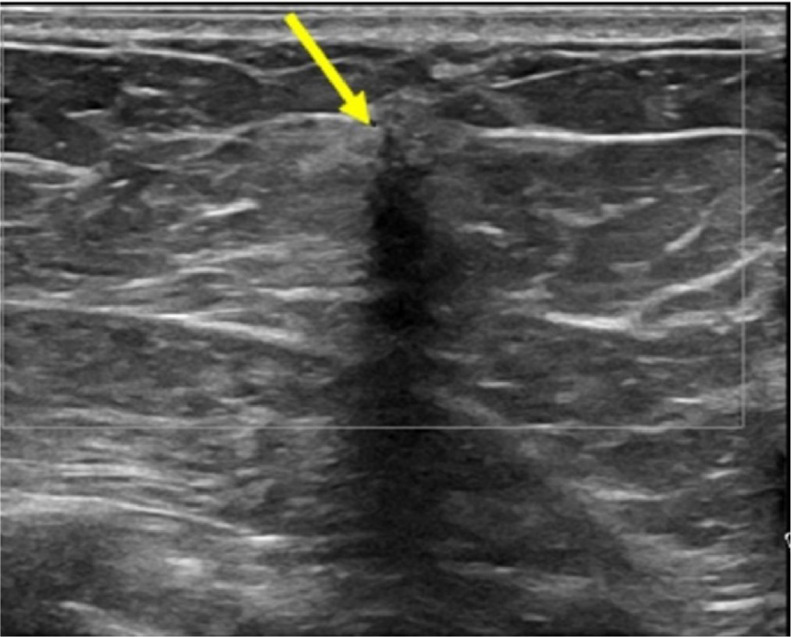
Spots were not performed since the mass was well seen on tomosynthesis images. An ultrasound (Fig. 2) was performed demonstrating a vertically oriented irregular mass with indistinct margins, hypoechoic echogenicity, and marked posterior acoustic shadowing in the upper outer quadrant of the right breast.
Fig. 2.
Right breast ultrasound demonstrates a vertically oriented irregular mass with indistinct margins, hypoechoic echogenicity, and marked posterior acoustic shadowing (arrow).
A metallic mark was placed at the sonographic finding, and the lateral mammographic view (Fig. 3) demonstrated the correlation of the mammographic and sonographic findings. This mass was categorized as BIRADS 5.
Fig. 3.
A lateral mammographic view demonstrating the correlation of the mammographic and sonographic findings.
The right axillary ultrasound did not show any suspicious lymphadenopathy. A 9G ultrasound-guided vacuum-assisted biopsy was then performed, as part of the Institution's protocol for small and highly suspicious lesions. Five samples were taken, and the biopsy results were consistent with a benign GCTB. Immunostains performed show the tumor cells to be positive for S100 and CD68 and negative for CK7. Post biopsy mammogram (Fig. 4) revealed hematoma and a well-positioned post biopsy clip (arrow).
Fig. 4.
A hematoma and a well-positioned post biopsy clip on a post-biopsy mammogram (arrow).
The lesion was locally excised, and the final pathology result yielded post biopsy changes, small residual granular cell tumor and no signs of malignancy. The radiology-pathology correlation was considered concordant and after a multidisciplinary discussion, the patient was discharged. A Follow-up mammogram in 1 year showed no suspicious abnormalities.
Discussion
GCTs are rare. According to a study at a single institution conducted over a span of 32 years, the overall incidence of GCTs in surgical specimens was 0.03% [5]. Some other studies suggest a prevalence of 1:617 among the screened population and 6.7:1000 cases in the total clinical population [1,6]. GCTB can occur in both sexes but is more common in women, with a female to male ratio ranging from 1.8 to 2.4 [7]. GCTB can occur in all age groups but is more common in women in their 40s to 60s [5,8]. However, there have been cases identified in patients as young as 14 years old [9,10]. It is also more prevalent in Black women, comprising about 60% to 70% of cases [8,11].
About 70% of cases of the GCTBs are detected by physical examination (palpation), 26% through screening and 4% during follow-up post breast malignancy [9,11,12]. Most of the palpable masses are painless, mobile, firm and elastic with some possible abnormal skin changes like thickening, tethering, dimpling, and retraction. Some patients have reported pain or pruritus. Axillary lymph adenopathy is uncommon and is reactive [8,9,11]. GCTBs are usually solitary, but multiple lesions can occur within the breast or in combination with an extramammary mass in 18% of the cases [12]. In 10% of the cases, there can be a concomitant malignancy, mostly ductal carcinoma [13,14]. Multiple GCTs should raise concern about associated syndromes such as neurofibromatosis type I and Noonan syndrome [15]. There is no known etiology or risk factors, but some authors have reported PTPN11 gene mutations in GCTs associated with Noonan syndrome [1]. In another study, GCT was found to be associated with germline PTEN mutations in patients with PTEN hamartoma tumor syndrome [16].
