Abstract
Microsomal glutathione transferase 3 (MGST3) regulates eicosanoid and glutathione metabolism. These processes are associated with oxidative stress and apoptosis, suggesting that MGST3 might play a role in the pathophysiology of Alzheimer's disease. Here, we report that knockdown (KD) of MGST3 in cell lines reduced the protein level of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) and the resulting amyloidogenesis. Interestingly, MGST3 KD did not alter intracellular reactive oxygen species level but selectively reduced the expression of apoptosis indicators which could be associated with the receptor of cysteinyl leukotrienes, the downstream metabolites of MGST3 in arachidonic acid pathway. We then showed that the effect of MGST3 on BACE1 was independent of cysteinyl leukotrienes but involved a translational mechanism. Further RNA-seq analysis identified that regulator of G-protein signaling 4 (RGS4) was a target gene of MGST3. Silencing of RGS4 inhibited BACE1 translation and prevented MGST3 KD–mediated reduction of BACE1. The potential mechanism was related to AKT activity, as the protein level of phosphorylated AKT was significantly reduced by silencing of MGST3 and RGS4, and the AKT inhibitor abolished the effect of MGST3/RGS4 on phosphorylated AKT and BACE1. Together, MGST3 regulated amyloidogenesis by controlling BACE1 protein expression, which was mediated by RGS4 and downstream AKT signaling pathway.
Keywords: MGST3, Alzheimer’s disease, BACE1, translation, RGS4, AKT
Alzheimer's disease (AD) is the most common cause of dementia, accounting for about 60%∼80% of all diagnosed cases, and the incidence increases with aging (1, 2). Irrespective of the complicated molecular mechanisms (3), the pathological hallmarks include extracellular β-amyloid (Aβ) deposition and intracellular Tau aggregation (4). It is suggested that amyloid precursor protein (APP) provides the only source of Aβ generation, in which beta-site APP cleaving enzyme 1 (BACE1)-mediated cleavage is a rate-limiting process (5). The elevated BACE1 protein and activity in the brain support that inhibiting BACE1 remains a promising therapeutic approach for AD (6, 7, 8, 9). On the other hand, important mechanisms related to AD, such as inflammation, oxidative stress, and intracellular calcium overload, all affect BACE1 expression (6, 7, 8). BACE1 may form an important intracellular hub, linking a variety of pathophysiological changes to Aβ aggregation. Therefore, investigating the regulation of BACE1 provides an important strategy for understanding the upstream mechanisms in AD.
Microsomal glutathione transferase 3 (MGST3) is member of microsomal prostaglandin E synthases super family that exhibits diversified functions in eicosanoid and GSH metabolism (10). It is reported that the GSH transferase and peroxidase activities of MGST3 are associated with detoxification, oxidative stress, inflammation, and apoptosis (11, 12, 13). In the brain, MGST3 is widely distributed, with particular enrichment in the hippocampus and brainstem (14). MGST3 expression level correlates with hippocampal size, and mutation of MGST3 contributes to spinal muscular dystrophy (15, 16), highlighting an important role of MGST3 in neuronal development and degenerative diseases.
We hypothesized that through mechanisms associated with oxidative stress and apoptosis, MGST3 could regulate BACE1 expression. Unexpectedly, MGST3 knockdown (KD) did not alter intracellular reactive oxygen species (ROS) level despite that selective apoptosis indicators were reduced. Although MSGT3 KD reduced the protein level of BACE1, this effect was not associated with downstream metabolites of MGST3 in arachidonic acid pathway but was prevented by translation inhibitors. We further identified that G-protein signaling 4 (RGS4), one of the target genes of MGST3, regulated BACE1 translation through AKT signaling pathway.
Results
MGST3 was involved in APP processing by regulating BACE1 protein expression
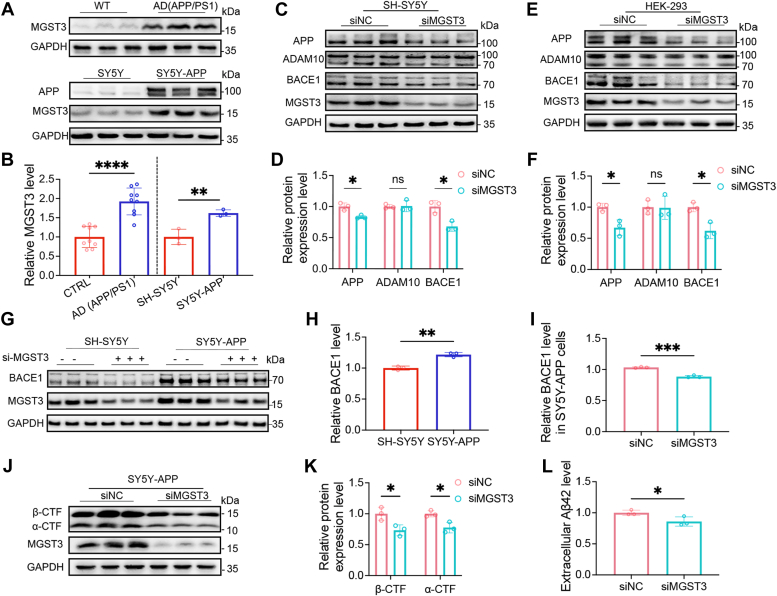
To determine whether MGST3 protein expression might be altered in AD, we first assessed MGST3 protein levels in the hippocampus of 10 months old APP/PS1 mice (APP/PS1) relative to age-matched WT mice. As shown in Figure 1, A and B, the hippocampal MGST3 protein levels were significantly increased in APP/PS1 mice compared with WT. Similarly, compared with SH-SY5Y cells, MGST3 protein level was significantly increased in SY5Y-APP cells, a cellular model of AD that stably overexpress human full-length APP695 leading to high intracellular Aβ generation (Fig. 1, A and B). In AD, amyloidogenesis refers to Aβ species resulting from the sequential cleavage of APP by BACE1 and γ-secretase, leading to the generation of C-terminal fragment β (β-CTF) and Aβ, respectively (9). This process is also regulated by the α-secretase a disintegrin and metalloproteinase domain–containing protein 10 (ADAM10), which catalyzes the α-site cleavage of APP with the production of α-CTF, thus promoting nonamyloidogenic pathway with the reduced Aβ level (17). To further understand the functional role of MGST3 in amyloidogenesis, we assessed the protein levels of APP, and the two catalytic enzymes BACE1 and ADAM10 in cells transiently transfected with MGST3 siRNA. As shown in Figure 1, C–F, in both SH-SY5Y and human embryonic kidney (HEK) cells, the protein levels of APP and BACE1 were significantly reduced, whereas those of ADAM10 were without change. Consistent with the elevated expression of MGST3 in SY5Y-APP cells (Fig. 1, A and B), BACE1 expression was also enhanced; and siMGST3 led to a significant reduction of BACE1 protein in SY5Y-APP cells (Fig. 1, G–I). Accordingly, the protein levels of the catalytic products of APP including β/α-CTFs and Aβ42 were decreased by MGST3 KD (Fig. 1, J–L). These results indicated that MGST3 regulated amyloidogenesis by controlling APP and BACE1 protein expression. Given that BACE1 is a rate-limiting enzyme in amyloidogenesis (18), we selected BACE1 for further investigation.
