Abstract
The tumor microenvironment (TME) is composed of different cellular and non-cellular elements. Constant interactions between tumor cells and the TME are responsible for tumor initiation, tumor progression, and responses to therapies. Immune cells in the TME can be classified into two broad categories, namely adaptive and innate immunity. Targeting these immune cells has attracted substantial research and clinical interest. Current research focuses on identifying key molecular players and developing targeted therapies. These approaches may offer more efficient ways of treating different cancers. In this review, we explore the heterogeneity of the TME in non-small cell lung cancer, summarize progress made in targeting the TME in preclinical and clinical studies, discuss the potential predictive value of the TME in immunotherapy, and highlight the promising effects of bispecific antibodies in the era of immunotherapy.
Keywords: Tumor microenvironment, Adaptive immune cell, Innate immune cell, Immunotherapy, Biomarker, Bispecific antibody
Introduction
Targeting the genetic alterations has contributed greatly to the treatment of non-small cell lung cancer (NSCLC).1,2 Increasing evidence indicates that the tumor microenvironment (TME) plays a pivotal role in tumorigenesis and treatment responses.3,4 The TME is complex and is composed of multiple cell types. Briefly, the TME includes the surrounding tumor vasculature, endothelial cells, fibroblasts, immune cells, and extracellular components.5 These components form an interactive cellular- and non-cellular-based network during tumor initiation, progression, and therapeutic responses.
Most research on TMEs has focused on innate and adaptive immune cells.6 Among the different kinds of adaptive immune cells, the roles of cytotoxic T lymphocytes in the TME have been studied most extensively.7 Current research mostly focuses on this subset owing to its cytotoxic ability. Based on the current understanding of the innate immune system, it is evident that the innate immune response is pivotal for tumor progression. Innate immune cells, especially myeloid cells, consist of antitumoral or protumoral cells such as tumor-associated macrophages (TAMs), dendritic cells (DCs), and myeloid-derived suppressor cells (MDSCs).8,9 The function and heterogeneity of each subset are discussed below [Fig. 1].
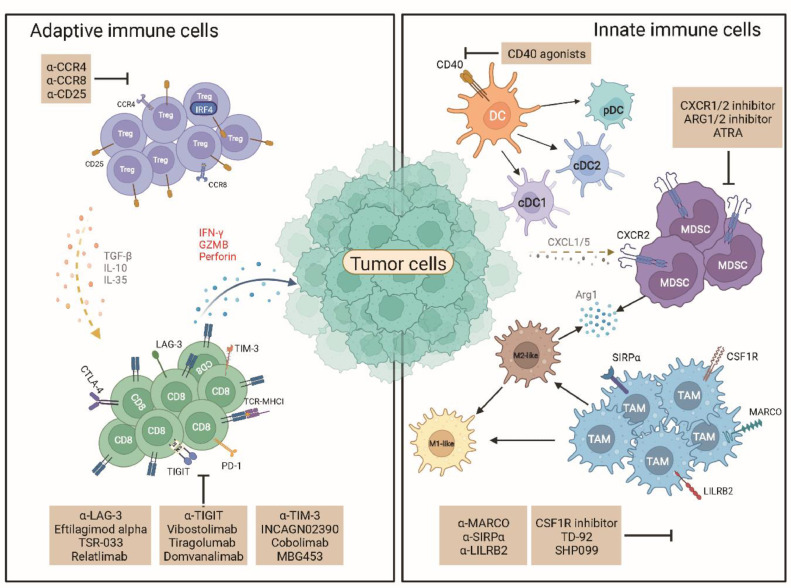
Fig. 1.
The adaptive and innate immune cells in the tumor microenvironment. The left part displays the adaptive immune cells, which include primarily CD8+ T cells and Tregs. CD8+ T cells release cytotoxic chemokines to kill tumor cells. However, CD8+ T cell expresses several immune checkpoints upon chronic antigen stimulation including PD-1, TIM-3, LAG-3, and TIGIT. This restricts the cytotoxic effect of CD8+ T cells. Treg was an immunosuppressive subset that could release IL-10 and TGF-β to suppress the function of T cells. The right part displays the innate immune cells. Macrophages, dendritic cells, and MDSC are major subsets of innate immune cells. These cells consist of both antitumor immune cells and pro-tumor immune cells. Macrophages and dendritic cells could be divided into subtypes based on specific markers and functions. MDSC produces Arg1 and iNOS to suppress the function of T cells. These innate immune cells could be targeted or reprogramed to support antitumor immunity. Arg1: Arginase 1; ATRA: All-trans retinoic acid; CCR4: C-C chemokine receptor type 4; CCR5: C-C chemokine receptor type 5; CCR8: C-C chemokine receptor type 8; cDC1: Type 1 conventional dendritic cells; cDC2: Type 2 conventional dendritic cells; CSF-1R: Colony stimulating factor 1 receptor; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; CXCL1/5: C-X-C motif chemokine ligand 1/5; CXCR1/2: C-X-C motif chemokine receptor 1/2; DC: Dendritic cell; GZMB: Granzyme B; IFN-γ: Interferon gamma; IL: Interleukin; IRF4: Interferon regulatory factor 4; LAG-3: Lymphocyte-activation gene 3; LILRB2: Leukocyte immunoglobulin-like receptor subfamily B member 2; MARCO: Macrophage receptor with collagenous structure; MDSC: Myeloid-derived suppressor cells; MHC I: Major histocompability complex I; PD-1: Programed death-1; pDC: Plasmacytoid DC; SHP-2: Src homology-2 domain-containing protein tyrosine phosphatase-2; SIRPα: Signal regulatory protein α; TAM: Tumor- associated macrophages; TCR: T cell receptor; TGF-β: Transforming growth factor beta; TIGIT: T cell immunoreceptor with immunoglobulin (Ig) and ITIM domains; TIM-3: T cell immunoglobulin and mucin domain-containing protein 3; Treg: Regulatory T cells.
Immunohistochemistry (IHC) and flow cytometry are frequently used to detect components of the TME. However, these technologies do not comprehensively reflect the complexity of the TME, and NSCLC is a highly heterogeneous disease.10 A deeper understanding of the TME can be achieved using highly developed technologies, such as single-cell RNA-sequencing (scRNA-seq),11 mass cytometry,12 and spatial transcriptomics.13,14 The TME of NSCLC has been systematically analyzed at single-cell resolution in several recent studies. In the next section, we discuss the heterogeneity of the tumor immune microenvironment in NSCLC.
TME of NSCLC
Adaptive immune subsets
CD8+ T cells
T cells in the TME experience chronic antigen stimulation, which leads to CD8+ T cell exhaustion. These cells express high levels of immune-checkpoint proteins, including programmed death-1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4),15 which hinder the cytotoxic effects of CD8+ T cells. However, T cell exhaustion is not a fixed state. A subset of progenitor exhausted cells has been identified in melanoma. This subset can differentiate into “terminally exhausted” tumor-infiltrating lymphocytes (TILs) and respond to anti-PD-1 therapy.16 Interestingly, Guo et al17 identified two CD8+ T cell clusters that probably precede exhaustion based on scRNA-seq data from 12,346 T cells from 14 treatment-naïve patients with NSCLC. The ratio of “pre-exhausted” to exhausted T cells was used to discern a favorable prognosis for a subset of patients with lung adenocarcinoma (LUAD). However, the association between “terminally exhausted” TILs and “pre-exhausted” T-cells requires further investigation. Recent findings by Liu et al18 revealed increased levels of precursor exhausted T cells (Texp) from responsive tumors after anti-PD-1-based therapies, which were absent in non-responsive tumors. The expansion of Texp cells probably results from: (1) local expansion and (2) replenishment by peripheral T cells. The results of a previous study highlight the role of precursor exhausted T cells in treatment responses and expand upon the concept of “clonal replacement”.19 In another study, a new subset of CD8+ T cells expressing CD45RO, eomesodermin (EOMES), FAS, Ki67, LAG-3, PD-1, and TIM-3 were termed burned-out CD8+ T cells (Ebo). Ebo CD8+ T cells share several features with exhausted T cells, but are highly proliferative, representing the overactive and apoptotic phenotype of these cells. The accumulation of Ebo CD8+ T cells in patients with advanced NSCLC is associated with resistance to anti-PD1 therapy.20 The reinvigoration of exhausted CD8+ T cells is the aim of immunotherapy. However, T cell differentiation is complex, and heterogeneity exists among different T cell subsets, as described above. Aging has also been reported to impair the expression of cytolytic molecules in CD8+ T cells.21 Thus, a comprehensive understanding of tumor-infiltrating T cells is urgently needed.