Although GCTBs are often benign, 1%-2% of cases can be malignant [15]. Malignant GCTBs can grow quickly, locally invade surrounding tissues, and spread to nearby axillary lymph nodes. They also show higher rates of local recurrence [3]. The breast can also be secondarily involved with metastasis from primary malignant GCT elsewhere. Recurrence and metastasis, however, can also occur with histologically benign or atypical GCTs [11]. The most common sites for metastasis are lungs and bones. Less common sites are liver, bowel, breast, thyroid, heart, pancreas, spleen, retroperitoneum, pharynx, mouth, neck, and brain [2].
On macroscopy, GCTBs present as firm solitary masses that frequently exhibit an infiltrative growth pattern with noncircumscribed margins, but some of them are well-circumscribed with a capsule [11]. When nonencapsulated, GCTBs may infiltrate into the surrounding tissues like fibrous tissue, adipose tissue, and pectoralis major muscle [11]. Due to the infiltrative growth pattern, GCTBs may resemble invasive breast carcinomas [9,12]. On microscopy, GCTBs show an infiltrative growth pattern in nests, cords, or sheets of large polygonal and occasionally spindled cells, which often have abundant eosinophilic finely granular cytoplasm and small nuclei surrounded by sclerotic stroma [11].
Both benign and malignant GCTs typically stain strongly positive for S-100, a neuro melanocytic marker, allowing differentiation from breast carcinomas, which are typically negative. Some GCTs have been reported during pregnancy [18], and the immunohistochemical profile shows no hormonal dependence. GCTs lack estrogen or progesterone receptor and cytokeratin expression [11]. Microscopically, GCTBs can resemble apocrine carcinomas, but GCTBs lack androgen receptors, and apocrine carcinoma lacks S-100 expression. AE1/3 epithelial markers can also help distinguish GCTB from invasive carcinoma because they stain negative in GCTBs [11].
Histologic characteristics that are indicative of malignant GCTB are prominent nucleoli, a tumor diameter greater than 5 cm, elevated mitotic activity, necrosis and nuclear or cellular pleomorphism. A high Ki-67 proliferation index may also suggest malignant GCT [11]. There are no specific imaging characteristics for GCTBs, however. They can mimic breast carcinoma [28] or be associated with a breast carcinoma [12,20].
On mammography, GCTBs often present as irregular masses in 75% of cases, circumscribed oval or round masses in 18% of cases, or masses with indistinct margins and architectural distortion in 8% of cases [11,19]. They can be either hyperdense, isodense, or have a hypodense rim [8,11,19]. Even if the mass is circumscribed, GCTB often appears infiltrative on microscopy. Careful review of GCTB images frequently demonstrates focally indistinct or spiculated margins. There are no associated calcifications, and they are smaller than 3 cm in size [8,11,19]. GCTBs are often reported in the upper inner quadrant of the breast in up to 83% of cases, which matches the cutaneous sensory branches of the supraclavicular nerve [7]; however, they have also been reported in all quadrants and in the axillary region. GCTB not only invades adjacent structures such as overlying skin or muscles [11], but it may also invade adjacent fibroadenomas or lymph nodes.
Sonographic findings of GCTBs are not specific. The sonographic appearance depends on the degree of the tumor infiltration and reactive fibrosis. The most reported findings are hyper vascularized hypo or hyperechoic solid masses with ill-defined margins and significant posterior shadowing [8,9,11,17]. Marked hypoechoic masses have been reported in 56% of cases, posterior acoustic shadowing in 48%, and mixed heterogeneous echotexture with areas of hyperechogenic or entirely hyperechoic in 44% of cases [9,11,19]. Rarely, they can be well circumscribed with posterior acoustic enhancement, which is reported in 36% of cases [19].
There are few reports on magnetic resonance imaging (MRI) findings of GCTBs. Most of the available reports are of oval or irregular masses with irregular margins, intermediate signal intensity on T1-weighted images, and mild hyperintensity on T2-weighted images with no surrounding edema [11,19]. After contrast injection, most GCTBs are described as having a homogeneous enhancement, but rim enhancement has also been reported. Kinetics is variable, and there are reports of slow and rapid enhancement [9,11,12]. On 18F- fluorodeoxyglucose positron emission computed tomography (18F-FDG PET/CT), GCTBs do not show any significant increased glucose metabolic activity. The reported standardized uptake values were 1.7 to 1.8 [9,21]. In one of the presented cases, an 18F-FDG PET/CT was performed because the patient's rectal carcinoma and GCTB demonstrated a low uptake, indicating a benign lesion.