Figure 1.
MGST3 inhibits amyloidogenesis by reducing BACE1 protein expression.A and B, representative Western blots (A) and quantification (B) of MGST3 protein in the hippocampus of WT) versus APP/PS1 mice at 10 months (n = 9) and in SH-SY5Y versus SY5Y-APP cells (n = 3 independent experiments), respectively. C and D, representative Western blots (C) and quantification (D) of APP, ADAM10, and BACE1 in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). E and F, representative Western blots (E) and quantification (F) of APP, ADAM10, and BACE1 in HEK-293 cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). G–I, representative Western blots (G) and quantification of BACE1 (H and I) in SH-SY5Y and SY5Y-APP cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h, respectively (n = 3 independent experiments). J and K, representative Western blots (J) and quantification (K) of β-CTF and α-CTF in SY5Y-APP cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). L, culture medium level of Aβ42 in SY5Y-APP cells treated with the scrambled (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. ADAM10, a disintegrin and metalloproteinase domain–containing protein 10; APP, amyloid precursor protein; BACE1, beta-site amyloid precursor protein cleaving enzyme 1; CFT, C-terminal fragment; HEK, human embryonic kidney; MGST, microsomal glutathione transferase 3.
MGST3 KD did not alter ROS level but inhibited apoptosis
One of the arachidonic acid metabolism pathways involves 5-lipoxygenase (5-LOX), which catalyzes the production of leukotriene A4 (19). In this pathway, LTC4 synthase (LTC4S) converts leukotriene A4 to LTC4, leading to the generation of cysteinyl leukotrienes (CysLTs) (13, 20). Importantly, MGST3 is considered as an isoenzyme of LTC4S (21). It is reported that LTC4 is known to mediate endoplasmic stress-induced ROS production (22), and an elevated level of MGST3 and CysLTs is accompanied with the increased expression of caspase3 in LLC-PK1 (porcine renal epithelial) cells treated with the plant extract aristolochic acid (12). Thus, we speculated that MGST3 might be involved in the regulation of ROS and apoptosis.
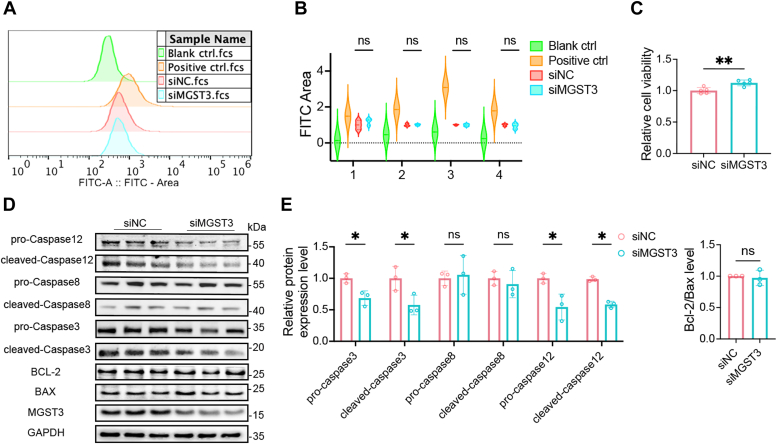
Surprisingly, as shown in Figure 2, A and B, silencing of MGST3 did not cause an alteration of ROS levels, whereas a dramatic increase of ROS was induced by a positive control ROS-UP (23). We further found that cell viability was rather enhanced by MGST3 KD (Fig. 2C), suggesting that MGST3 KD inhibited apoptosis. Evidences indicate that apoptosis involves a variety of caspases (24). In particular, caspase8 regulates inflammation through apoptosis induction (25), caspase12 is activated during apoptosis induced by endoplasmic reticulum (ER) stress (26), and caspase3 serves as one of the important apoptosis executioners (27). To further understand the potential role of MGST3 in apoptosis, we assessed in SH-SY5Y cells the effect of siMGST3 on protein levels of these caspases, along with Bcl-2 and BAX which correlate with mitochondrial apoptotic pathway (28, 29). As shown in Figure 2, D and E, MGST3 KD significantly reduced the expression of procaspase and cleaved-caspase 3/12, without affecting that of caspase8 and the ratio of Bcl-2/BAX, suggesting that MGST3 selectively affected ER stress associated apoptosis, which was in line with the subcellular localization of MGST3 in ER and mitochondria (30).
Figure 2.
MGST3 KD does not alterreactive oxygen specieslevel but inhibits apoptosis.A and B, the reactive oxygen species level in living HEK-293 cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 4 independent experiments). C, relative cell viability of SH-SY5Y cells treated with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 5 independent experiments). D and E, representative Western blots (D) and quantification (E) of pro/cleaved-caspase 3, pro/cleaved-caspase 8, pro/cleaved-caspase 12, BCL-2, and BAX in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA(siMGST3) for 48 h (n = 3 independent experiments). HEK, human embryonic kidney; KD, knockdown; MGST, microsomal glutathione transferase 3.
MGST3-mediated regulation of BACE1 was independent of downstream metabolites in 5-LOX pathway but involved a translation mechanism
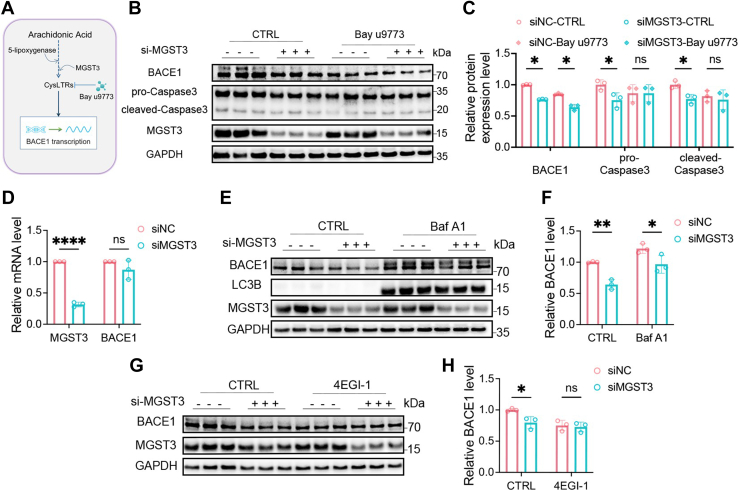
CysLTs activate CysLTs receptors (CysLTRs) and promote BACE1 transcription via NF-κB signaling (8, 31). We then tested whether CysLTs might mediate MGST3-induced regulation of BACE1 by using CysLTR inhibitor BAY u9773 (22, 32). As shown in Figure 3, A–C, BAY u9773 alone significantly reduced protein levels of BACE1 and pro/cleaved-caspase3, indicating that CysLTs indeed regulated BACE1 expression and apoptosis under basal condition. However, MGST3 KD–induced reduction of BACE1 protein was not prevented by BAY u9773 in SH-SY5Y cells, despite that the effect of MGST3 on pro/cleaved-caspase3 was blocked by BAY u9773. These results indicated that the effect of MGST3 on apoptosis instead of BACE1 was dependent on the downstream metabolites in 5-LOX pathway.
