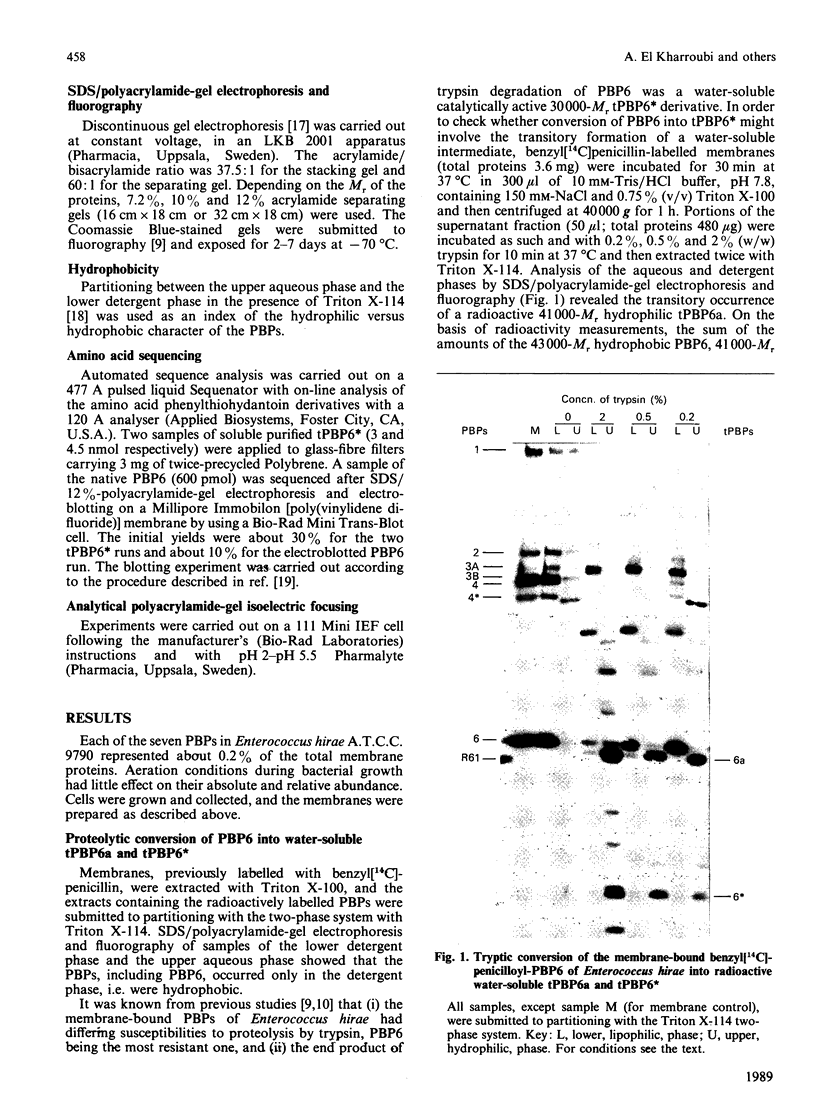
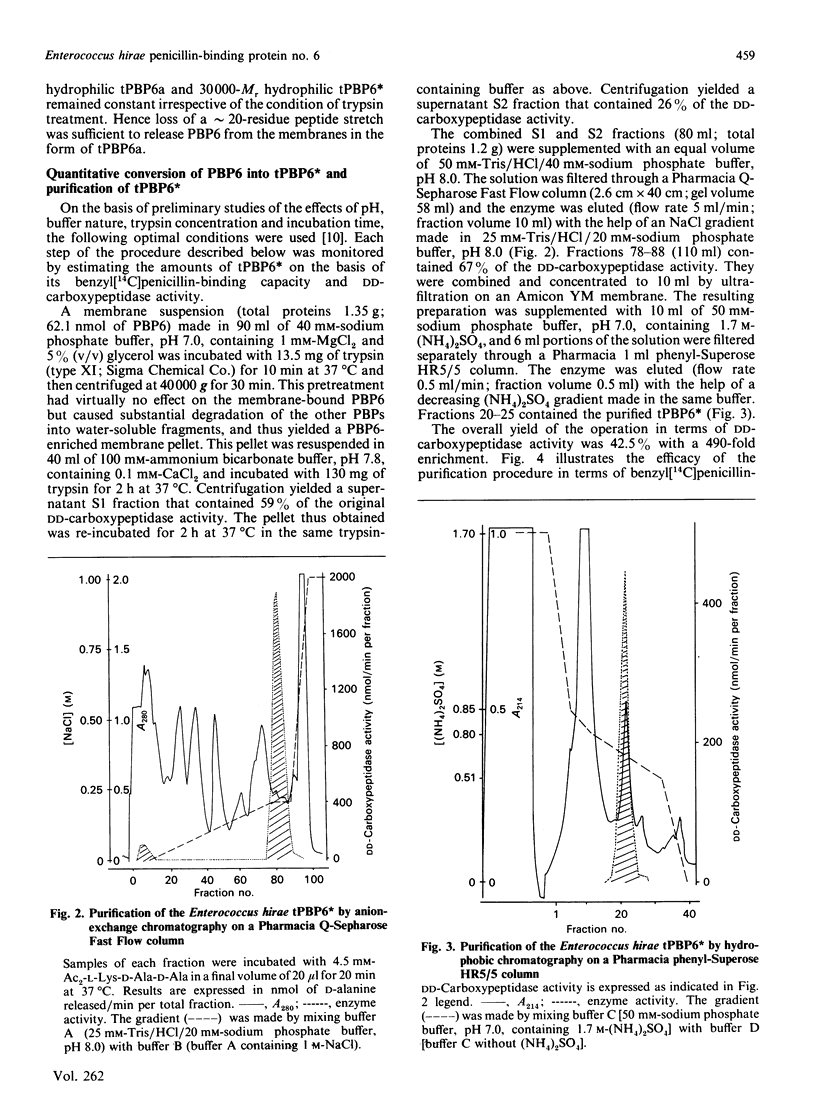
Abstract
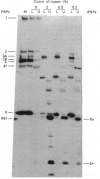
The membrane-bound 43,000-Mr penicillin-binding protein no. 6 (PBP6) of Enterococcus hirae consists of a 30,000-Mr DD-peptidase/penicillin-binding domain and a approximately 130-residue C-terminal appendage. Removal of this appendage by trypsin proteolysis has no marked effect on the catalytic activity and penicillin-binding capacity of the PBP. Anchorage of the PBP in the membrane appears to be mediated by a short 15-20-residue stretch at the C-terminal end of the appendage. The sequence of the 50-residue N-terminal region of the PBP shows high degree of homology with the sequences of the corresponding regions of the PBPs5 of Escherichia coli and Bacillus subtilis. On this basis the active-site serine residue occurs at position 35 in the enterococcal PBP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Broome-Smith J., Spratt B. G. An amino acid substitution that blocks the deacylation step in the enzyme mechanism of penicillin-binding protein 5 of Escherichia coli. FEBS Lett. 1984 Jan 9;165(2):185–189. doi: 10.1016/0014-5793(84)80166-0. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. The penicillin-binding proteins in Streptococcus faecalis ATCC 9790. Eur J Biochem. 1980 Sep;110(2):445–456. doi: 10.1111/j.1432-1033.1980.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Perkins H. R. The exchange reaction of peptides R-D-alanyl-D-alanine with D-[14C]alanine to R-D-alanyl-D-[14C]alanine and D-alanine, catalysed by the membranes of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1977 May 2;75(1):225–229. doi: 10.1111/j.1432-1033.1977.tb11521.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Perkins H. R., Polacheck I., Shockman G. D., Ghuysen J. M. Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1974 May 15;44(2):459–468. doi: 10.1111/j.1432-1033.1974.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Dideberg O., Charlier P., Wéry J. P., Dehottay P., Dusart J., Erpicum T., Frère J. M., Ghuysen J. M. The crystal structure of the beta-lactamase of Streptomyces albus G at 0.3 nm resolution. Biochem J. 1987 Aug 1;245(3):911–913. doi: 10.1042/bj2450911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Piron-Fraipont C., Joris B., Dusart J., Urdea M. S., Martial J. A., Frère J. M., Ghuysen J. M. Primary structure of the Streptomyces R61 extracellular DD-peptidase. 1. Cloning into Streptomyces lividans and nucleotide sequence of the gene. Eur J Biochem. 1987 Feb 2;162(3):509–518. doi: 10.1111/j.1432-1033.1987.tb10669.x. [DOI] [PubMed] [Google Scholar]