Regulatory T cells (Tregs)
Tregs display robust immunosuppressive function in CD4-positive (CD4+) T cells, which were first found to maintain immunological self-tolerance and homeostasis.22 Tregs are defined as CD25+ forkhead box protein 3 (FOXP3)+ cells based on analysis of their surface markers.23 Tregs are frequently found in the TME and can be classified into different groups based on their distinct features. Three groups of tumor-associated Tregs have been identified previously, namely tumor-resident Tregs, tissue-resident Tregs, and circulating Tregs.24, 25, 26 Tumor-associated Tregs account for 10–50% of CD4+ T cells in tumors, which is profoundly higher than that found in peripheral blood from healthy individuals (2–5%). The immunosuppressive effect of Tregs involves the release of immunosuppressive cytokines, including transforming growth factor β (TGF-β), interleukin 10 (IL-10), and IL-35.27,28 Increased Treg infiltration influences invasion, metastasis, recurrence, drug responses, and the prognosis of patients with multiple cancers by inhibiting the cytotoxic role of CD8+ T cells.28 The prevalence of Tregs in the TME indicates that targeting Tregs may improve the efficacy of NSCLC treatment. This strategy for targeting Tregs is discussed in the next section.
Innate immune cells
DCs
Myeloid cells comprise both antitumor and immunosuppressive cells.29 DCs are the most common antigen-presenting myeloid cells.30 DCs can be classified into at least three subsets, based on specific markers and functions, including plasmacytoid DCs (pDCs), conventional type 1 DCs (cDC1s), and conventional type 2 DCs (cDC2s).31 Among these subtypes, cDC1 plays a pivotal role in initiating T cell responses and is associated with survival in patients with cancer.32. Leader et al33 profiled 361,929 cells from 35 early stage NSCLC lesions, and 49 immune clusters were identified. DCs are mainly composed of cDC1s, cDC2s, mature DCs enriched in regulatory molecules (mregDCs), and DC3s. DC3 is the most prevalent DC subtype found in tumors and is characterized as CD14+/CD163+.33 In another study, Zilionis et al34 identified four subsets of DCs in lung cancer: human pDC and hDC1–3. The hDC1 and hDC2 subsets mapped well to the cDC1 and cDC2 populations. hDC3 displayed an “activated” DC phenotype.34 Both studies identified four DC clusters. The cDC1 and cDC2 populations were identified in accordance with the previous study.35 However, disparate definitions exist for distinct subtypes, which may reflect differences in the analytical methods used and the markers selected.
Macrophages
In the study by Leader et al,33 the distributions of different types of macrophages were analyzed simultaneously. Based on the expression of marker genes, macrophages can be classified into alveolar macrophages, interstitial macrophages, monocyte-derived macrophages, and azurocidin 1 (AZU1)+ macrophages. Alveolar macrophages are the predominant subset in the TME, whereas interstitial macrophages are depleted. Zilionis et al34 defined nine transcriptional states in human macrophages, which were classified as hMø1–9 cells. Macrophages can be classified as M1 macrophages (with a pro-inflammatory phenotype) and M2 macrophages (with an anti-inflammatory phenotype), based on the expression of specific markers.36,37 Among the nine subsets of macrophages mentioned above, no unique M1 phenotype macrophages were found. The M2 gene-expression signature was found with the hMø3, hMø6, and hMø8 subsets.34 These data suggest that immunosuppressive M2 macrophages occupy the TME. Targeting this subset of macrophages may improve treatment efficacies.
MDSCs
MDSCs represent another type of immunosuppressive myeloid cells in the TME.38,39 MDSCs can be further divided into polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs).40 Infiltrating MDSCs can suppress the function of T cells through various mechanisms, such as programed death-ligand 1 (PD-L1) expression 41,42 and the secretion of immunosuppressive cytokines such as IL-10 and TGF-β.43, 44, 45 Previously, MDSCs and inflammatory factors were profiled in the peripheral blood and resectable tumors of 49 patients with NSCLC. More C-C chemokine receptor type 5 (CCR5)-expressing circulating M-MDSCs were found in the peripheral blood of patients with lung cancer than in that from healthy donors. The frequency of M- and PMN-MDSCs was higher in tumor tissues than in the peripheral blood.46 Dysregulated MDSC differentiation and chemoattraction may contribute to a higher abundance of MDSCs in the TME. The results of a previous study showed that higher levels of CD45+ erythroid progenitor cells (EPCs, CD71+TER119+ cells) were found in anemic patients with cancer.47 The same study revealed that CD45+ EPCs could transdifferentiate into erythroid–myeloid hybrid cell (EDMC) populations. This process was dependent on granulocyte-macrophage colony-stimulating factor produced by tumor cells. EDMCs displayed robust immunosuppressive activity and reduced efficacy with anti-PD-1/PD-L1 treatment.48 Thus, targeting the differential chemotaxis of MDSCs may prevent MDSC infiltration and improve immunotherapy.
As discussed above, scRNA-seq provides a high-resolution map of the TME. Immune cells can be subdivided into clusters for more precise characterization. Such information can help identify specific immune subsets that influence tumor progression or treatment responses, as well as potential targets.
Targeting TME to improve immunotherapy against NSCLC
Immune cells inside the TME can be identified using specific markers, including chemokine receptors, immunosuppressive molecules, and functional markers. The corresponding immune cells can be depleted or inhibited by targeting these markers with antibodies or inhibitors, respectively. Some molecules have entered clinical trials and achieved great success. In this section, we discuss the potential for targeting these immune cells in the TME [Tables 1 and 2].
Table 1.
Preclinical studies that target the tumor microenvironment.
| Immune cell(s) | Agent(s) | Target(s) | Model(s) | Effect(s) | Reference(s) |
|---|---|---|---|---|---|
| Tregs | Docetaxel | NA | In vitro | Docetaxel-based chemotherapy decreased Treg after four cycles of treatment | 67 |
| Anti-CD25 antibody | CD25 | LLC model | Deplete Tregs; inhibit tumor growth when combined with radiation; increase the effector T cells | 68 | |
| Anti-CD25 antibody | CD25 | MC38 and EO771 model; LL/2 and 4T1 model | Deplete Tregs; the antitumor effect was associated with CD8+ T and NK cell infiltration level | 69 | |
| Nb-Fc1B0 (CCR8 antibody) | CCR8 | LLC-OVA model | Deplete CCR8+ Tregs; inhibit tumor growth when combined with anti-PD1 | 70 | |
| DCs | Anti-CD40 agonist antibody | CD40 | LLC model | Induce E-cadherin+ inflammatory DCs; enhance antitumor immunity; limit the tumor growth | 76 |
| Macrophages | Targeting specific markers on marcophages | ||||
| Anti-MARCO antibody | MARCO | In vitro | Recover cytolytic activity of NK cells and T cells | 78 | |
| TD-92 | CSF-1R | LLC model | Decrease the expression of CSF-1R on macrophages; inhibit tumor growth combined with PD-1 | 79 | |
| CSF-1R inhibitor | CSF-1R | KrasG12D/+; p53 −/− NSCLC model | Improve the efficacy of anti-angiogenic blockade and ICB | 81 | |
| SIRPα-Fc | SIRPα/CD47 signaling | LLC model | Sensitize the tumor to VEGF blockade | 83 | |
| Reprogramming of macrophages | |||||
| SHP099 | SHP-2 | 344SQ NSCLC adenocarcinoma | Repolarization of M2 TAMs to the antitumor M1 phenotype; triple therapy with radiation and anti-PD-L1 significantly inhibits the primary tumor growth | 85 | |
| Anti-LILRB2 antibody | LILRB2 | LLC model | Enhance TNF-α release; combination of PD-L1 and LILRB2 significantly reduces tumor size | 88 | |
| Anlotinib | VEGFR, FGFR, PDGFR, and c-kit | LLC model | Expand M1-like TAM; inhibit tumor growth when combined with αPD-1 | 87 | |
| MDSCs | CXCR1/2 inhibition | CXCR1/2 | KP mouse model | Deplete the subsets of gMDSCs; combination with SHP099 further inhibits tumor growth with anti-PD-1 | 92 |
| ATRA | NA | LKB1-deficient murine models | Suppress the percentage and function of gMDSCs; inhibit tumor growth when combined with anti-PD-1 | 93 | |
| ARG1/2 inhibitor | ARG1 | KrasG12D lung cancer model | Inhibit the function of MDSC; inhibit tumor growth and enhance T cell numbers and function | 94 | |
ARG1: Arginase 1; ATRA: All-trans retinoic acid; CCR8: C-C chemokine receptor type 8; CSF-1R: Colony-stimulating factor 1 receptor; CXCR1/2: C-X-C motif chemokine receptor 1/2; DC: Dendritic cells; FGFR: Fibroblast growth factor receptor; gMDSC: Granulocytic MDSC; ICB: Immune checkpoint blockade; KP: KrasG12D/+ and p53−/−; LKB1: Liver kinase B1; LILRB2: Leukocyte immunoglobulin-like receptor subfamily B member 2; LLC: Lewis lung cancer; LLC-OVA: LLC-ovalbumin; LILRB2: Leukocyte immunoglobulin-like receptor subfamily B member 2; MARCO: Macrophage receptor with collagenous structure; MDSC: Myeloid-derived suppressor cells; NA: Not available; NK: Natural killer; NSCLC: Non-small cell lung cancer; PD-1: Programed death-1; PDGFR: Platelet-derived growth factor receptor; PD-L1: Programmed death-ligand 1; SHP-2: Src homology-2 domain-containing protein tyrosine phosphatase-2; SIRPα: Signal regulatory protein α; TAMs: Tumor-associated macrophages; TNF-α: Tumor necrosis factor-α; Tregs: Regulatory T cells; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor.