Figure 3.
MGST3-mediated regulation of BACE1 is independent of downstream metabolites in 5-LOX pathway but dependent on translation.A, AA metabolite CysLTs in 5-LOX pathway activate CysLTRs and promote BACE1 transcription. B and C, representative Western blots (B) and quantification (C) of BACE1, caspase3 in SH-SY5Y cells transfected with the scrambled control (siNC), or MGST3 siRNA (siMGST3) for 24 h, followed by dimethyl sulfoxide (DMSO) or the CysLT receptor antagonist BAY u9773 (1 μM) treatment for 24 h. D, relative BACE1 mRNA level in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). E and F, representative Western blots (E) and quantification (F) of BACE1 protein levels in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 24 h, followed by treatment of DMSO or Baf A1 (100 nM) for additional 24 h (n = 3 independent experiments). G and H, representative Western blots (G) and quantification (H) of BACE1 protein levels in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 24 h, followed by treatment of DMSO or 4EGI-1(25 μM) for additional 24 h (n = 3 independent experiments). Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 (two-way ANOVA). 5-LOX, 5-lipoxygenase; AA, arachidonic acid; Baf A1, bafilomycin A1; BACE1, beta-site amyloid precursor protein cleaving enzyme 1; CysLT, cysteinyl leukotriene; CysLTR, CysLT receptor; MGST, microsomal glutathione transferase 3.
The reduced BACE1 protein by MGST3 KD could be a result of an alteration of transcription, translation, and protein degradation mechanisms. Thus, we assessed mRNA levels of BACE1 in SH-SY5Y cells. As shown in Figure 3D, BACE1 mRNA was not significantly altered by MGST3 silencing. We further showed that in cells treated with lysosome inhibitor bafilomycin A1 (Baf A1) that significantly increased the basal protein level of BACE1, MGST3 siRNA caused a significant reduction of BACE1 protein (Fig. 3, E and F). However, translation inhibitors 4EGI-1, which decreased the basal protein level of BACE1, prevented the reduction of BACE1 by MGST3 KD (Fig. 3, G and H). These results indicated that regulation of BACE1 by MGST3 was associated with translation mechanism (33).
RGS4 was a target gene of MGST3 and regulated BACE1 translation
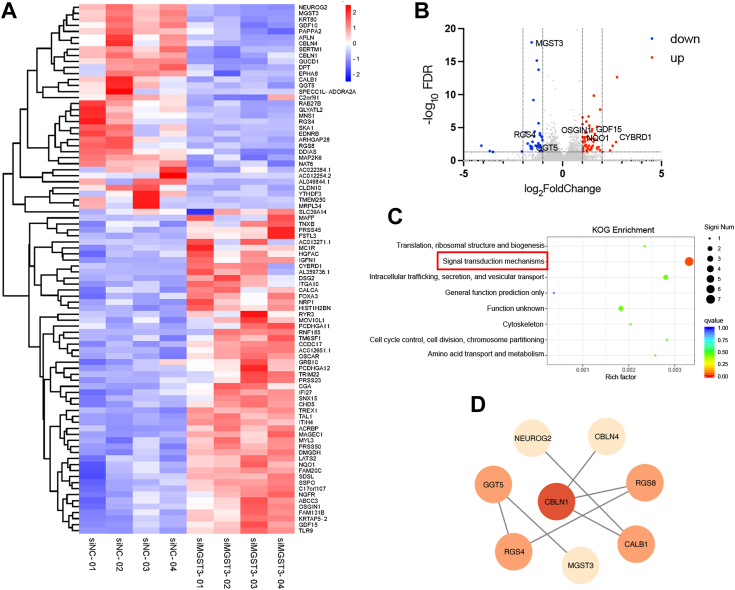
To further understand the potential mechanisms that MGST3 controlled BACE1 expression, we performed RNA-seq in SH-SY5Y cells transfected with MGST3 siRNA. A total of 88 differentially expressed genes (DEGs) (fold change >2 and p < 0.05) were identified (Fig. 4, A and B). Of these DEGs, 54 were upregulated and 34 downregulated. Those upregulated included growth differentiation factor 15 (GDF15), NAD(P)H quinone dehydrogenase 1 (NQ01), and cytochrome B reductase 1, whereas downregulated included regulator of RGS4, neurogenin 2, and mitochondrial ribosomal protein L34. EuKaryotic Orthologous Groups analysis revealed that “signal transduction pathways” was among those mostly enriched (Fig. 4C). Further protein–protein interaction analysis through online String software identified RGS4 and RGS8 could plan an important role in this “signal transduction pathway” (Fig. 4D). RGS family proteins are critical for a variety of cellular processes, including homeostasis, response to stimuli and signaling (34). Compared with RGS8, RGS4 is highly enriched in neurons and implicated in Parkinson disease (35, 36). Importantly, RGS4 is negatively correlated with immune cell infiltration and exhibits diagnostic potential for AD (37), whereas the potential mechanisms have not been well understood.
Figure 4.
MGST3-induced DEGs are enriched in signal transduction mechanisms.A, cluster map of DEGs in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 4 biological replicates). Red, upregulated; blue, downregulated. B, volcano map shows red and green dots that represent upregulated and downregulated genes with significant differences, respectively, whereas gray dots represent genes with no significant differences. C, KOG enrichment map shows that “signal transduction mechanisms” is significantly enriched. D, protein–protein interaction network diagram of downregulated DEGs generated by online String software. Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. DEG, differentially expressed gene; KOG, EuKaryotic Orthologous Groups; MGST, microsomal glutathione transferase 3.
Thus, we tested whether RGS4 may play a role in AD by regulating BACE1. As shown in Figure 5, A–D, the protein level of RGS4 was significantly increased in the hippocampus of APP/PS1 mice and SY5Y-APP cells, in a similar manner to that of MGST3 (Fig. 1, A–D), suggesting a functional connection between MGST3 and RGS4. Moreover, RGS4 KD resulted in a significant decrease in protein levels of BACE1, downstream CTFs, and extracellular Aβ42 (Fig. 5, E–G). In support, immunohistochemical signals of BACE1 were also significantly reduced by RGS4 KD (Fig. 5, H and I). We further showed that translation inhibitor 4EGI-1 significantly attenuated the effect of RGS4 KD on BACE1 (Fig. 5, J and K). These results indicated that RGS4 controlled BACE1 expression through a translational mechanism.