Core biopsy is the more common method to achieve definitive diagnosis [22]. Analysis of the histologic features combined with the immunohistochemical stains as discussed above allow the correct diagnosis and differentiation from breast carcinomas [9]. Sometimes it can be difficult to distinguish malignant from a benign CGT on core biopsy because some nonrepresentative sampling can occur. Benign GCTs can also demonstrate both vascular and perineural invasion, but these histologic findings do not necessarily indicate a malignancy or an adverse prognosis [23]. A GCT can eventually develop patchy areas of malignancy, which combined with a nonrepresentative sampling, might require a complete excisional biopsy to establish a correct diagnosis. One differential diagnosis of GCT is a traumatic granular cell neuroma, which can occur in mastectomy beds, close to surgical scars, mimicking a recurrence. They can be indistinguishable in terms of histology and immunohistochemistry [15], but they are both benign. The main differential diagnosis is invasive breast carcinomas, specifically scirrhous carcinomas, since they have completely different treatment, outcomes, and prognosis. The correct diagnosis can be achieved by combining histologic, immunohistochemical, imaging and clinical features [8]. A careful evaluation of a patient's personal history, family history, and risk for breast cancer can be useful to address eventual discordant biopsy results and clinical evolution.
Excision of a GCTB tumor remains the standard treatment for both benign or malignant GCTBs, and it is often curative. Due to the infiltrative growth pattern of this tumor, wide surgical margins have been shown to diminish its recurrence risk [8]. Local excision of lymph nodes or sentinel lymph node biopsy is not indicated except in cases of malignant GCTB [8,9]. Given the lack of conducted randomized clinical trials, there is no current standard chemotherapy regimen or adjuvant radiation therapy for malignant or metastatic GCTs [8]. Benign GCTs show excellent prognosis even when recurrence is present [8,24]. Recurrence has been reported in 2% to 8% of cases, even after excision of the tumor with wide margins [24]. After removal of a GCT, some authors recommend that long term follow-up of 10 years should be performed [25], but there is still no clear consensus on this matter [11]. Malignant GCTs have a worse prognosis, with 74% and 65% survival rates at 5 years and 10 years, respectively. Malignant GCTs show a recurrence rate of 32% to 41% and metastasis rate of 11% to 62% between 3 and 37 months after diagnosis [8]. GCTs with distant metastases at diagnosis have 0% survival at 5 years compared to an 81% survival rate in those without metastasis [8].
Conclusion
GCTBs are rare. Most cases are palpable and benign, and the most common imaging feature is a small irregular and spiculated mass on mammograms with a marked posterior shadowing on ultrasound, making the differentiation from a malignancy difficult. Even benign GCTBs can invade local structures and adjacent lesions. The diagnosis can be achieved with a core needle biopsy using the appropriate immunohistochemical panel and should be intensively positive for S-100 and negative for both progesterone and estrogen receptors. Excision with wide margins is the standard treatment and recurrences can occur. Overall, the prognosis is excellent even when there is recurrence. There are currently few reported studies on the genetic predisposition of this tumor. Some associated mutations with GCTBs under investigation include PTPN11 and PTEN.
Patient consent
Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Patel A, Lefemine V, Yousuf SM, Abou-Samra W. Granular cell tumour of the pectoral muscle mimicking breast cancer. Cases J. 2008;1(1):142. doi: 10.1186/1757-1626-1-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ordóñez NG. Granular cell tumor: a review and update. Adv Anat Pathol. 1999;6(4):186–203. doi: 10.1097/00125480-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Omar L, Pfeifer CM, Kulkarni S, Sharma P, Sengupta A, Kwon JK. Granular cell tumor in a premenstrual female breast. Clin Imaging. 2018;52:334–336. doi: 10.1016/j.clinimag.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Neelon D. Treasure Island (FL): StatPearls Publishing. JanWalter Reed National Military Medical Center; Bethesda, Maryland: 2024. Granular cell tumor.https://www.ncbi.nlm.nih.gov/books/NBK563150/ Available at. (Accessed: February 01, 2024) [Google Scholar]