Table 2.
Clinical trials targeting the tumor microenvironment.
| Target(s) | Agent(s) | Combination | Phase | NCT number | Condition(s) | Status | Result(s) |
|---|---|---|---|---|---|---|---|
| TIGIT | Vibostolimab | Anti-PD-1 (pembrolizumab) | I | NCT02964013 | Advanced solid tumors (NSCLC) | Active, not recruiting | NA |
| Tiragolumab | Anti-PD-L1 (atezolizumab) | II | NCT03563716 | NSCLC | Active, not recruiting | The combination therapy achieved an ORR of 37% | |
| Domvanalimab | Anti-PD-1 (zimberelimab) | II | NCT04791839 | NSCLC | Recruiting | NA | |
| TIM-3 | Eftilagimod alpha | Anti-PD-1 (pembrolizumab) | II | NCT03625323 | NSCLC | Active, not recruiting | NA |
| TSR-033 | Anti-PD-1 (dostarlimab)/docetaxel | I | NCT03250832 | Advanced solid tumors (NSCLC) | Active, not recruiting | NA | |
| Relatlimab | Anti-PD-1 (nivolumab) | II | NCT04623775 | NSCLC | Recruiting | NA | |
| Relatlimab | Anti-PD-1 (nivolumab) | II | NCT04205552 | NSCLC | Recruiting | NA | |
| LAG-3 | TSR-022 | Anti-PD-1 (TSR-042)/carboplatin-pemetrexed | I | NCT03307785 | Advanced cancer (NSCLC) | Active, not recruiting | NA |
| INCAGN02390 | NA | I | NCT03652077 | Advanced malignancies (NSCLC) | Completed | NA | |
| Cobolimab | Anti-PD-1 (dostarlimab)/docetaxel | II/III | NCT04655976 | Advanced NSCLC | Recruiting | NA | |
| MBG453 | Anti-PD-1 (PDR001) | I/II | NCT02608268 | Advanced malignancies (NSCLC) | Active, not recruiting | NA | |
| CCR4 | Mogamulizumab | NA | I | NCT01929486 | Advanced or recurrent cancer | Unknown | Well-tolerated but induces limited clinical efficacy |
| Mogamulizumab | Docetaxel | I | NCT02358473 | NSCLC | Completed | NA | |
| Mogamulizumab | Nivolumab | I | NCT02476123 | Advanced solid tumors (NSCLC) | Completed | Acceptable safety profile and antitumor activity | |
| Mogamulizumab | Anti-PD-L1 (durvalumab)/anti-CTLA-4 (tremelimumab) | I | NCT02301130 | Advanced solid tumors (NSCLC) | Completed | Acceptable safety profile, but did not result in potent antitumor efficacy | |
| CSF-1R | Cabiralizumab | CD40 agonist (APX005M)/anti-PD-1 (nivolumab) | I | NCT03502330 | NSCLC | Recruiting | NA |
| Arg1 | INCB001158 | Anti-PD-1 (pembrolizumab) | I/II | NCT02903914 | Advanced/metastatic solid tumors | Active, not recruiting | Well tolerated and showed responses |
Arg1: Arginase 1; CCR4: C-C chemokine receptor type 4; CSF-1R: Colony-stimulating factor 1 receptor; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; LAG-3: Lymphocyte-activation gene 3; NA: Not available; NSCLC: Non-small cell lung cancer; PD-1: Programmed death-1; PD-L1: Programmed death-ligand 1; TIGIT: T cell immunoreceptor with immunoglobulin (Ig) and ITIM domains; TIM-3: T cell immunoglobulin and mucin domain-containing protein 3.
Targeting immune checkpoints
Immune checkpoint inhibitors have achieved great success in treating NSCLC. Well-established immune checkpoint inhibitors, including anti-PD-1 and anti-CTLA-4 agents, have been approved for monotherapy and combination therapy (chemotherapy and immunotherapy).49, 50, 51, 52, 53, 54, 55, 56 In-depth research in the field of immuno-oncology has identified several novel immune checkpoints, including T cell immunoreceptor with immunoglobulin (Ig) and ITIM domains (TIGIT), TIM-3, and LAG-3. The corresponding inhibitors have been developed and entered into clinical trials.
TIGIT
TIGIT belongs to the poliovirus receptor/nectin family. TIGIT is expressed by various types of immune cells, including effector CD8+ T cells, natural killer (NK) cells, and regulatory CD4+ T cells.57 TIGIT suppresses antitumor immunity by inhibiting the antitumor effects of CD8+ T cells and NK cells.58 In a bilaterally established model of LUAD, triple therapy with RadScopal + anti-TIGIT + anti-PD1 suppressed the growth of both primary and secondary tumors and significantly prolonged survival.59 Vibostolimab is an anti-TIGIT antibody. A phase I study (NCT02964013) was conducted to investigate the safety and efficacy of vibostolimab in treating advanced solid tumors. Monotherapy or vibostolimab plus pembrolizumab was well-tolerated. In anti-PD-1/PD-L1-naïve NSCLC, combination therapy achieved 26% of the confirmed objective response rate (ORR); in anti-PD-1/PD-L1-refractory NSCLC, the ORRs following monotherapy and combination therapy were both 3%. These results demonstrate that vibostolimab exhibited antitumor activity in advanced solid tumors.60 Recently, the results of the phase II CITYSCAPE trial (NCT03563716) were released. Combined treatment with the TIGIT inhibitor, tiragolumab, and atezolizumab achieved an ORR of 37%, compared with an ORR of 21% in NSCLC patients administered atezolizumab monotherapy. The ORR of the PD-L1 high-expression subgroup in patients receiving combination treatment was 66%. These results highlight the potential role of tiragolumab in combination with the anti-PD-L1 antibody.61 Regarding ongoing trials, anti-PD-1 (zimberelimab) in combination with anti-TIGIT (domvanalimab) was launched in 30 previously treated patients with NSCLC (NCT04791839).
TIM-3
TIM-3 serves as a co-inhibitory receptor that primarily inhibits T-cell function.62 INCAGN02390 is a novel antibody targeting TIM-3. The results of a preclinical study demonstrated that interferon gamma (IFN-γ) production increased following treatment with INCAGN02390. A phase I study of INCAGN02390 is ongoing for patients with advanced solid tumors (NCT03652077). The efficacy of combinations containing anti-TIM-3 (cobalolimab), anti-PD-1 (dostarlimab) and docetaxel-based chemotherapy is under observation in clinical trial NCT04655976. Another trial is being conducted to explore the efficacy of combination therapy of anti-PD-1 and anti-TIM3 with platinum-based doublet chemotherapy (NCT03307785). The preliminary results of combination therapy have been reported in a phase II dose-expansion trial (NCT02608268). In that study, 16 patients with melanoma and 17 patients with NSCLC were enrolled and treated with antibodies against TIM-3 (MBG453) and PD-1 (spartalizumab). Combination therapy is safe, but its efficacy is limited.63
LAG3
LAG-3 is a novel inhibitory coreceptor that can inhibit T-cell activation and function.64 Eftilagimod alpha, a soluble LAG-3 protein, directly targets LAG-3. Related clinical trials have been conducted for metastatic melanoma,65 metastatic breast cancer,66 and NSCLC (NCT03625323). TSR-033 is a LAG-3 antibody. A phase 1 study of TSR-033, alone or in combination with anti-PD-1, is ongoing for patients with advanced solid tumors (NCT03250832). Another anti-LAG-3 antibody, relatlimab, is under investigation in different clinical trials (NCT04623775 and NCT04205552).
Targeting Tregs
Depleting Tregs is an appropriate approach for enhancing the therapeutic efficacy of immunotherapy, and docetaxel is widely used in NSCLC treatment. Four cycles of docetaxel treatment decreased the levels of Tregs. In vitro experiments confirmed these results.67 CD25 is a surface marker of Tregs. Targeting CD25 with a specific antibody efficiently depleted Tregs and inhibited tumor regression. In Lewis lung cancer (LLC) models, low-dose radiotherapy and anti-CD25 antibodies efficiently depleted Tregs and increased the levels of effector T cells. In addition, the growth of irradiated and distal non-irradiated tumor cells was significantly inhibited.68 In this model, anti-CD25 treatment alone did not efficiently inhibit tumor growth. This finding is in accordance with the results of the following discussed study. Anti-CD25 was effective in MC38 and EO771 mouse models, whereas minimal effects were observed in LL/2 and 4T1 mouse models. This discrepancy was attributed to the differences in the infiltration levels of CD8+ T and NK cells.69 These findings may suggest that the depletion of Tregs alone is insufficient for eliminating tumors and that cytotoxic cells are essential for tumor control.