Figure 5.
Knockdown of RGS4 inhibits BACE1 translation.A and B, representative Western blots (A) and quantification (B) of RGS4 protein in the hippocampus of WT and APP/PS1 mice at 10 months (n = 9), respectively. C and D, representative Western blots (C) and quantification (D) of RGS4 protein in SH-SY5Y and SY5Y-APP cells (n = 3 independent experiments). E and F, representative Western blots (E) and quantification (F) of BACE1, β-CTF, and α-CTF in SY5Y-APP cells transfected with the scrambled control (siNC) or RGS4 siRNA (siRGS4) for 48 h (n = 3 independent experiments). G, culture medium level of Aβ42 in SY5Y-APP cells treated with the scrambled (siNC) or RGS4 siRNA (siRGS4) for 48 h (n = 3 independent experiments). H and I, representative immunofluorescent images of RGS4 and BACE1 in SH-SY5Y cells transfected with the scrambled control (siNC) or RGS4 siRNA (siRGS4) for 48 h (100× objective lens, 10× eyepieces, the scale bar represents 1 μm, n = 9). J and K, representative Western blots (J) and quantification (K) of BACE1 protein levels in SH-SY5Y cells transfected with the control (siNC) or RGS4 siRNA for 24 h, followed by treatment of dimethyl sulfoxide or translation inhibitor 4EGI-1 (25 μM) for additional 24 h (n = 3 independent experiments). Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. APP, amyloid precursor protein; BACE1, beta-site amyloid precursor protein cleaving enzyme 1; CFT, C-terminal fragment; MGST, microsomal glutathione transferase 3; RGS4, regulator of G-protein signaling 4.
RGS4 mediated the regulation of BACE1 by MGST3
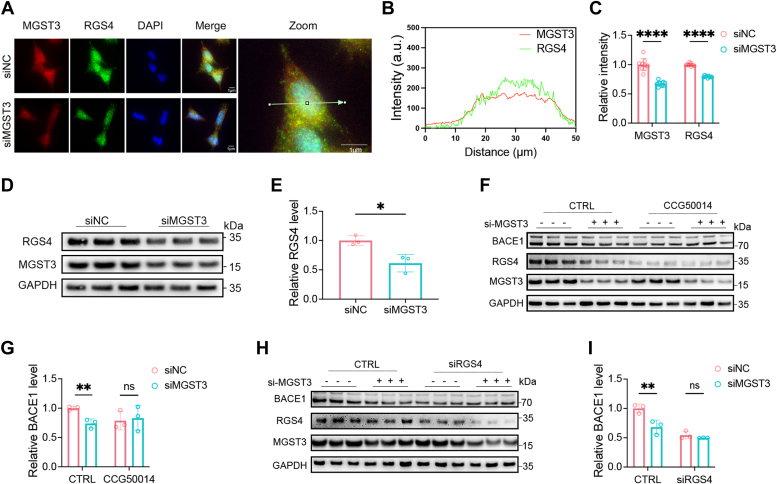
To determine whether RGS4 might contribute to the regulation of BACE1 by MGST3, we assessed the protein expression of RGS4 in SH-SY5Y cells transfected with MGST3 siRNA. As shown in Figure 6, A–C, RGS4 and MGST3 were partially colocalized, with a significant reduction of RGS4 signals in cells transfected with MGST3 siRNA, which was further confirmed by Western blotting analysis (Fig. 6, D and E). Moreover, in cells treated with the RGS4 inhibitor CCG50014 (38), BACE1 proteins level was significantly reduced; and in the presence of CCG50014 no further reduction of BACE1 protein was found by MGST3 KD (Fig. 6, F and G). Accordingly, RGS4 KD reduced BACE1 protein and further prevented MGST3-induced reduction of BACE1 (Fig. 6, H and I). These results indicated that MGST3-induced regulation of BACE1 was dependent on RGS4.
Figure 6.
MGST3-mediated regulation of BACE1 is dependent on RGS4.A–C, analysis of immunofluorescent images of MGST3 (red) and RGS4 (green) in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (100× objective lens, 10× eyepieces, the scale bar represents 1 μm, n = 9). MSGT3 and RGS4 are relatively colocalized in some area (A and B), whereas RGS4 signal is significantly reduced by MSGT3 knockdown (C). D and E, representative Western blots (D) and quantification (E) of RGS4 in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 48 h (n = 3 independent experiments). F and G, representative Western blots (F) and quantification (G) of BACE1 protein levels in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 24 h, followed by treatment of dimethyl sulfoxide or RGS4 inhibitor CCG50014 (30 nM) for 24 h (n = 3 independent experiments). H and I, representative Western blots (H) and quantification (I) of BACE1 protein levels in SH-SY5Y cell scoped with the scrambled control (siNC) or MGST3 siRNA (siMGST3) cotransfected with RGS4 siRNA (siRGS4) for 48 h (n = 3 independent experiments). Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. BACE1, beta-site amyloid precursor protein cleaving enzyme 1; MGST, microsomal glutathione transferase 3; RGS4, regulator of G-protein signaling 4.
AKT signaling was involved in BACE1 regulation by MGST3 and RGS4
We have previously reported that PI3K/AKT signaling plays a key role in BACE1 translation that is regulated by matrix metallopeptidase 13 (39). As AKT is also essential for RGS4 in regulating zebrafish motility and melanoma cell proliferation (40, 41), we speculated that RGS4 could regulate BACE1 translation through AKT activity. As shown in Figure 7, A–D, RGS4 inhibitor CCG50014 and RGS4 KD significantly reduced the level of phosphorylated AKT (p-AKT at Ser473) (42), which was without further alteration when cells were transfected with MGST3 siRNA. LY294002 is a wide-spectrum PI3K inhibitor, which is known to inhibit p-AKT at Ser473 (43). Thus, we assessed the effect of MGST3/RGS4 on BACE1 protein in cells treated with LY294002. As shown in Figure 7, E and F, in LY294002 treated cells, a significant reduction of BACE1 and p-AKT levels were found. Accordingly, the protein level of Aβ42 that is downstream of BACE1 was significantly reduced by LY294002 in SY5Y-APP cells (Fig. 7G). We finally showed that siMGST3-induced reduction of p-AKT and BACE1 was blocked by LY294002 (Fig. 7, H–J), which also prevented the effect of siRGS4 on BACE1 and p-AKT (Fig. 7, K–M). These results indicated that the activity of AKT critically linked MGST3 and the associated RGS4 signaling with BACE1 translation.
Figure 7.
