Abstract
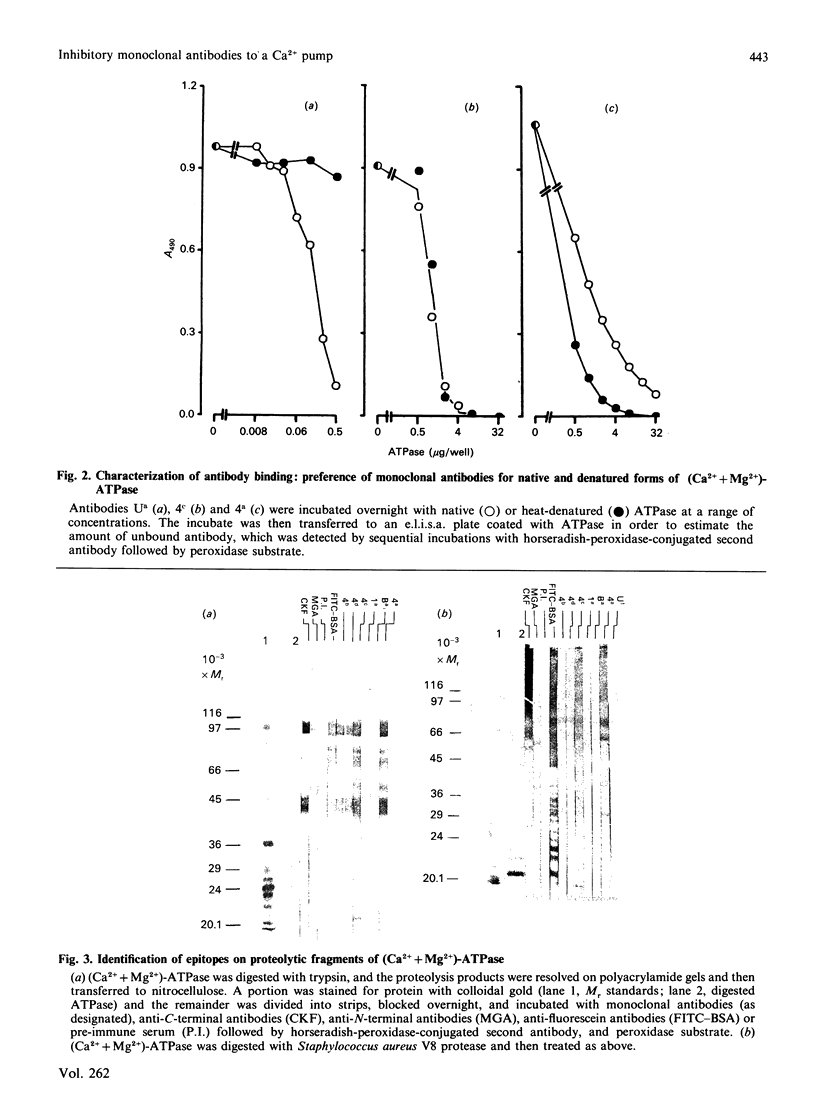
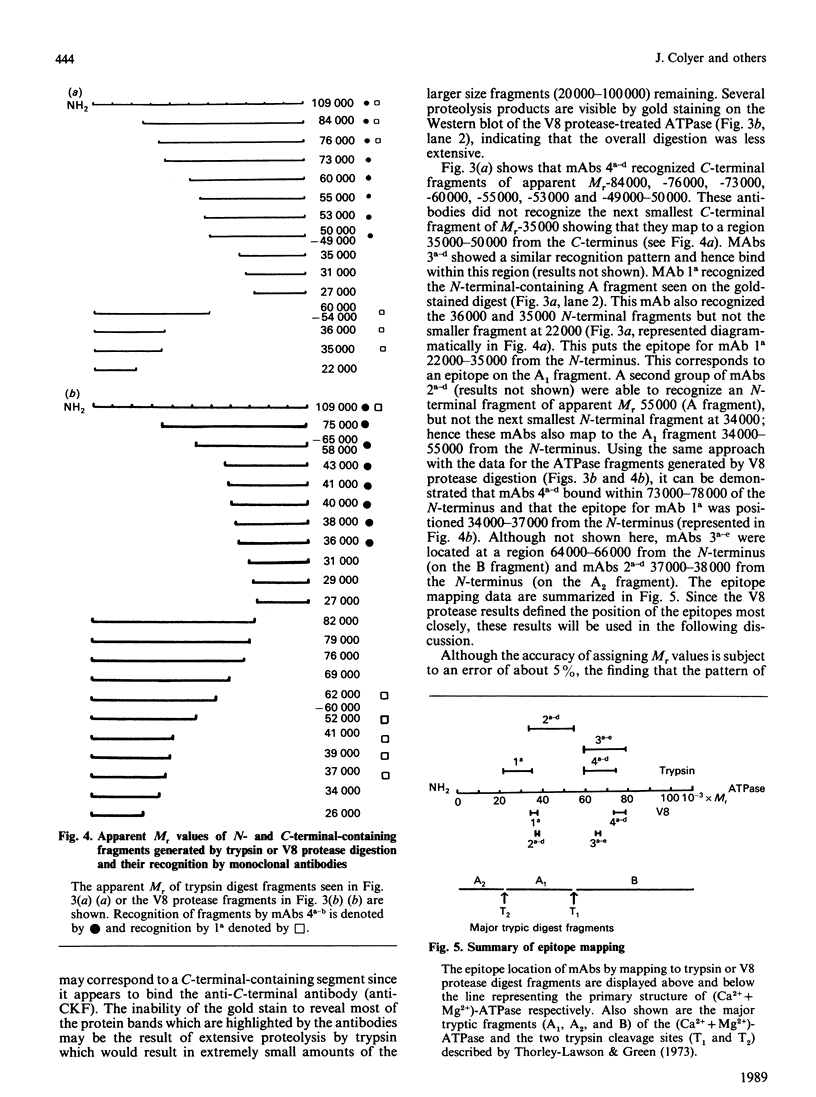
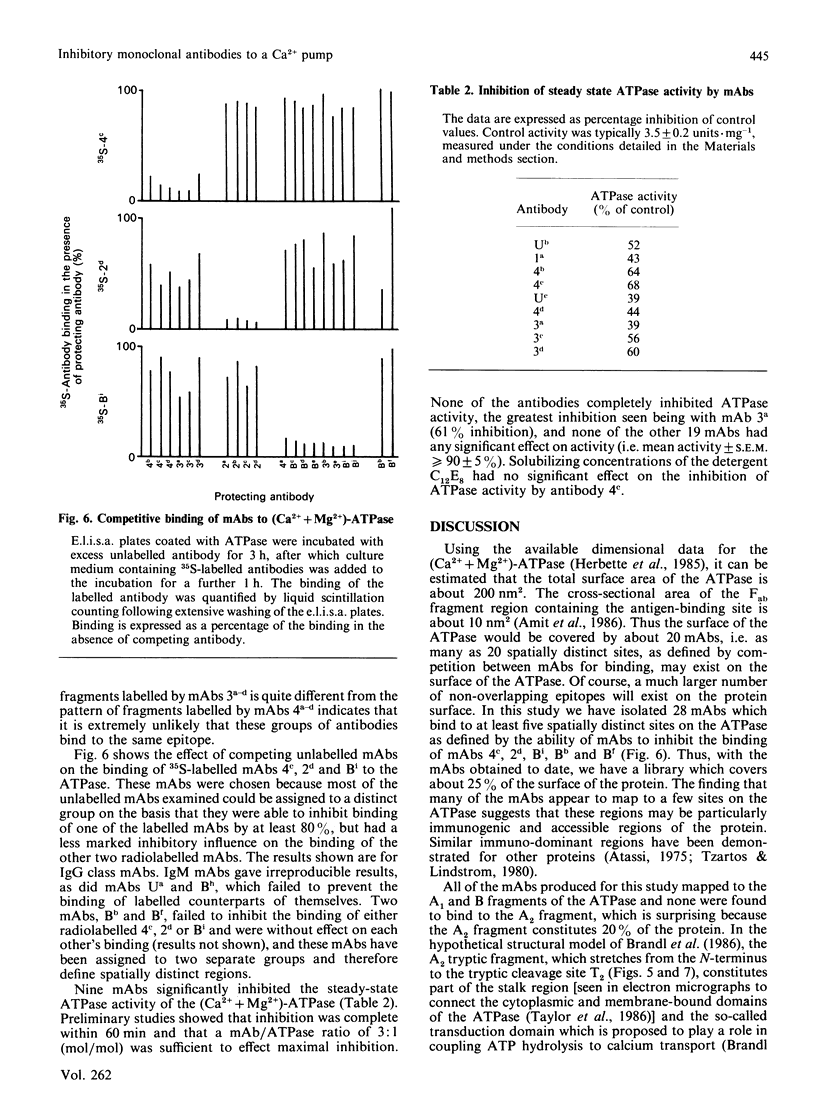
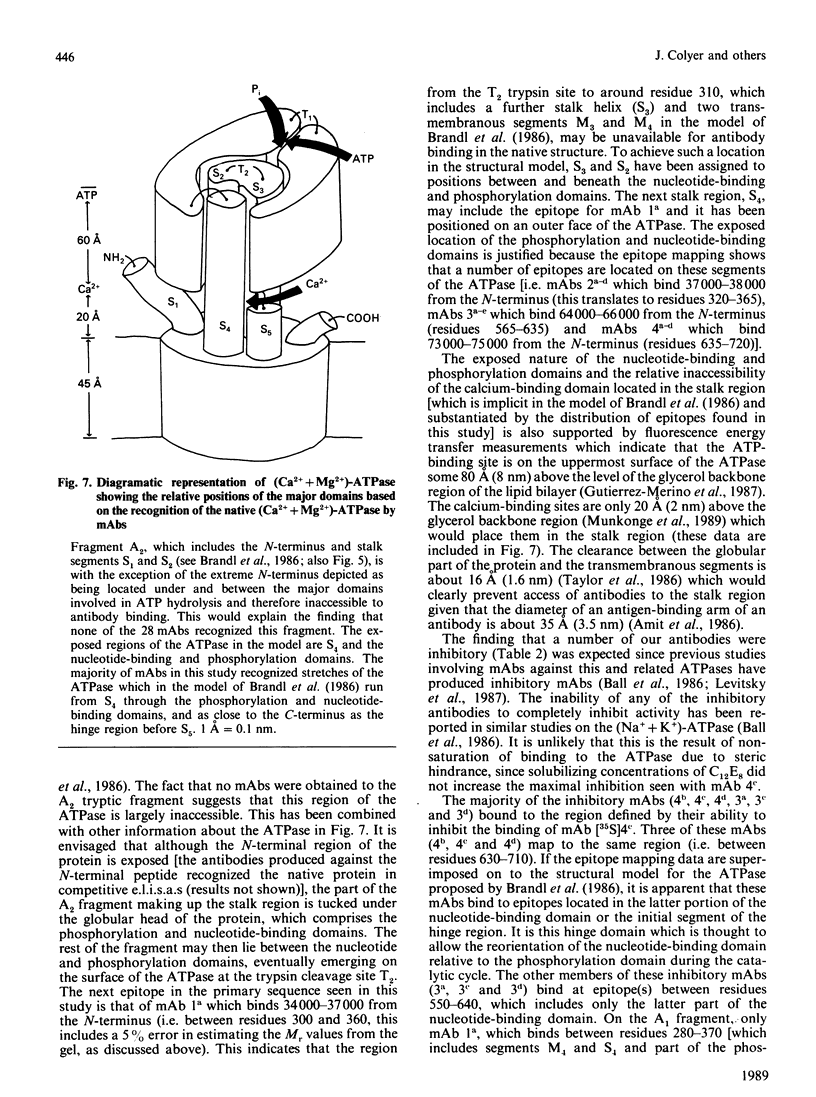
A total of 28 monoclonal antibodies have been raised against the (Ca2+ + Mg2+)-ATPase of rabbit skeletal muscle sarcoplasmic reticulum. Epitope mapping, using protein fragments generated by proteolysis, indicates that these antibodies include examples binding to at least four distinct epitopes on the A1 and B tryptic fragments of the ATPase. Competition data also show that the 28 antibodies are directed against at least five spatially distinct regions. Altogether, nine inhibitory antibodies were produced: six of these inhibitory antibodies mapped to the same spatial region, although they appear to bind to two distinct epitopes located within the hinge region and the nucleotide-binding domains of current structural models; one antibody bound to an epitope located within the phosphorylation