C-C chemokine receptor type 8 (CCR8) is expressed in a subset of Tregs and CCR8 expression correlates with more advanced diseases.26 Single-cell analysis of the T cell compartment in LLC-ovalbumin (LLC-OVA) tumors revealed distinct CCR8 expression in Tregs. Nb-Fc1B is an antibody that blocks CCR8, along with antibody-dependent cell-mediated cytotoxicity (ADCC). Treating LLC-OVA tumor-bearing mice with Nb-Fc1B successfully depleted CCR8+ Tregs in an NK cell-dependent manner. When combined with anti-PD1, tumor growth was largely inhibited.70 C-C chemokine receptor type 4 (CCR4) is highly expressed on the surface of inhibitory Tregs. KW-0761 is a CCR4 monoclonal antibody that can deplete CCR4+ Tregs via ADCC. In patients with lung cancer, even low-dose KW-0761 can deplete Tregs. Monotherapy with KW-0761 (mogamulizumab) is safe and well-tolerated.71 Clinical trials are ongoing to test the efficacy of mogamulizumab combined with anti-PD1/anti-PD-L1. In the phase I clinical trial NCT02476123, combined mogamulizumab and nivolumab treatment was safe and well tolerated. The antitumor effect was moderate in multiple types of tumors. For NSCLC, three partial responses and three disease stabilizations were observed in 15 patients.72 In another phase I/II study, 114 patients diagnosed with locally advanced or metastatic solid tumors were enrolled. Patients were treated with mogamulizumab (1 mg/kg) plus nivolumab (240 mg). These doses were acceptable and tolerable. However, no additional effect was observed with combination therapy, and only a 10.5% ORR was observed.73 In another study (NCT02301130), mogamulizumab was combined with durvalumab. However, the ORR was only 5.3% with the combination treatment.74
Targeting DCs
Targeting DC activation with different approaches provides therapeutic options. Several factors are required for the development and maturation of DCs, including FMS-like tyrosine kinase 3 ligand and CD40.75 Targeting these factors may enhance the function of DCs. Anti-CD40 treatment induced E-cadherin+ inflammatory DCs in the lungs of Rag1-knockout mice. The LLC model showed slower growth after the transfer of E-cadherin+ DCs. The TME was reprogrammed with increased T helper 1 (Th1) and Th17 cell responses and reduced Treg cells. In addition, antitumor CD103+CD8+ T cells and tumor-specific CD8+ T cell responses were also enhanced. This finding highlights the potential of using anti-CD40 to improve the efficiency of immunotherapy in patients.76
Targeting macrophages
Targeting specific markers on macrophages
Fleur et al77 observed the expression levels of scavenger receptors and macrophage receptor with collagenous structure (MARCO) in a distinct subpopulation of TAMs in a cohort of 352 patients with NSCLC. This cluster of macrophages was located close to the tumor cell nests. Co-expression of PD-L1 was observed in MARCO+ macrophages. MARCO+ macrophages suppress the activity of cytotoxic T and NK cells. Targeting MARCO with a specific antibody reversed the effect of MARCO+ macrophages, resulting in the recovery of the cytolytic activities of NK and T cells.78 TD-92 is a novel erlotinib derivative. Combined anti-PD-1 and TD-92 treatment significantly reduced tumor growth and increased survival in a model of LLC. Analysis of the microenvironment revealed a significant decline in pro-tumorigenic CD11b+F4/80+ macrophages. Mechanistically, TD-92 can decrease the expression of colony-stimulating factor 1 receptor (CSF-1R) in macrophages.79 Using a genetically engineered mouse model of KrasG12D/+; p53−/− NSCLC (KP model),80 monocyte- and alveolar-origin TAMs were found to be associated with resistance to immune-checkpoint blockade (ICB). These subsets of macrophages facilitate the infiltration of PD-1+ regulatory T cells. Combination treatment with the CSF-1R inhibitor and cisplatin depleted both subsets of macrophages, thereby improving the efficacy of anti-angiogenic blockade and immunotherapy.81 The cluster of differentiation 47/signal-regulatory protein alpha (CD47/SIRPα) pathway could inhibit phagocytosis, which inhibits the function of macrophages.82 Recent data revealed that CD47 upregulation resulted in resistance to vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) inhibitors. Blocking CD47 signaling with SIRPα-Fc sensitized tumors to VEGF blockade, and this effect was reversed by eliminating macrophages in vivo.83 These findings suggest that blocking CD47/SIRPα signaling in macrophages could enhance phagocytosis and suppress tumor growth.
Reprograming macrophages
Macrophage repolarization has been shown to be effective in controlling tumor growth. SHP099 is a unique inhibitor that selectively blocks the activity of Src homology-2 domain-containing protein tyrosine phosphatase-2 (SHP-2),84 which is widely expressed in immune cells. In a mouse model of anti-PD-1-resistant NSCLC, triple therapy with radiation, anti-PD-L1, and SHP099 significantly inhibited primary tumor growth. Abscopal effects were only observed with triple therapy. The observed effect was related to the repolarization of M2 TAMs to the antitumor M1 phenotype. When macrophages were depleted, the effect of triple therapy was diminished.85 Anlotinib is an oral tyrosine-kinase receptor inhibitor approved for treating lung cancer as a third-line treatment.86 In a mouse model of LLC, anlotinib treatment reduced the frequency of M2-like TAMs, whereas the abundance of the population of M1-like TAMs increased. When combined with αPD-1, tumor growth was significantly inhibited compared with either treatment alone.87 TAMs isolated from patient biopsies ubiquitously express leukocyte immunoglobulin-like receptor subfamily B member 2 (LILRB2). Ex vivo treatment with an anti-LILRB2 antibody enhanced tumor necrosis factor (TNF)-α release, whereas lower levels of the cell-surface markers CD163, CD209, and CD14 were observed. These findings suggest that TAMs can be reprogramed by LILRB2 blockade. Furthermore, combined inhibition of PD-L1 and LILRB2 led to significantly reduced tumor sizes in LLC tumor-bearing mice,88 indicating that LILRB2 is a potential target for treating lung cancer.
Clinical trials targeting macrophages have been initiated based on these preclinical studies. Recently, the efficiencies of the CD40 agonist, APX005M (sotigalimab), and the CSF-1R inhibitor, cabiralizumab, were tested in a phase I trial with or without nivolumab. The patients enrolled in the clinical trial had melanoma, kidney cancer, or NSCLC. The combination treatments were well tolerated, although the dosing frequency and sequence require further trials.89
Targeting MDSCs
Various researchers have reported different approaches for inhibiting MDSC infiltration or impacting MDSC function.90 C-X-C motif chemokine receptor 2 (CXCR2) is a trafficking receptor expressed on MDSCs,91 which can be attracted by C-X-C motif chemokine ligand 1/5 (CXCL1 and CXCL5). In a mouse model of KP, SHP2 inhibition promoted granulocytic MDSC (gMDSC) infiltration by elevating the expression levels of CXCL1 and CXCL5, which limits the use of SHP2 inhibitors. CXCR1/2 inhibition with an SHP2 inhibitor depleted subsets of gMDSCs, which further inhibited tumor growth.92 These data suggest that inhibiting MDSC attraction could provide further benefits when SHP2 inhibitor is used. In liver kinase B1 (LKB1)-deficient lung cancer cells, the production of Glu-Leu-Arg (ELR)+ CXC chemokines was significantly upregulated, which led to the local enrichment of gMDSCs within the TME. All-trans-retinoic acid (ATRA) suppressed the percentage and function of gMDSCs in LKB-1 mutant model. Treatment of tumor-bearing mice with ATAR can enhance the infiltration and function of CD8+ T cells. When combined with anti-PD-1, tumor growth was efficiently inhibited.93 Arginase 1 (Arg1) can contribute to the immunosuppressive function of MDSCs. Thus, inhibiting Arg1 may represent a new therapeutic strategy. In vitro treatment with an Arg1/2 inhibitor (compound 9) restored T cell function when co-cultured with MDSCs. In a model of KrasG12D lung cancer, treatment with compound 9 inhibited tumor growth and enhanced the abundance and function of T cells.94 INCB001158 is a novel arginase inhibitor. A phase I clinical trial was conducted for patients with advanced/metastatic solid tumors to evaluate the efficiency of INCB001158 alone or in combination with pembrolizumab.63
TME components as biomarkers for NSCLC immunotherapy
The complex functions of different immune subsets can influence the responses of patients with NSCLC to immunotherapy. Thus, these components can potentially be used to predict the progression and responses of patients. Blood or surgical tumor samples are frequently used to detect the presence and percentage of specific types of immune cells [Table 3].
Table 3.