- Ellerbrok H., Hakenbeck R. Penicillin-binding proteins of Streptococcus pneumoniae: characterization of tryptic peptides containing the beta-lactam-binding site. Eur J Biochem. 1984 Nov 2;144(3):637–641. doi: 10.1111/j.1432-1033.1984.tb08512.x. [DOI] [PubMed] [Google Scholar]
- Ferreira L. C., Schwarz U., Keck W., Charlier P., Dideberg O., Ghuysen J. M. Properties and crystallization of a genetically engineered, water-soluble derivative of penicillin-binding protein 5 of Escherichia coli K12. Eur J Biochem. 1988 Jan 15;171(1-2):11–16. doi: 10.1111/j.1432-1033.1988.tb13751.x. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Bacterial active-site serine penicillin-interactive proteins and domains: mechanism, structure, and evolution. Rev Infect Dis. 1988 Jul-Aug;10(4):726–732. doi: 10.1093/clinids/10.4.726. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Nguyen-Distèche M., Coyette J. Active-site-serine D-alanyl-D-alanine-cleaving-peptidase-catalysed acyl-transfer reactions. Procedures for studying the penicillin-binding proteins of bacterial plasma membranes. Biochem J. 1986 Apr 1;235(1):159–165. doi: 10.1042/bj2350159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg O., Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science. 1987 May 8;236(4802):694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M. Analysis of the membrane-binding domain of penicillin-binding protein 5 of Escherichia coli. Mol Microbiol. 1988 Sep;2(5):563–568. doi: 10.1111/j.1365-2958.1988.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. A., Dideberg O., Charlier P., Wery J. P., Libert M., Moews P. C., Knox J. R., Duez C., Fraipont C., Joris B. On the origin of bacterial resistance to penicillin: comparison of a beta-lactamase and a penicillin target. Science. 1986 Mar 21;231(4744):1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Nguyen-Distèche M., Pirlot S., Veithen A., Bourguignon C., Ghuysen J. M. Streptomyces K15 DD-peptidase-catalysed reactions with suicide beta-lactam carbonyl donors. Biochem J. 1986 Apr 1;235(1):177–182. doi: 10.1042/bj2350177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nguyen-Distèche M., Leyh-Bouille M., Pirlot S., Frère J. M., Ghuysen J. M. Streptomyces K15 DD-peptidase-catalysed reactions with ester and amide carbonyl donors. Biochem J. 1986 Apr 1;235(1):167–176. doi: 10.1042/bj2350167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Roberts A. N., Johnstone K., Piggot P. J., Winter G., Ellar D. J. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J Bacteriol. 1986 Jul;167(1):257–264. doi: 10.1128/jb.167.1.257-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Primary structure of the COOH-terminal membranous segment of a penicillin-sensitive enzyme purified from two Bacilli. J Biol Chem. 1981 Feb 25;256(4):2067–2077. [PubMed] [Google Scholar]