PI3K/AKT pathway is involved in BACE1 regulation by MGST3/RGS4.A and B, representative Western blots (A) and quantification (B) of p-AKT/AKT in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 24 h, followed by treatment of DMSO or CCG50014 (30 nM) for 24 h (n = 3 independent experiments). C and D, representative Western blots (C) and quantification (D) of p-AKT/AKT in SH-SY5Y cells cotransfected with MGST3 siRNA (siMGST3) and RGS4 siRNA (siRGS4) for 48 h (n = 3 independent experiments). E and F, representative Western blots (E) and quantification (F) of BACE1 in SY5Y-APP cells treated with DMSO (1:1000) or PI3K inhibitor LY294002 (10 μM) for 24 h (n = 3 independent experiments). G, culture medium level of Aβ42 in SY5Y-APP cells treated with DMSO or PI3K inhibitor LY294002 (10 μM) for 24 h (n = 3 independent experiments). H–J, representative Western blots (H) and quantification of p-AKT/AKT (I) and BACE1 (J) in SH-SY5Y cells transfected with the scrambled control (siNC) or MGST3 siRNA (siMGST3) for 24 h, followed by treatment of DMSO or PI3K inhibitor LY294002 (10 μM) for 24 h (n = 3 independent experiments). K–M, representative Western blots (K) and quantification of p-AKT/AKT (L) and BACE1 (M) in SH-SY5Y cells transfected with the scrambled control (siNC) or RGS4 siRNA (siRGS4) for 24 h, followed by treatment of DMSO or LY294002 (10 μM) for 24 h (n = 3 independent experiments). Data are expressed as means ± SD. n.s. no significant difference. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Aβ, β-amyloid; APP, amyloid precursor protein; MGST, microsomal glutathione transferase 3; BACE1, beta-site amyloid precursor protein cleaving enzyme 1; DMSO, dimethyl sulfoxide; RGS4, regulator of G-protein signaling 4.
Discussion
In the brain of AD, BACE1 protein rather than mRNA level is consistently increased (44), suggesting an important role of posttranscriptional regulation that underlies the pathophysiology. We have previously reported that BACE1 translation is regulated by mitochondrial Tu translation elongation factor, RNA-binding proteins DDX17 and FXR1, and TNFAIP3-interacting protein 2 (45, 46, 47, 48). In the present study, the role of MGST3 in amyloidogenesis is revealed by that KD of MGST3 inhibits BACE1 translation, which is mediated by downstream RGS4 and AKT signaling. Moreover, the enhanced expression in both animal and cellular model highlights the functional significance of MGST3 in the pathogenesis of AD.
In our study, MGST3 KD does not change ROS level but inhibits apoptosis. On the one hand, ROS can be generated by cellular systems including plasma membrane, mitochondria, and ER (49), whereas antioxidant enzymes including superoxide dismutase, catalase and GSH peroxidase, and nonenzymatic antioxidants such as GSH, cooperatively work against oxidative state (50). In our study, several ROS-related genes are differentially altered by MGST3 KD, which include GGT5 that are downregulated, and NQ01, OSGIN1, and GDF15 that are upregulated (Fig. 4). Previous studies demonstrate that GGT5 and NQ01 reduces ROS (51, 52), whereas OSGIN1 elevates ROS level (53). Interestingly, the effect of GDF15 on ROS is bidirectional depending on cell types (54, 55, 56). Although it is reported that LTC4 triggers ROS production (22), the DEGs by MGST3 could collectively maintain a relatively stable ROS level in SH-SY5Y cells. On the other hand, apoptosis can be induced by not only ROS but also other pathways including ER stress and mitochondrial damage (57, 58). In our study, siMGST3 reduces the protein level of caspase12, suggesting an involvement of ER stress (26). Moreover, the effect of MGST3 on apoptosis measured by caspase3 protein level is attenuated by CysLTRs inhibitor BAY u9773 (Fig. 3, B and C), supporting the LTC4S activity of MGST3 in association with CysLTs generation. In line with this, an elevation of MGST3 and CysLTs protein level is with the increased expression of caspase3 (12). Interestingly, two independent studies demonstrate that human but not rat MGST3 exhibits the catalytic property of LTC4 synthetase (59, 60). Although the potential mechanisms underlying this discrepancy are currently unclear, the function of MGST3 is tested in human cells in the present study. It is tempting to speculate that MGST3 regulates apoptosis though its catalytic activity.
The present study reveals that MGST3 regulates BACE1 expression via a translational mechanism, as revealed by that translation inhibitors 4EGI-1 block the effect of MGST3 on BACE1 protein (Fig. 3, G and H). We also show that a transcription mechanism is unlikely, as there is no statistical difference on BACE1 mRNA level by MGST3 KD, and inhibition of CysLTR that is known to activate NF-κB signaling which in turn promotes BACE1 transcription (61), does not prevent MGST3 KD-induced reduction of BACE1 protein (Fig. 3, A–D). Moreover, Golgi-localized γ-ear–containing ARF-binding protein 3 is an adaptor protein that promotes BACE1 trafficking and lysosomal degradation (62). It is reported that caspase3 stabilizes BACE1 protein by cleaving Golgi-localized γ-ear–containing ARF-binding protein 3 (63). As caspase3 expression is changed by MGST3 in our study, it could be possible that MGST3 might regulate BACE1 protein degradation through lysosome. However, lysosomal inhibitor Baf A1 fails to prevent MGST3-induced reduction of BACE1 (Fig. 3, E and F). In line with the role of MGST3 in translation, the downstream signal protein RGS4 also regulates BACE1 protein via a translation mechanism (Fig. 5, J and K). It is worth noting that the reduction of Aβ level is not as overt as that of BACE1 (Fig. 1L). In sporadic AD, Aβ accumulates slowly and continuously within a range of 15 to 20 years (64), which could be attributed to the fact that BACE1 binds to APP with low affinity leading to Aβ generation with relatively low efficiency (65). In our study, the cell model overexpresses human WT APP695, we speculate that BACE1 inhibition might alter Aβ level to a less extent in cells within 48 h. Nonetheless, β-CTF level is also significantly reduced by siMGST3 (Fig. 1, J and K), which is more relevant to neuronal death in the brain of AD patients (66). The importance of MGST3 in AD could involve mechanisms not limited to Aβ.
RGS4 serves as an important regulator in G protein–coupled receptor (GPCR) signaling (67, 68). In the brain, RGS4 blocks opioid release and long-term depression via metabolic glutamate receptor mGluR5 (69), and upregulation of RGS4 contributes to the development of Parkinson disease by inhibiting autoreceptor function in interneurons (70). Conversely, RGS4 gene deficits or polymorphism is associated with schizophrenia (71, 72), suggesting a diversified function of RGS4 in the central nervous system. In our study, through RNA-seq we identify that RGS4 is one of the important DEGs downstream of MGST3. We also provide evidence that RGS4 directly controls amyloidogenesis by regulating BACE1 and corresponding CTFs/Aβ levels (Fig. 5). As pharmacological and molecular inhibition of RGS4 prevents MSGT3-induced regulation BACE1 (Fig. 6, F–I), it is tempting to speculate that RGS4 mediates the effect of MGST3 on BACE1.