Prognostic value of the tumor microenvironment.
| Subset(s) | Marker(s) | Localization | Detection time | Detection method | Association with prognosis/response | Reference(s) |
|---|---|---|---|---|---|---|
| TAAbs | NA | P | Baseline | ELISA | Better prognosis | 95 |
| NLR | NA | P | Baseline | FACS | High NLR associated with worse survival | 96,97 |
| NLR | NA | P | After treatment | FACS | Increase associated with worse survival | 98 |
| TCRβ chains | Complementarity-determining region 3 | P | Baseline/after treatment | TCRβ sequencing | High diversity/TCR clonality associated with better prognosis | 111 |
| Tregs | CD4+ CD25+ CD127lo FoxP3+ | P | 7 days after treatment | FACS | Decrease in responder | 100 |
| Tregs | CD4+ CD25+ CD45RA− FoxP3+ | P | 7 days after treatment | FACS | Increase associated with a better response | 101 |
| TILs | PD-L1+ TIL+ | I | Baseline | IHC | Better prognosis | 107 |
| TILs | PD-L1– TIL– | I | Baseline | IHC | Poor prognosis | 107 |
| TILs | CD8+ SP142+/CD8A+ CD274+ | I | Baseline | IHC/RNA-seq | Better prognosis | 108 |
| Interferon-γ gene signature | CD8A, GZMA, GZMB, IFN-γ, EOMES, CXCL9, CXCL10, and TBX21 | I | Baseline | RNA-seq | Better prognosis | 112 |
| Interferon-γ gene signature | T cell-inflamed GEP panel | I | Baseline | RNA-seq | Better prognosis | 113 |
| Tregs | IRF4 | I | Baseline | RNA-seq | Poor prognosis | 118 |
| DCs | DC-LAMP | TLS | Baseline | IHC | Better prognosis | 120 |
| DCs | DC signature | I | Baseline | RNA-seq | Better prognosis | 121 |
| Macrophages | CD68/CD163 | I | Baseline | IHC | Poor prognosis | 122 |
| Macrophages | SIRPα/CD68 | I | Baseline | IHC | Poor prognosis | 123 |
| Macrophages | CD163/PD-1 | I | Baseline | IHC | Poor prognosis | 124 |
| Macrophages | TREM2 | I | Baseline | IHC | Poor prognosis | 125 |
| Plasma cells | Plasma cells signature | I | Baseline | RNA-seq | Better prognosis | 126 |
CXCL9: C-X-C motif chemokine receptor 9; CXCL10: C-X-C motif chemokine receptor 10; DCs: Dendritic cells; DC-LAMP: Dendritic cell lysosomal associated membrane glycoprotein; ELISA: Enzyme-linked immunosorbent assay; EOMES: Eomesodermin; FACS: Fluorescence-activated cell sorting; FoxP3: Forkhead box P3; GEP: Gene expression profile; GZMA: Granzyme A; GZMB: Granzyme B; I: Intratumoral; IFN-γ: Interferon gamma; IHC: Immunohistochemistry; IRF4: Interferon regulatory factor 4; NA: Not available; NLR: Neutrophil-to-lymphocyte ratio; P: Peripheral blood; PD-1: Programed death-1; PD-L1: Programed death-ligand 1; RNA-seq: RNA-sequencing; SIRPα: Signal regulatory protein α; TAAbs: Tumor associated autoantibodies; TBX21: T-box protein 21; TCR: T cell receptor; TILs: Tumor-infiltrating lymphocytes; TLS: Tertiary lymphoid structures; Treg: Regulatory T cells; TREM2: Triggering receptor expressed on myeloid cells-2.
Biomarkers in the peripheral blood
Multiple immune markers in the peripheral blood have been tested. Recent results showed that tumor-associated autoantibodies (TAAbs) could be used to predict the efficacy of immunotherapy in patients with advanced NSCLC.95 Neutrophil-to-lymphocyte ratios (NLRs) have been used to predict the outcomes of ICB. High baseline NLRs have been linked to a worse overall survival (OS) and progression-free survival (PFS) in multiple types of tumors, including NSCLC.96,97 An increased NLR after treatment has been associated with worse outcomes.98 In another study, blood samples from 53 patients treated with nivolumab were analyzed to determine the abundances of gMDSCs, neutrophils, eosinophils, and lymphocytes. The results demonstrated that high baseline levels of gMDSCs, low absolute neutrophil counts, high eosinophil counts, and low NLRs predicted significant improvements in the OS and PFS.99 CD4+CD25+CD127loFoxP3+ Treg cells were associated with treatment responses in patients with advanced NSCLC administered anti-PD-1/PD-L1 therapy. The frequency of circulating Treg cells significantly decreased 7 days after treatment in the responder group.100 However, the results of another study indicated that high frequencies of circulating Treg cells (CD4+CD25+CD45RA−FoxP3+) at one week after anti-PD-1 therapy correlated with a better response.101 This discrepancy may reflect the different surface markers examined in both studies, and the lack of CD45RA expression may indirectly reflect an effective antitumor immune response.102
TME-related biomarkers
Tumor-infiltrating lymphocytes
TILs, especially CD8+ T cells, have been associated with an improved prognosis.103 Kim et al104 demonstrated that PD-L1 expression was associated with increased TILs in pulmonary squamous carcinoma. Thus, a new classification of TMEs based on TILs and PD-L1 levels was proposed. Patients can be classified into four types, namely type I (PD-L1+TIL+), type II (PD-L1−TIL−), type III (PD-L1+TIL−), and type IV (PD-L1−TIL+).105,106 Previous analysis indicated that type-II patients had a very poor prognosis, whereas type-I had the best prognosis.107 The combined predictive value of CD8+ TIL levels and PD-L1 expression was tested in a cohort of 85 patients treated with nivolumab. Patients with high levels of CD8+ TILs (CD8A) and PD-L1 (CD274), as determined either by IHC or messenger RNA (mRNA)-expression analysis, were classified as having a longer PFS.108 As CD8+ TILs are the most efficient cytotoxic cells, a higher CD8-to-CD3 ratio has been associated with longer PFS and OS in patients with NSCLC who received nivolumab.109 Previously, a low incidence of PD-1 expression among CD8+ cells was associated with a prolonged PFS.109 An effective antitumor immune response requires T cell receptor (TCR) binding to the major histocompatibility complex/antigen short-peptide complex.110 In a study, the complementarity-determining region 3 of TCR chains was sequenced as a predictive biomarker in patients with NSCLC who were treated with ICB. The results showed that high baseline PD-1+CD8+ TCR diversity and increased PD-1+CD8+ TCR clonality after ICB treatment were associated with a prolonged PFS.111
Successful antitumor immunity is dependent on effector chemokines, including IFN-γ. In a phase II study of NSCLC (POPLAR), a gene signature for effector T cells and IFN-γ was defined (CD8A, granzyme A [GZMA], granzyme B [GZMB], IFN-γ, EOMES, C-X-C motif chemokine receptor 9 [CXCL9], C-X-C motif chemokine receptor 10 [CXCL10], and T-Box transcription factor 21 [TBX21]). Patients with high expression levels of these genes displayed improved OS after atezolizumab treatment.112 In another study, a pan-tumor T cell-inflamed panel (18 genes) was constructed, reflecting inflammatory IFN-γ responses and increasing the accuracy of prognosis for ICB treatment.113 Based on weighted correlation-network analysis of CD8-associated genes, LUAD can be classified into two molecular subtypes. A 10-gene signature risk model was constructed, and patients with higher risk scores had lower survival rates.114
Evidence has shown that PD-1+ regulatory T cells are associated with cancer hyperprogression following treatment with PD-1 inhibitors.115 Additionally, the PD-1-expression balance between effector and regulatory T cells may serve as a biomarker for the effectiveness of PD-1 blockade therapy. Group R was proposed as the responder group, based on PD-1 expression. Group R was defined as having PD-1 positivity in ≥40% of CD8+ TILs and a PD-1-expression ratio in CD8+ T cells to Treg cells among TILs of ≥1. Patients in group R had a significantly longer PFS.116 Interferon regulatory factor 4 (IRF4) is a transcription factor involved in T cell differentiation and metabolism.117 IRF4 is specifically expressed by a subset of intratumoral CD4+ effector Treg cells in patients with NSCLC, according to single-cell analysis of tumors, adjacent lung tissues, and blood samples from these patients. The abundance of IRF4 Tregs was correlated with a poor prognosis.118
DCs
DCs are indispensable for successful antitumor immunity. Mature DCs are characterized by the expression of dendritic cell lysosome-associated membrane glycoprotein (DC-LAMP).119 In a retrospective analysis of 376 patients with NSCLC, the density of mature DCs correlated with a longer OS. This cluster of mature DCs was localized in tertiary lymphoid structures (TLSs). When combined with the stromal CD8+ cell density, patients with the highest risk of death could be specifically identified.120 In a cohort of patients with lung cancer, the DC signature could predict an improved survival benefit for treatment with atezolizumab, but not docetaxel. Moreover, the study showed that the DC gene signature could predict survival for a patient subgroup with PD-L1+ immune cells, but not for a PD-L1− subgroup.121
Macrophages
The prognostic value of M2 macrophages was examined in 349 patients with NSCLC. CD68 and CD163 expressions were detected to identify M2 macrophages. The results demonstrated that an elevated M2 ratio (CD163+/CD68+) predicted a poor OS.122 In another cohort of 98 patients with NSCLC, the expression levels of CD47, SIRPα, and CD68 expression were detected. A high CD68+ Mφ-score predicted an improved OS. However, SIRPα+CD68+ macrophages have been associated with a poor prognosis, indicating that SIRPα is a potential target.123 The above-mentioned MARCO+ macrophages predicted a weak trend toward worse survival, but did not reach statistical significance.77 PD-1 is frequently expressed in activated T lymphocytes. However, PD-1 can also be expressed by TAMs. In 213 human LUADs, PD-1 was preferentially expressed by CD163+ TAMs in the tumor stroma. Survival analysis showed that stromal PD-1+ TAM infiltration predicted poor survival.124 A subset of triggering receptor expressed on myeloid cells-2 (TREM2)+ TAMs was identified based on data deposited in The Cancer Genome Atlas and single-cell sequencing datasets. In a cohort of 40 patients with NSCLC who received PD-1 based ICB treatment, the TREM2+TAMhi group showed poor survival.125 These results may provide new biomarkers for determining the prognosis of patients receiving immunotherapy in clinical practice.