- 5.Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13(4):301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 6.Irshad A, Pope TL, Ackerman SJ, Panzegrau B. Characterization of sonographic and mammographic features of granular cell tumors of the breast and estimation of their incidence. J Ultrasound Med. 2008;27(3):467–475. doi: 10.7863/jum.2008.27.3.467. [DOI] [PubMed] [Google Scholar]
- 7.Mariscal A, Perea RJ, Castellá E, Rull M. Granular cell tumor of the breast in a male patient. AJR Am J Roentgenol. 1995;165(1):63–64. doi: 10.2214/ajr.165.1.7785634. [DOI] [PubMed] [Google Scholar]
- 8.Neelon D, Lannan F, Childs J. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2023. Granular cell tumor. [PubMed] [Google Scholar]
- 9.Brown AC, Audisio RA, Regitnig P. Granular cell tumour of the breast. Surg Oncol. 2011;20(2):97–105. doi: 10.1016/j.suronc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.De Simone N, Aggon A, Christy C. Granular cell tumor of the breast: clinical and pathologic characteristics of a rare case in a 14-year-old girl. J Clin Oncol. 2011;29(22):e656–e657. doi: 10.1200/JCO.2011.35.9448. [DOI] [PubMed] [Google Scholar]
- 11.Ghannam SM, Carter GJ, Villatoro TM, Berg WA. Granular cell tumor of the breast: radiologic–pathologic correlation. J Breast Imaging. 2021;3(4):473–481. doi: 10.1093/jbi/wbab041. [DOI] [PubMed] [Google Scholar]
- 12.Meani F, Di Lascio S, Wandschneider W, Montagna G, Vitale V, Zehbe S, et al. Granular cell tumor of the breast: a multidisciplinary challenge. Crit Rev Oncol Hematol. 2019;144:102828. doi: 10.1016/j.critrevonc.2019.102828. [DOI] [PubMed] [Google Scholar]
- 13.Delaloye JF, Seraj F, Guillou L, Genton CY, Anciaux-Le Teno D, Schnyder P, et al. Granular cell tumor of the breast: a diagnostic pitfall. Breast. 2002;11(4):316–319. doi: 10.1054/brst.2002.0421. [DOI] [PubMed] [Google Scholar]
- 14.Al-Ahmadie H, Hasselgren PO, Yassin R, Mutema G. Colocalized granular cell tumor and infiltrating ductal carcinoma of the breast. Arch Pathol Lab Med. 2002;126(6):731–733. doi: 10.5858/2002-126-0731-CGCTAI. [DOI] [PubMed] [Google Scholar]
- 15.Neelon D, Lannan F, Childs J. Granular cell tumor. PubMed. Published 2023. https://www.ncbi.nlm.nih.gov/books/NBK563150/. [PubMed]
- 16.Marchese C, Montera M, Torrini M, Goldoni F, Mareni C, Forni M, et al. Granular cell tumor in a PHTS patient with a novel germline PTEN mutation. Am J Med Genet A. 2003;120A(2):286–288. doi: 10.1002/ajmg.a.20179. Jul 15. [DOI] [PubMed] [Google Scholar]
- 17.Adeniran A, Al-Ahmadie H, Mahoney MC, Robinson-Smith TM. Granular cell tumor of the breast: a series of 17 cases and review of the literature. Breast J. 2004;10(6):528–531. doi: 10.1111/j.1075-122X.2004.21525.x. [DOI] [PubMed] [Google Scholar]
- 18.Kommoss F, Mercer L, Schmidt RA, Talerman A. Granular cell tumor of the breast mimicking carcinoma in pregnancy. Obstet Gynecol. 1989;73(5 Pt 2):898–900. [PubMed] [Google Scholar]
- 19.Abreu N, Filipe J, André S, Marques JC. Granular cell tumor of the breast: correlations between imaging and pathology findings. Radiol Bras. 2020;53(2):105–111. doi: 10.1590/0100-3984.2019.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergel A, Yucel AF, Karaca AS, Aydin I, Sahin DA, Demirbag N. A therapeutic and diagnostic dilemma: granular cell tumor of the breast. Case Rep Med. 2011;2011 doi: 10.1155/2011/972168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoess C, Freitag K, Kolben M, Allgayer B, Laemmer-Skarke I, Nathrath WB, et al. FDG PET evaluation of granular cell tumor of the breast. J Nucl Med. 1998;39(8):1398–1401. [PubMed] [Google Scholar]
- 22.Tan PH, Ellis I, Allison K, Brogi EB, Fox S, Lakhani S, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 23.Battistella M, Cribier B, Feugeas JP, Roux J, Le Pelletier F, Pinquier L, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67(1):19–25. doi: 10.1136/jclinpath-2013-201642. [DOI] [PubMed] [Google Scholar]
- 24.Quiroz-Rodriguez G, Robles-Vidal C, Guzmán-Navarro L, Ortiz-Hidalgo C. Granular cell (Abrikossof) tumor of the breast. Breast J. 2006;12(5):494. doi: 10.1111/j.1075-122X.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 25.Althausen AM, Kowalski DP, Ludwig ME, Curry SL, Greene JF. Granular cell tumors: a new clinically important histologic finding. Gynecol Oncol. 2000;77(2):310–313. doi: 10.1006/gyno.2000.5767. [DOI] [PubMed] [Google Scholar]