AKT is a central mediator that connects upstream growth factors and GPCRs with a plethora of downstream effectors in almost every organ system (73). A potential role of AKT in RGS4 signaling is demonstrated by that RGS4 regulates brain-derived nerve growth factor expression (74), and that RGS4 interacts with Gα-protein involved in GPCR cycle (34), resulting in an altered AKT activity (75, 76). In our study, inhibition of RGS4 leads to a reduced level of p-AKT, which is in line with prior reports (40, 77). Moreover, MGST3-mediated reduction of p-AKT and BACE1 protein is blocked by CCG50014 and siRGS4, indicating that RGS4 mediates the effect of MGST3 on AKT. Indeed, AKT signaling regulates cap-dependent translation in eukaryotes by acting on the formation of translation–initiation complexes (78, 79). As PI3K-AKT signaling also contributes to the regulation of BACE1 translation and Aβ (39, 80), and PI3K inhibitor attenuates MGST3/RGS4-mediated regulation of BACE1 (Fig. 7), we conclude that PI3K–AKT pathway is critical for BACE1 regulation by MGST3 and RGS4.
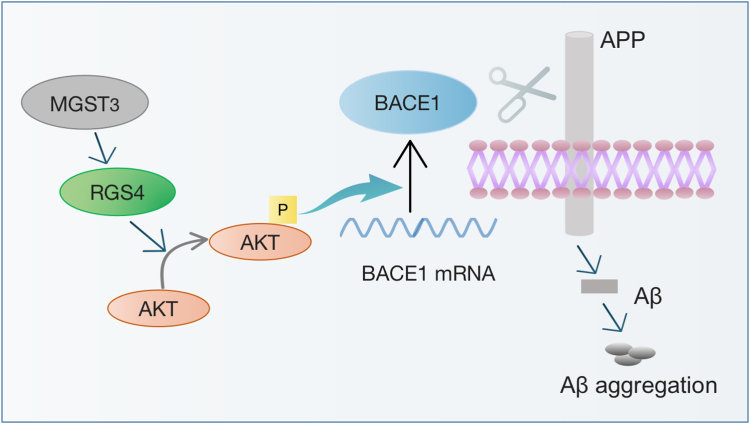
We propose a model which MGST3 regulates BACE1 expression. The enhanced MGST3 protein in the hippocampus of AD promotes RGS4 expression, which enhances BACE1 translation via p-AKT signaling, leading to an enhanced APP processing and Aβ generation (Fig. 8).
Figure 8.
Schematic diagram depicts that MGST3 regulates BACE1 translation through RGS4–AKT pathway. BACE1, beta-site amyloid precursor protein cleaving enzyme 1; MGST, microsomal glutathione transferase 3; RGS, regulator of G-protein signaling.
Experimental procedures
Animal model
All mouse generation, husbandry, and experimental procedures were approved by the Commission of Chongqing Medical University for ethics of experiments on animals, in accordance with international standards. APP/PS1 mice expressing Swedish APP and Presenilin1 delta exon 9 mutations (APP/PS1, RRID: MMRRC_034832-JAX) were purchased from the Model Animal Research Centre of Nanjing University. All animals were feed in 12 h light/12 h dark cycles with free access to food and water in the Animal Center of Chongqing Medical University.
Antibodies and reagents
Antibodies against MGST3 (ab192254, 1:5000 for Western blotting), ADAM10 (ab1997, 1:1000), BACE1 (ab2077, 1:1000 for Western blotting, 1:100 for immunofluorescence),and LC3B(ab48394, 1:1000)were purchased from Abcam. MGST3 antibody (PA5-97969, 1:100 for immunofluorescence) was purchased from Thermo Fisher Scientific Co. APP/CTFs (A8717, 1:1000) was purchased from Sigma-Aldrich. Caspase 3 (19677-1-AP, 1:1000), BAX (50599-2-Ig, 1:1000), Bcl2 (12789-1-AP, 1:1000), GAPDH (60003-2-Ig, 1:4000), phospho-AKT (Ser473) (66444-1-Ig, 1:100), and AKT (10176-2-AP, 1:1000) were from Proteintech. Caspase 8 (sc-56070, 1:300) and RGS4 (sc-398348, 1:300 for Western blotting, 1:50 for immunofluorescence) were from Santa Cruz Biotechnology (Santa Cruz). Caspase 12 (160136, 1:500) was from ZEN-BIOSCIENCE.
Cell culture and chemicals
HEK293 cells (RRID: CVCL_0045; American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Cat# 11965092) supplemented with 10% of FBS (HyClone, Cat# SV30087.03). Human neuroblastoma cells (SH-SY5Y, RRID: CVCL_0019; The European Collection of Authenticated Cell Cultures) were cultured in F-12 nutrient medium (Gibco, Cat# 11,220,033) with 10% of fetal bovine serum (Gibco, Cat# 16,140,071). SH-SY5Y stably expresses full-length human APP695 (SY5Y-APP) was created as previously described (48) and were cultured in added extra 200 mg/ml of G418 (Sigma-Aldrich, Cat# N6386). All cells were incubated in an incubator at 37 °C and 5% CO2.
4EGI1 (α-[2-[4-(3,4-Dichlorophenyl)-2-thiazolyl] hydrazinylidene] -2-nitro-benzenepropanoic acid), BAY u9773 [6(R)-(4′-carboxyphenylthio)-5(S)-hydroxy-7(E), 9(E), 11(Z),14(Z)-eicosatetraenoic acid], Baf A1, γ-secretase inhibitor (DAPT), CCG-50014, and LY294002 were purchased from MedChemExpress (Monmouth Junction, Cat#HY-19831, Cat#HY-107069, Cat#HY-100558, Cat#HY-13027, Cat#HY-13509, Cat#HY-10108).
siRNA transfection
HEK-293 cells were transfected with Lipofectamine 2000 Transfection Reagent (Invitrogen, Cat# 11668019), SH-SY5Y, and SY5Y-APP cells were transfected with Lipofectamine 3000 Transfection Reagent (Invitrogen, Cat# L3000015) and with Opti-MEM Reduced Serum Media (Invitrogen, Cat# 31985088) according to the manufacturer's protocol.
The siRNA oligonucleotides for human MGST3 were purchased from GenePharma. The siRNA oligonucleotides for human RGS4 were purchased from Youbia. The nontargeting siRNA was used as the negative control. The following siRNA oligonucleotide sequences were used for human MGST3: #1 5′-GCAUCUUGGUUGGGUUAAATT-3′, #2 5′-GACGAGTTCTTTATGCTTA -3′, #3 5′-GCATCTTGGTTGGGTTAAA-3′ (81); RGS4: 5′-CCUCAAGUCUCGAUUCUAU-3′ and 5′-GAAGGAGCCAAGAGUUCA-3′ (82); negative control: 5′-UUCUCCGAACGUGUCACGUTT -3′.