TLSs
The results of numerous studies have demonstrated that TLSs are associated with favorable survival across a wide range of cancers.126,127 Recent data showed that B and plasma cell signals were associated with an OS benefit in patients treated with atezolizumab based on the transcriptomes of POPLAR and OAK (clinical trials of atezolizumab vs. chemotherapy in NSCLC). In addition, B cell, germinal center B cell, and plasma cell signatures were determined from scRNA-seq data. The results indicated a pivotal role of plasma cells in the OS benefit from atezolizumab.126
Perspective of the TME as target for NSCLC
Monoclonal antibodies targeting immune checkpoints elicit significant responses during NSCLC treatment.128 However, the TME is complex, and the interaction between different pathways hinders the success of immunotherapy. In addition, the adverse effects of antibodies restrict their clinical use. The emergence of bispecific antibodies may expand the current applications of cancer immunotherapy.129 In this section, we discuss the current bispecific antibodies that are being used in preclinical and clinical studies [Table 4].
Table 4.
Recent advances in bispecific antibodies in NSCLC.
| Targets | BsAb | Status | Phase | NCT number | Conditions |
|---|---|---|---|---|---|
| PD-1/PD-L1 | IBI318 | Recruiting | I | NCT04777084 | Advanced NSCLC |
| PD-1/LAG-3 | MGD013 | Active, not recruiting | I | NCT03219268 | Advanced solid tumors (NSCLC) |
| PD-1/LAG-3 | RO7247669 | Recruiting | I | NCT04140500 | Advanced and/or metastatic solid tumors (NSCLC) |
| PD-1/TIM-3 | AZD7789 | Recruiting | I/II | NCT04931654 | Advanced or metastatic solid cancer (NSCLC) |
| PD-1/TIM-3 | RO7121661 | Active, not recruiting | I | NCT03708328 | Advanced and/or metastatic solid tumors (NSCLC) |
| PD-1/CTLA-4 | AK104 | Recruiting | II | NCT05215067 | Advanced NSCLC |
| PD-1/CTLA-4 | MGD019 | Recruiting | I | NCT03761017 | Unresectable/metastatic cancer (NSCLC) |
| PD-1/VEGF | AK112 | Recruiting | I/II | NCT04900363 | NSCLC |
| PD-L1/CD27 | CDX-527 | Recruiting | I | NCT04440943 | Advanced malignancies (NSCLC) |
| PD-L1/41BB | GEN1046 | Recruiting | I/II | NCT03917381 | Solid tumors (NSCLC) |
| PD-L1/41BB | INBRX-105 | Recruiting | I | NCT03809624 | Metastatic solid tumors (NSCLC) |
| CD47/PD-L1 | PF-07257876 | Recruiting | I | NCT04881045 | Advanced tumors (NSCLC) |
| CD3/B7H4 a | GEN1047 | Recruiting | I/II | NCT05180474 | Malignant solid tumors (NSCLC) |
| TIGIT/PD-L1 | HLX301 | Recruiting | I/II | NCT05102214 | Locally advanced or metastatic solid tumors (NSCLC) |
| CTLA-4/LAG-3 | XmAb®22841 | Recruiting | I | NCT03849469 | Advanced solid tumors (NSCLC) |
BsAb: Bispecific antibody; CD47: Cluster of differentiation 47; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; LAG-3: Lymphocyte-activation gene 3; NSCLC: Non-small cell lung cancer; PD-1: Programed death-1; PD-L1: Programed death-ligand 1; TIGIT: T cell immunoreceptor with immunoglobulin (Ig) and ITIM domains; TIM-3: T cell immunoglobulin and mucin domain-containing protein 3; VEGF: Vascular endothelial growth factor.
Preclinical studies
MEDI5752 is a monovalent bispecific antibody that inhibits both PD-1 and CTLA4 in PD-1+ activated T cells. In vivo experiments demonstrated that MEDI5752 accumulated in tumors at higher levels than an isotype-matched control monoclonal antibody. When 10 mg/kg MEDI5752 was administered to mice, the antitumor effect was strong, with 60% of the mice showing complete tumor clearance. This was superior to a single treatment using anti-PD-1 or anti-CTLA4 monoclonal antibodies.130 In two patients with advanced gastric adenocarcinoma and clear cell carcinoma of the kidney, treatment with MEDI5752 achieved robust partial responses.130 Bispecific anti-PD-L1/PD-1 antibodies have been reported in a patent (US2019010232). The antitumor effect was examined in a model of lung carcinoma. The v3.2 antibody delayed tumor growth significantly at a dose of 0.02 mg/kg, compared with the delay found with an anti-PD-L1 antibody, an anti-PD-1 antibody, or a combination of both.131 A bispecific antibody targeting CD47 and CTLA4 was constructed, which preferentially depleted inducible T-cell co-stimulator high (ICOShi) immunosuppressive Treg cells in the TME. Treatment with the CD47/CTLA4 bispecific antibody significantly inhibited tumor growth in MC38 and CT26 models in a T cell-dependent manner.132
In addition to the dual inhibition of immune checkpoints, the simultaneous inhibition of immunosuppressive cells and promotion of systemic T cell responses is also a promising strategy. N809 is a bifunctional agent that contains an IL-15 superagonist fused with two αPD-L1 domains. In 4T1 and MC38 mouse models, treatment with N809 inhibited tumor growth and metastasis. The TME was characterized by enhanced NK and CD8+ T cell activation and reduced Tregs, M-MDSCs, and M2-like macrophages after treatment.133 Stimulation of CD3-mediated signaling has been used to treat hematologic malignancies. Based on these observations, a bispecific antibody that simultaneously targets CD3ε and PD-L1 was developed. PD-L1 × CD3 generated a superior antitumor effect in a mouse model than treatment with a single agent. Mechanistically, this effect was due to the blockade of PD-L1 on DCs,134 in accordance with a previous report showing that PD-L1 on DCs inhibits T cell activation.135,136
Clinical trials
Several bispecific antibodies have been investigated in clinical trials with promising results in preclinical studies. These include IBI318-bispecific PD-1/PD-L1 antibody (NCT04777084), MGD013-bispecific PD-1/LAG-3 antibody (NCT03219268), RO7247669-bispecific PD-1/LAG-3 antibody (NCT04140500), AZD7789-bispecific PD-1/TIM-3 antibody (NCT04931654), RO7121661-bispecific PD-1/TIM-3 antibody (NCT03708328), AK104-bispecific PD-1/CTLA-4 antibody (NCT05215067), MGD019-bispecific PD-1/CTLA-4 (NCT03761017), AK112-bispecific PD-1/VEGF antibody (NCT04900363), CDX-527 -bispecific PD-L1/CD27 antibody137 (NCT04440943), GEN1046-bispecific PD-L1/41BB antibody (NCT03917381), INBRX-105-bispecific PD-L1/41BB antibody (NCT03809624), PF-07257876-bispecific CD47/PD-L1 antibody (NCT04881045), GEN1047-bispecific CD3/B7H4 antibody (NCT05180474), XmAb®22841-bispecific CTLA-4/LAG-3 antibody (NCT03849469), and HLX301-bispecific TIGIT/PD-L1 antibody (NCT05102214). Some clinical trials have already shown preliminary results. The bispecific AK104 antibody displayed acceptable safety and four patients showed objective responses among 25 response-evaluable patients.138 The GEN1046 bispecific antibody targeting PD-L1 and 4-1BB achieved a 65.6% disease-control rate with multiple types of tumors.139 Although these results are from a phase I study, we anticipate that continuing trials will provide more promising results.
Conclusion
Increasing the survival and treatment response of patients with NSCLC (the most common and deadly cancer worldwide) remains a research hotspot. Although immunotherapy has changed the treatment landscape for NSCLC, some patients do not benefit from current treatments. The TME is considered a crucial determinant of treatment responses.140 In this review, we discuss the heterogeneity of the TME in NSCLC. Immune cells inside the TME are in a mixed state. Thus, a more accurate portrayal of the immune microenvironment is required. With technological advancements, we should better understand the TME in the future and identify novel targets for drug development. Strategies targeting the immune microenvironment do not focus on the global depletion of all immunosuppressive cells in the TME. The solution should be precisely targeted to distinct cell types using specific antibodies and inhibitors, as mentioned in this review. Bolstering the antitumor phenotype of immune cells may have additional effects. In patients with advanced disease, monotherapy may provide insufficient efficacy. When combined with different drugs (antibodies or inhibitors), chemotherapy or radiation, promising results have been achieved in clinical trials. However, these side effects were non-negligible. Bispecific antibodies have provided new hope. They can simultaneously target two molecules without causing severe side effects. An increasing number of prospective clinical trials are ongoing and have shown promising results. We anticipate that more targeted molecules will be chosen for the development of bispecific antibodies for cancer immunotherapy in the future.