domain and the stalk-transmembranous region designated M4S4 by Brandl, Green, Korczak & MacLennan [(1986) Cell 44, 597-607]. Two of the inhibitory antibodies recognized assembled epitopes exclusively and could not be mapped. Binding to four of the five identified spatial regions was without effect on activity. These data show that the inhibition of catalytic activity by monoclonal antibodies is achieved only by binding to defined regions of the ATPase and they may therefore provide useful probes of structure-function relationships.
Full text
PDF

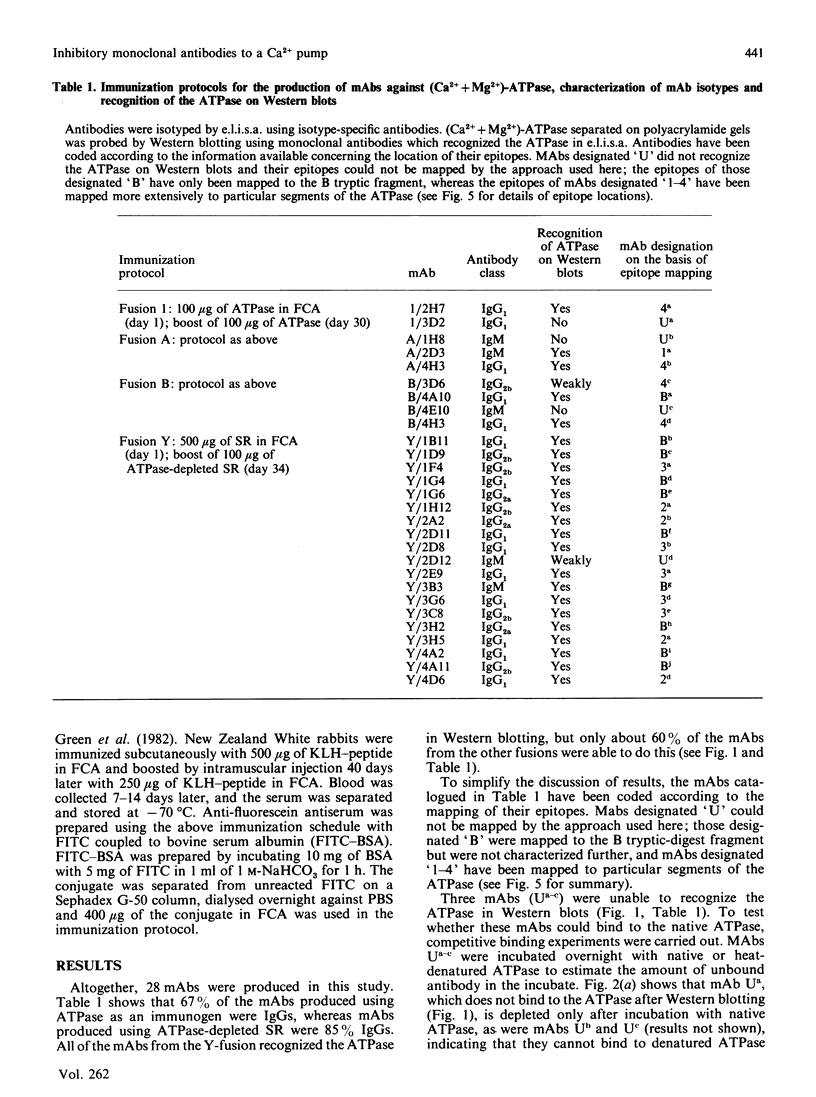
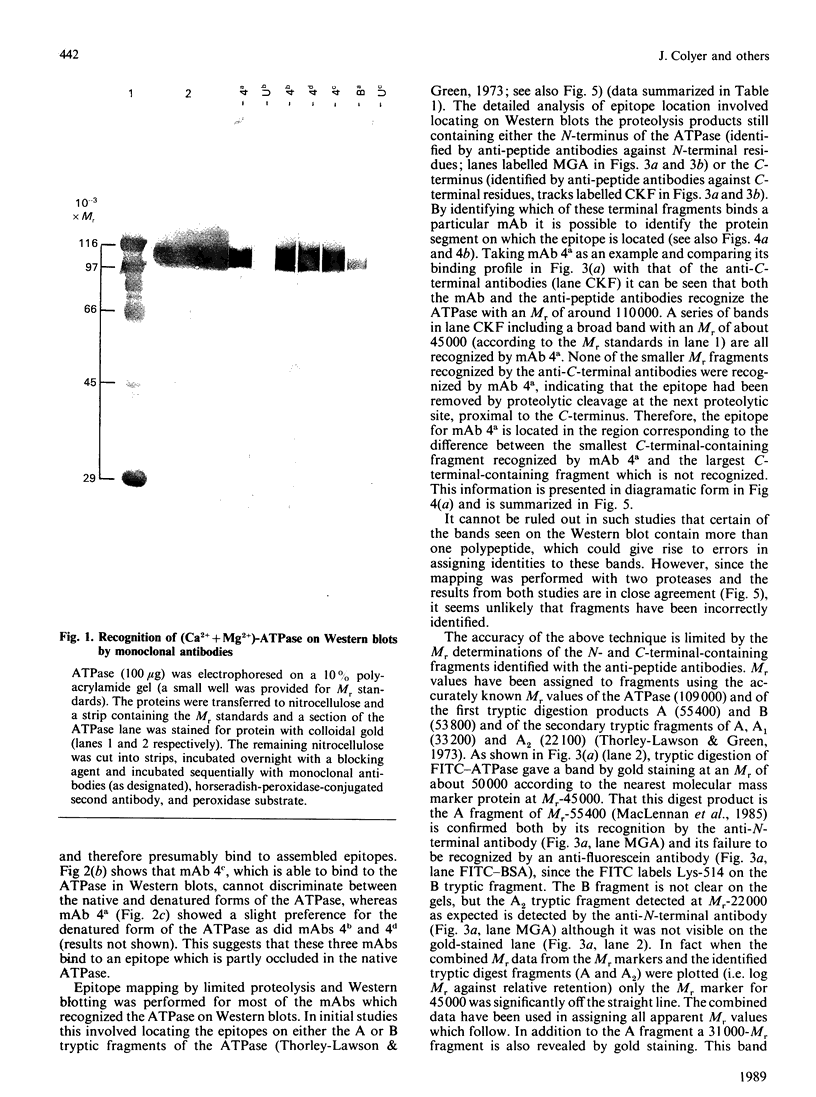

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr Uncoupling of ATP binding to Na+,K+-ATPase from its stimulation of ouabain binding: studies of the inhibition of Na+,K+-ATPase by a monoclonal antibody. Biochemistry. 1986 Nov 4;25(22):7155–7162. doi: 10.1021/bi00370a058. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dulhunty A. F., Banyard M. R., Medveczky C. J. Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J Membr Biol. 1987;99(2):79–92. doi: 10.1007/BF01871228. [DOI] [PubMed] [Google Scholar]
- East J. M., Lee A. G. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 1982 Aug 17;21(17):4144–4151. doi: 10.1021/bi00260a035. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. Conformational transitions in the Ca2+ + Mg2+-activated ATPase and the binding of Ca2+ ions. Biochem J. 1986 Jul 1;237(1):197–206. doi: 10.1042/bj2370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gould G. W., East J. M., Froud R. J., McWhirter J. M., Stefanova H. I., Lee A. G. A kinetic model for the Ca2+ + Mg2+-activated ATPase of sarcoplasmic reticulum. Biochem J. 1986 Jul 1;237(1):217–227. doi: 10.1042/bj2370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Merino C., Munkonge F., Mata A. M., East J. M., Levinson B. L., Napier R. M., Lee A. G. The position of the ATP binding site on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1987 Feb 26;897(2):207–216. doi: 10.1016/0005-2736(87)90417-2. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Herbette L., DeFoor P., Fleischer S., Pascolini D., Scarpa A., Blasie J. K. The separate profile structures of the functional calcium pump protein and the phospholipid bilayer within isolated sarcoplasmic reticulum membranes determined by X-ray and neutron diffraction. Biochim Biophys Acta. 1985 Jul 11;817(1):103–122. doi: 10.1016/0005-2736(85)90073-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitsky D. O., Syrbu S. I., Cherepakhin V. V., Rokhlin O. V. Monoclonal antibodies to dog heart sarcoplasmic reticulum. Antibodies that inhibit Ca2+-pump systems of cardiac and skeletal muscles. Eur J Biochem. 1987 Apr 15;164(2):477–484. doi: 10.1111/j.1432-1033.1987.tb11081.x. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Moeremans M., Daneels G., De Mey J. Sensitive colloidal metal (gold or silver) staining of protein blots on nitrocellulose membranes. Anal Biochem. 1985 Mar;145(2):315–321. doi: 10.1016/0003-2697(85)90368-9. [DOI] [PubMed] [Google Scholar]
- Munkonge F., East J. M., Lee A. G. Positions of the sites labeled by N-cyclohexyl-N'-(4-dimethylamino-1-naphthyl)carbodiimide on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1989 Feb 13;979(1):113–120. doi: 10.1016/0005-2736(89)90530-0. [DOI] [PubMed] [Google Scholar]
- Petithory J. R., Jencks W. P. Phosphorylation of the calcium adenosinetriphosphatase of sarcoplasmic reticulum: rate-limiting conformational change followed by rapid phosphoryl transfer. Biochemistry. 1986 Aug 12;25(16):4493–4497. doi: 10.1021/bi00364a006. [DOI] [PubMed] [Google Scholar]
- Schenk D. B., Hubert J. J., Leffert H. L. Use of a monoclonal antibody to quantify (Na+,K+)-ATPase activity and sites in normal and regenerating rat liver. J Biol Chem. 1984 Dec 10;259(23):14941–14951. [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Dux L., Martonosi A. Three-dimensional reconstruction of negatively stained crystals of the Ca2+-ATPase from muscle sarcoplasmic reticulum. J Mol Biol. 1986 Feb 5;187(3):417–427. doi: 10.1016/0022-2836(86)90442-0. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E., MacDonald G., Phillips L., Jorgensen A. O., MacLennan D. H. Monoclonal antibodies to the Ca2+ + Mg2+-dependent ATPase of sarcoplasmic reticulum identify polymorphic forms of the enzyme and indicate the presence in the enzyme of a classical high-affinity Ca2+ binding site. J Bioenerg Biomembr. 1984 Dec;16(5-6):441–464. doi: 10.1007/BF00743238. [DOI] [PubMed] [Google Scholar]
- de Ancos J. G., Inesi G. Patterns of proteolytic cleavage and carbodiimide derivatization in sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1988 Mar 8;27(5):1793–1803. doi: 10.1021/bi00405a061. [DOI] [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]