Western blotting
Animal tissues and culture cells were homogenized in ice-cold radio immunoprecipitation assay lysis buffer (1% of Triton X-100, 0.5% of sodium deoxycholate, 0.1% of SDS, 150 mM of NaCl, 1 mM of EDTA, and 50 mM of Tris) supplemented with protease inhibitors (Beyotime, Cat# WB-0181) and phosphatase inhibitors mixture (Beyotime, Cat# P1081), and then centrifuged at 13,000 rpm for 20 min at 4 °C. Bicinchoninic acid protein assay kit (Beyotime, Cat# P0011) was used to measure the protein concentrations. Western blotting was performed as previously described (48). Samples were loaded onto 7.5% SDS-PAGE gels (EpiZyme, Cat#PG111) and a specific 16.5% Tris-tricine gel (Solarbio, Cat#P1320) was used for CTFs detection in cells treated with DAPT (250 nM) for 24 h (83, 84). The separated proteins were transferred to 0.22 μm polyvinylidene fluoride membranes (Bio-Rad, Cat# 162-0177). The specific protein bands were visualized using electrochemiluminescence reagent (Beyotime, Cat# P0018M) by Fusion FX5 image analysis system (Vilber Lourmat). Relative protein intensities were calculated by Quantity One software (Bio-Rad, https://www.bio-rad.com) normalized to GAPDH.
Enzyme-linked immunosorbent assay
SY5Y-APP cells were seeded in a 96-well plate at a density of 5000 cells and cultured for 24 h, followed by incubation with siNC or siMGST3 for further 48 h. Culture medium were collected and the level of Aβ42 were measured by the ELISA kits according to the manufacturer's specification (Elabscience, Cat# E-EL-H0543).
Flow cytometric assay
Reactive oxygen species assay kit (Beyotime, Cat#S0033S) was used to determine ROS in living cells. ROSup was used as the positive control wells half an hour in advance. After removing serum-containing medium, cells were added with 2,7-dichlorodihydrofluorescein diacetate (no blank control) at 1:1000 and incubated at 37 °C for 30 min. Cells were then washed three times in PBS, digested by 0.25% of trypsin, centrifugated with high-speed at 4 °C for 5 min, and resuspended in PBS with propidium iodide (Beyotime, Cat#ST511, 1:100). Fluorescence was detected at 488/525 nm.
Cell viability assay
A CCK-8 cell counting kit (MedChem Express) was used. SH-SY5Y cells were seeded in a 96-well plate at a density of 5000 cells and cultured for 24 h, followed by incubation with siNC or siMGST3 for further 48 h. The CCK-8 solution was then added to culture medium and incubated at 37 °C for 1 h, and the absorbance at 450 nm was determined by a Spectra Max 340 PC (Molecular Devices).
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the Total RNA Extractor (Trizol) kit (B511311) according to the manufacturer’s protocol, and treated with RNase-free DNase I to remove genomic DNA contamination. The complementary DNA was synthesized by the 5 × HiScript II Select quantitative reverse transcription (qRT) Super Mix II (R233-01-AC, Vazyme, Cat# R233-01) according to the manufacturer's protocol. The primers for human MGST3, BACE1, and GAPDH were as follows: (human MGST3-forward, 5′- ACCTGAAAATGGGCACATCT-3′; reverse,5′-TGGTAAACACCTCCAACAGCTAGA-3′ (85). BACE1-forward: 5′- CCGGCGGGAGTGGTATTATGAAGT-3’; R: GATGGTGATGCGGAAGGACTGATT. GAPD-forward, 5′- CCTCTGACTTCAACAGCGAC-3′; reverse, 5′- CCTCTTGTGCTCTTGCTGG-3′). AceQ qPCR SYBR Green Master Mix (Q111-02, Vazyme, Cat# Q111-02) was used to perform the qRT-PCR reactions. The qRT-PCR reaction mixture (20 μl) consisted of 10 μl of SYBR, 5.2 μl of nuclease-free water, 0.4 μl of each primer, and 4 μl of diluted complementary DNA. Reactions were performed according to the following steps: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 34 s; the melting curve was run after RT-PCR. The Ct value for each sample was calculated, and the relative mRNA level of BACE1 was normalized to that of GAPDH.
RNA-seq assay
SH-SY5Y cells were treated with control or MGST3 siRNA for 48 h and then collected to extract total RNA using the Total RNA Extractor(Trizol)kit (B511311, Sangon). A total amount of 1 μg RNA was used for RNA sampling. Sequencing libraries were generated by using VAHTSTM mRNA-seq V2 Library Prep Kit for Illumina. Raw reads were filtered by Trimmomatic (version 0.36) and clean reads mapped to reference genome by HISAT2 (version 2.0) with default parameters. Alignment results were analyzed by RSeQC (version 2.6.1), and the homogeneity distribution and the genome structure by Qualimap (version 2.2.1). BEDTools (version 2.26.0) was used to statistical analysis the gene coverage ratio. DESeq2 (version 1.12.4) was used to determine DEGs between samples.
Immunofluorescence
SH-SY5Y cells were seeded in confocal dishes and transfected with siRNA of control, MGST3, or RGS4 for 48 h. The cells were washed three times with PBS, fixed at 4% paraformaldehyde at 37 °C for 30 min, washed again, permeabilized with 0.3% Triton X-100 at room temperature for 10 min, washed, and then blocked with 10% donkey serum at 37 °C for 30 min. Cells were incubated cells with the primary antibody against MGST3 (1:100), RGS4 (1:50), and BACE1 (1:100) in PBS at 4 °C for 18 h. Cells were then washed with PBS for three times and incubated with IFKine green donkey anti-goat IgG (H + L) (1:200, A24231) (Abbkine) and IFKine red donkey anti-rabbit IgG (H + L) (1:200, A24421) (Abbkine) at 37 °C for 1 h. After washing, cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole Fluoromount-G (Southern Biotech, Cat# 0100-20). Images were acquired using confocal microscope (Leica TCS SP8 X).
Statistical analysis
All experiments were replicated at least three times. All data were obtained from at least three independent replicate experiments and were presented as mean ± SD. Data were statistically analyzed by GraphPad Prism version 9.3.0 (GraphPad Software, https://www.graphpad-prism.cn/?c=p&a=shoplist). Comparisons between two groups were analyzed by unpaired Student’s t test. Multiple comparisons were made using one-way and two-way ANOVAs. The differences were statistically significant when p <0.05.
Data availability
The data that support the findings of this study are included within the article and its supporting information.
Supporting information
This article contains supporting information.
Conflict of interests
The authors declare that they have no conflicts of with the contents of this article.
Acknowledgments
Author contributions
Y. P., J. Y., F. X., and G. C. methodology; Y. P. and J. Y. investigation; Y. P., J. Y., and X. C. formal analysis; Q. P., C. L., L. W., X. X., and X. C. supervision; Y. P. and G. C. writing–original draft; Y. P. and G. C. conceptualization; Y. P., J. Y., C. L., L. W., and X. X. software; J. Y., L. W., and F. X. resources; J. Y. visualization; Q. P. project administration; Q. P., F. X., and G. C. validation; C. L. data curation; G. C. funding acquisition; G. C. writing–review and editing.