Acknowledgments
Acknowledgments
The figures are created with Biorender.com.
Funding
This study was conducted with support from the National Natural Science Foundation of China (No. 82073147 to Q. J.) and supported by Foundation from Third Military Medical University (TMMU) (No. 2018XLC1008).
Conflicts of interest
None.
Edited by: Peifang Wei
References
- 1.Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Zhang T, Xu Q. Therapeutic advances in non-small cell lung cancer: focus on clinical development of targeted therapy and immunotherapy. MedComm (2020) 2021;2:692–729. doi: 10.1002/mco2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli C, Trapani D, Viale G, et al. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 8.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadeghi Rad H, Monkman J, Warkiani ME, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev. 2021;41:1474–1498. doi: 10.1002/med.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Q, Wu W, Wang Y, et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018;9:5361. doi: 10.1038/s41467-018-07767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Q, Chu H, Jin Z, Long H, Zhu B. High-throughput single-сell sequencing in cancer research. Signal Transduct Target Ther. 2022;7:145. doi: 10.1038/s41392-022-00990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann FJ, Bendall SC. Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat Rev Rheumatol. 2020;16:87–99. doi: 10.1038/s41584-019-0338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao A, Barkley D, França GS, Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature. 2021;596:211–220. doi: 10.1038/s41586-021-03634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang N, Li X, Wang R, Ding Z. Spatial transcriptomics and proteomics technologies for deconvoluting the tumor microenvironment. Biotechnol J. 2021;16 doi: 10.1002/biot.202100041. [DOI] [PubMed] [Google Scholar]
- 15.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 16.Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Zhang Y, Zheng L, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Hu X, Feng K, et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat Cancer. 2022;3:108–121. doi: 10.1038/s43018-021-00292-8. [DOI] [PubMed] [Google Scholar]
- 19.Yost KE, Satpathy AT, Wells DK, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanmamed MF, Nie X, Desai SS, et al. A burned-out CD8+ T-cell subset expands in the tumor microenvironment and curbs cancer immunotherapy. Cancer Discov. 2021;11:1700–1715. doi: 10.1158/2159-8290.CD-20-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Z, Jia Q, Chen J, et al. Impaired cytolytic activity and loss of clonal neoantigens in elderly patients with lung adenocarcinoma. J Thorac Oncol. 2019;14:857–866. doi: 10.1016/j.jtho.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Goswami TK, Singh M, Dhawan M, et al. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders – advances and challenges. Hum Vaccin Immunother. 2022;18 doi: 10.1080/21645515.2022.2035117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteside TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018;22:353–363. doi: 10.1080/14728222.2018.1451514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoadley KA, Yau C, Hinoue T, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plitas G, Konopacki C, Wu K, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression – implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 29.De Vlaeminck Y, González-Rascón A, Goyvaerts C, Breckpot K. Cancer-associated myeloid regulatory cells. Front Immunol. 2016;7:113. doi: 10.3389/fimmu.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 31.Verneau J, Sautés-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020;48 doi: 10.1016/j.smim.2020.101410. [DOI] [PubMed] [Google Scholar]
- 32.Böttcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4:784–792. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leader AM, Grout JA, Maier BB, et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell. 2021;39:1594–1609.e12. doi: 10.1016/j.ccell.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zilionis R, Engblom C, Pfirschke C, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garris CS, Luke JJ. Dendritic cells, the T-cell-inflamed tumor microenvironment, and immunotherapy treatment response. Clin Cancer Res. 2020;26:3901–3907. doi: 10.1158/1078-0432.CCR-19-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu K, Lin K, Li X, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 2020;11:1731. doi: 10.3389/fimmu.2020.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 2020;13:10. doi: 10.1186/s13045-020-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng JN, Yuan YX, Zhu B, Jia Q. Myeloid-derived suppressor cells: a multifaceted accomplice in tumor progression. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.740827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–82. doi: 10.1016/j.coi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CR, Kwak Y, Yang T, et al. Myeloid-derived suppressor cells are controlled by regulatory T cells via TGF-beta during murine colitis. Cell Rep. 2016;17:3219–3232. doi: 10.1016/j.celrep.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 44.Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol. 2011;2:29. doi: 10.3389/fimmu.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–651. doi: 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamauchi Y, Safi S, Blattner C, et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am J Respir Crit Care Med. 2018;198:777–787. doi: 10.1164/rccm.201708-1707OC. [DOI] [PubMed] [Google Scholar]
- 47.Zhao L, He R, Long H, et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat Med. 2018;24:1536–1544. doi: 10.1038/s41591-018-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long H, Jia Q, Wang L, et al. Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell. 2022;40:674–693.e7. doi: 10.1016/j.ccell.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Xiong A, Wang J, Zhou C. Immunotherapy in the first-line treatment of NSCLC: current status and future directions in China. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.757993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 51.Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2021;148:2313–2320. doi: 10.1002/ijc.33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 55.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 56.Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 Tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39:2327–2338. doi: 10.1200/JCO.20.03579. [DOI] [PubMed] [Google Scholar]
- 57.Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38:20–28. doi: 10.1016/j.it.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 59.Barsoumian HB, Sezen D, Menon H, et al. High plus low dose radiation strategy in combination with TIGIT and PD1 blockade to promote systemic antitumor responses. Cancers. 2022;14:221. doi: 10.3390/cancers14010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niu J, Maurice-Dror C, Lee DH, et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer☆. Ann Oncol. 2022;33:169–180. doi: 10.1016/j.annonc.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Horvath L, Pircher A. ASCO 2020 non-small lung cancer (NSCLC) personal highlights. Memo. 2021;14:66–69. doi: 10.1007/s12254-020-00673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genova C, Dellepiane C, Carrega P, et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.799455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atkinson V, Khattak A, Haydon A, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dirix L, Triebel F. AIPAC: a phase IIb study of eftilagimod alpha (IMP321 or LAG-3Ig) added to weekly paclitaxel in patients with metastatic breast cancer. Future Oncol. 2019;15:1963–1973. doi: 10.2217/fon-2018-0807. [DOI] [PubMed] [Google Scholar]
- 67.Li JY, Duan XF, Wang LP, et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res. 2014;2014 doi: 10.1155/2014/286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Son CH, Bae JH, Shin DY, et al. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys. 2015;92:390–398. doi: 10.1016/j.ijrobp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Kurebayashi Y, Olkowski CP, Lane KC, et al. Rapid depletion of intratumoral regulatory T cells induces synchronized CD8 T- and NK-cell activation and IFNgamma-dependent tumor vessel regression. Cancer Res. 2021;81:3092–3104. doi: 10.1158/0008-5472.CAN-20-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Damme H, Dombrecht B, Kiss M, et al. Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurose K, Ohue Y, Wada H, et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin Cancer Res. 2015;21:4327–4336. doi: 10.1158/1078-0432.CCR-15-0357. [DOI] [PubMed] [Google Scholar]
- 72.Doi T, Muro K, Ishii H, et al. A phase i study of the anti-cc chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25:6614–6622. doi: 10.1158/1078-0432.CCR-19-1090. [DOI] [PubMed] [Google Scholar]
- 73.Hong DS, Rixe O, Chiu VK, et al. Mogamulizumab in combination with nivolumab in a phase I/II study of patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2022;28:479–488. doi: 10.1158/1078-0432.CCR-21-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zamarin D, Hamid O, Nayak-Kapoor A, et al. Mogamulizumab in combination with durvalumab or tremelimumab in patients with advanced solid tumors: a phase I study. Clin Cancer Res. 2020;26:4531–4541. doi: 10.1158/1078-0432.CCR-20-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol. 2019;348:1–68. doi: 10.1016/bs.ircmb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Hu X, Hu Y, et al. Anti-CD40-induced inflammatory E-cadherin+ dendritic cells enhance T cell responses and antitumour immunity in murine Lewis lung carcinoma. J Exp Clin Cancer Res. 2015;34:11. doi: 10.1186/s13046-015-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.La Fleur L, Boura VF, Alexeyenko A, et al. Expression of scavenger receptor MARCO defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int J Cancer. 2018;143:1741–1752. doi: 10.1002/ijc.31545. [DOI] [PubMed] [Google Scholar]
- 78.La Fleur L, Botling J, He F, et al. Targeting MARCO and IL37R on immunosuppressive macrophages in lung cancer blocks regulatory T cells and supports cytotoxic lymphocyte function. Cancer Res. 2021;81:956–967. doi: 10.1158/0008-5472.CAN-20-1885. [DOI] [PubMed] [Google Scholar]
- 79.Shih CT, Shiau CW, Chen YL, et al. TD-92, a novel erlotinib derivative, depletes tumor-associated macrophages in non-small cell lung cancer via down-regulation of CSF-1R and enhances the anti-tumor effects of anti-PD-1. Cancer Lett. 2021;498:142–151. doi: 10.1016/j.canlet.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 80.Sheridan C, Downward J. Overview of KRAS-driven genetically engineered mouse models of non-small cell lung cancer. Curr Protoc Pharmacol. 2015;70:14.35.1–14.35.16. doi: 10.1002/0471141755.ph1435s70. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Usatorre A, Kadioglu E, Boivin G, et al. Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models. Sci Transl Med. 2021;13:eabd1616. doi: 10.1126/scitranslmed.abd1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Zhao C, Liu Y, et al. Recent advances of tumor therapy based on the CD47-SIRPalpha axis. Mol Pharm. 2022;19:1273–1293. doi: 10.1021/acs.molpharmaceut.2c00073. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Wang Y, Fan J, et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J Immunother Cancer. 2019;7:346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu J, Zhang H, Zhao G, Wang R. Allosteric inhibitors of SHP2: an updated patent review (2015-2020) Curr Med Chem. 2021;28:3825–3842. doi: 10.2174/1568011817666200928114851. [DOI] [PubMed] [Google Scholar]
- 85.Chen D, Barsoumian HB, Yang L, et al. SHP-2 and PD-L1 inhibition combined with radiotherapy enhances systemic antitumor effects in an Anti-PD-1-resistant model of non-small cell lung cancer. Cancer Immunol Res. 2020;8:883–894. doi: 10.1158/2326-6066.CIR-19-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569–1575. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69:2523–2532. doi: 10.1007/s00262-020-02641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen HM, van der Touw W, Wang YS, et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Invest. 2018;128:5647–5662. doi: 10.1172/JCI97570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiss SA, Djureinovic D, Jessel S, et al. A phase I study of APX005M and cabiralizumab with or without nivolumab in patients with melanoma, kidney cancer, or non-small cell lung cancer resistant to anti-PD-1/PD-L1. Clin Cancer Res. 2021;27:4757–4767. doi: 10.1158/1078-0432.CCR-21-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9:561. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007974. :237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang KH, Li S, Khodadadi-Jamayran A, et al. Combined inhibition of SHP2 and CXCR1/2 promotes antitumor T-cell response in NSCLC. Cancer Discov. 2022;12:47–61. doi: 10.1158/2159-8290.CD-21-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li R, Salehi-Rad R, Crosson W, et al. Inhibition of granulocytic myeloid-derived suppressor cells overcomes resistance to immune checkpoint inhibition in LKB1-deficient non-small cell lung cancer. Cancer Res. 2021;81:3295–3308. doi: 10.1158/0008-5472.CAN-20-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miret JJ, Kirschmeier P, Koyama S, et al. Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity. J Immunother Cancer. 2019;7:32. doi: 10.1186/s40425-019-0504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou J, Zhao J, Jia Q, et al. Peripheral blood autoantibodies against to tumor-associated antigen predict clinical outcome to immune checkpoint inhibitor-based treatment in advanced non-small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.625578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Zhang Z, Hu Y, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) may predict the outcomes of advanced non-small-cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs) Front Oncol. 2020;10:654. doi: 10.3389/fonc.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother. 2020;69:2513–2522. doi: 10.1007/s00262-020-02637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Passaro A, Mancuso P, Gandini S, et al. Gr-MDSC-linked asset as a potential immune biomarker in pretreated NSCLC receiving nivolumab as second-line therapy. Clin Transl Oncol. 2020;22:603–611. doi: 10.1007/s12094-019-02166-z. [DOI] [PubMed] [Google Scholar]
- 100.Kang DH, Chung C, Sun P, et al. Circulating regulatory T cells predict efficacy and atypical responses in lung cancer patients treated with PD-1/PD-L1 inhibitors. Cancer Immunol Immunother. 2022;71:579–588. doi: 10.1007/s00262-021-03018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koh J, Hur JY, Lee KY, et al. Regulatory (FoxP3+) T cells and TGF-beta predict the response to anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci Rep. 2020;10:18994. doi: 10.1038/s41598-020-76130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 103.Bremnes RM, Busund LT, Kilvær TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 104.Kim MY, Koh J, Kim S. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24–33. doi: 10.1016/j.lungcan.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Cui S, Dong L, Qian J, Ye L, Jiang L. Classifying non-small cell lung cancer by status of programmed cell death ligand 1 and tumor-infiltrating lymphocytes on tumor cells. J Cancer. 2018;9:129–134. doi: 10.7150/jca.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on t-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fumet JD, Richard C, Ledys F, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119:950–960. doi: 10.1038/s41416-018-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8+ tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res. 2018;24:407–419. doi: 10.1158/1078-0432.CCR-17-2156. [DOI] [PubMed] [Google Scholar]
- 110.Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104:1–4. doi: 10.1016/s0092-8674(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 111.Han J, Duan J, Bai H, et al. TCR repertoire diversity of peripheral PD-1+CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res. 2020;8:146–154. doi: 10.1158/2326-6066.CIR-19-0398. [DOI] [PubMed] [Google Scholar]
- 112.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 113.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M, Ma J, Guo Q, Ding S, Wang Y, Pu H. CD8+ T cell-associated gene signature correlates with prognosis risk and immunotherapy response in patients with lung adenocarcinoma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.806877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 117.Huber M, Lohoff M. IRF4 at the crossroads of effector T-cell fate decision. Eur J Immunol. 2014;44:1886–1895. doi: 10.1002/eji.201344279. [DOI] [PubMed] [Google Scholar]
- 118.Alvisi G, Brummelman J, Puccio S, et al. IRF4 instructs effector Treg differentiation and immune suppression in human cancer. J Clin Invest. 2020;130:3137–3150. doi: 10.1172/JCI130426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Saint-Vis B, Vincent J, Vandenabeele S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 120.Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 121.Mayoux M, Roller A, Pulko V, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12:eaav7431. doi: 10.1126/scitranslmed.aav7431. [DOI] [PubMed] [Google Scholar]
- 122.Hwang I, Kim JW, Ylaya K, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443. doi: 10.1186/s12967-020-02618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giatromanolaki A, Mitrakas A, Anestopoulos I, et al. Expression of CD47 and SIRPalpha macrophage immune-checkpoint pathway in non-small-cell lung cancer. Cancers. 2022;14:1801. doi: 10.3390/cancers14071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L, Cao MF, Xiao JF, et al. Stromal PD-1+ tumor-associated macrophages predict poor prognosis in lung adenocarcinoma. Hum Pathol. 2020;97:68–79. doi: 10.1016/j.humpath.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H, Liu Z, Wen H, et al. Immunosuppressive TREM2(+) macrophages are associated with undesirable prognosis and responses to anti-PD-1 immunotherapy in non-small cell lung cancer. Cancer Immunol Immunother. 2022;71:2511–2522. doi: 10.1007/s00262-022-03173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. 2022;19:441–457. doi: 10.1038/s41571-022-00619-z. [DOI] [PubMed] [Google Scholar]
- 127.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375:eabf9419. doi: 10.1126/science.abf9419. [DOI] [PubMed] [Google Scholar]
- 128.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 129.Rader C. Bispecific antibodies in cancer immunotherapy. Curr Opin Biotechnol. 2020;65:9–16. doi: 10.1016/j.copbio.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dovedi SJ, Elder MJ, Yang C, et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 2021;11:1100–1117. doi: 10.1158/2159-8290.CD-20-1445. [DOI] [PubMed] [Google Scholar]
- 131.Villa-Ruano N, Guerrero-González T, Gómez-Conde E, Perez-Santos M. Bispecific anti-PD-L1/PD-1 antibodies for advanced solid tumors: a patent evaluation of US2019010232. Expert Opin Ther Pat. 2020;30:723–727. doi: 10.1080/13543776.2020.1810238. [DOI] [PubMed] [Google Scholar]
- 132.Zhang A, Ren Z, Tseng KF, et al. Dual targeting of CTLA-4 and CD47 on Treg cells promotes immunity against solid tumors. Sci Transl Med. 2021;13:eabg8693. doi: 10.1126/scitranslmed.abg8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knudson KM, Hicks KC, Ozawa Y, Schlom J, Gameiro SR. Functional and mechanistic advantage of the use of a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu L, Chen J, Bae J, et al. Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells. Nat Biomed Eng. 2021;5:1261–1273. doi: 10.1038/s41551-021-00800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 2018;128:580–588. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dammeijer F, van Gulijk M, Mulder EE, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020;38:685–700. doi: 10.1016/j.ccell.2020.09.001. .e8. [DOI] [PubMed] [Google Scholar]
- 137.Vitale LA, He LZ, Thomas LJ, et al. Development of CDX-527: a bispecific antibody combining PD-1 blockade and CD27 costimulation for cancer immunotherapy. Cancer Immunol Immunother. 2020;69:2125–2137. doi: 10.1007/s00262-020-02610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berezhnoy A, Sumrow BJ, Stahl K, et al. Development and preliminary clinical activity of PD-1-guided CTLA-4 blocking bispecific DART molecule. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muik A, Garralda E, Altintas I, et al. Preclinical characterization and phase I trial results of a bispecific antibody targeting PD-L1 and 4-1BB (GEN1046) in patients with advanced refractory solid tumors. Cancer Discov. 2022;12:1248–1265. doi: 10.1158/2159-8290.CD-21-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022;21:529–540. doi: 10.1038/s41573-022-00493-5. [DOI] [PubMed] [Google Scholar]