Funding and additional information
This work was supported by NSFC (81971030, 82271461) to G. C.
Reviewed by members of the JBC Editorial Board. Edited by Qi-Qun Tang
Contributor Information
Fei Xiao, Email: feixiao81@126.com.
Guojun Chen, Email: GJChen@hospital.cqmu.edu.cn.
Supporting information
References
- 1.Crous-Bou M., Minguillon C., Gramunt N., Molinuevo J.L. Alzheimer's disease prevention: from risk factors to early intervention. Alzheimers Res. Ther. 2017;9:71. doi: 10.1186/s13195-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 3.Yarns B.C., Holiday K.A., Carlson D.M., Cosgrove C.K., Melrose R.J. Pathophysiology of Alzheimer's disease. Psychiatr. Clin. North Am. 2022;45:663–676. doi: 10.1016/j.psc.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari C., Sorbi S. The complexity of Alzheimer's disease: an evolving puzzle. Physiol. Rev. 2021;101:1047–1081. doi: 10.1152/physrev.00015.2020. [DOI] [PubMed] [Google Scholar]
- 5.Yan R., Vassar R. Targeting the beta secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol. 2014;13:319–329. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katherine R., Sadleir W.A.E., Kaufman R.J., Osten P., Vassar R. Genetic inhibition of phosphorylation of the translation initiation factor eIF2a does not block ab-dependent elevation of BACE1 and APP levels or reduce amyloid pathology in a mouse model of Alzheimer’s disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das B., Yan R. Role of BACE1 in Alzheimer's synaptic function. Transl. Neurodegener. 2017;6:23. doi: 10.1186/s40035-017-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X.Y., Tang S.S., Hu M., Long Y., Li Y.Q., Liao M.X., et al. Leukotriene D4 induces amyloid-beta generation via CysLT(1)R-mediated NF-kappaB pathways in primary neurons. Neurochem. Int. 2013;62:340–347. doi: 10.1016/j.neuint.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Hampel H., Vassar R., De Strooper B., Hardy J., Willem M., Singh N., et al. The β-secretase BACE1 in Alzheimer's disease. Biol. Psychiatry. 2021;89:745–756. doi: 10.1016/j.biopsych.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsson P.J., Morgenstern R., Mancini J., Ford-Hutchinson A., Persson B. Common structural features of MAPEG -- a widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 1999;8:689–692. doi: 10.1110/ps.8.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K., Xin Y., Ymer S.I., Werther G.A., Russo V.C. Subtractive hybridisation screen identifies genes regulated by glucose deprivation in human neuroblastoma cells. Brain Res. 2007;1170:129–139. doi: 10.1016/j.brainres.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Yang H., Dou Y., Zheng X., Tan Y., Cheng J., Li L., et al. Cysteinyl leukotrienes synthesis is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Toxicology. 2011;287:38–45. doi: 10.1016/j.tox.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsson P.J., Mancini J.A., Ford-Hutchinson A.W. Identification and characterization of a novel human microsomal glutathione S-transferase with leukotriene C4 synthase activity and significant sequence identity to 5-lipoxygenase-activating protein and leukotriene C4 synthase. J. Biol. Chem. 1996;271:22203–22210. doi: 10.1074/jbc.271.36.22203. [DOI] [PubMed] [Google Scholar]
- 14.Fetissov S.O., O S., Jakobsson P.-J., Samuelsson B., Haeggström J.Z., Hökfelt T. Expression of microsomal glutathione S-transferase type 3 mRNA in the rat nervous system. Neuroscience. 2002;115:891–897. doi: 10.1016/s0306-4522(02)00411-6. [DOI] [PubMed] [Google Scholar]
- 15.David G., Ashbrook R.W.W., Lu L., Stein J.L., Hibar D.P., Nichols T.E., et al. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. BMC Genomics. 2014;15:850. doi: 10.1186/1471-2164-15-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre S., L B., Reboullet S., Clermont O., Burlet P., Viollet L., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 17.Yuan X.Z., Sun S., Tan C.C., Yu J.T., Tan L. The role of ADAM10 in Alzheimer's disease. J. Alzheimer's Dis. 2017;58:303–322. doi: 10.3233/JAD-170061. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer's disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werz O., Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.O Schröder M.S., Qiu H., Jakobsson P.-J., Haeggström J.Z., Haeggström J.Z. Microsomal glutathione S-transferases: selective up-regulation of leukotriene C4 synthase during lipopolysaccharide-induced pyresis. Cell Mol. Life Sci. 2005;62:87–94. doi: 10.1007/s00018-004-4366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam B.K. Leukotriene C(4) synthase. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:111–116. doi: 10.1016/s0952-3278(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 22.Dvash E., Har-Tal M., Barak S., Meir O., Rubinstein M. Leukotriene C4 is the major trigger of stress-induced oxidative DNA damage. Nat. Commun. 2015;6 doi: 10.1038/ncomms10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin S., Zhang Q.Y., Kang X.M., Wang J.X., Zhao W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010;21:263–268. doi: 10.1093/annonc/mdp499. [DOI] [PubMed] [Google Scholar]
- 24.Sahoo G., Samal D., Khandayataray P., Murthy M.K. A review on caspases: key regulators of biological activities and apoptosis. Mol. Neurobiol. 2023;60:5805–5837. doi: 10.1007/s12035-023-03433-5. [DOI] [PubMed] [Google Scholar]
- 25.Han J.H., Park J., Kang T.B., Lee K.H. Regulation of caspase-8 activity at the crossroads of pro-inflammation and anti-inflammation. Int. J. Mol. Sci. 2021;22:3318. doi: 10.3390/ijms22073318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoneda T., Imaizumi K., Oono K., Yui D., Gomi F., Katayama T., et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 27.Nagata S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 28.Reed J.C. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 29.Wong W.W., Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life. 2008;60:390–397. doi: 10.1002/iub.51. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.Y., Kang M.G., Park J.S., Lee G., Ting A.Y., Rhee H.W. APEX fingerprinting reveals the subcellular localization of proteins of interest. Cell Rep. 2016;15:1837–1847. doi: 10.1016/j.celrep.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 31.Tang S.S., Wang X.Y., Hong H., Long Y., Li Y.Q., Xiang G.Q., et al. Leukotriene D4 induces cognitive impairment through enhancement of CysLT(1) R-mediated amyloid-beta generation in mice. Neuropharmacology. 2013;65:182–192. doi: 10.1016/j.neuropharm.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Tudhope S.R., Cuthbert N.J., Abram T.S., Jennings M.A., Maxey R.J., Thompson A.M., et al. BAY u9773, a novel antagonist of cysteinyl-leukotrienes with activity against two receptor subtypes. Eur. J. Pharmacol. 1994;3:317–323. doi: 10.1016/0014-2999(94)00485-4. [DOI] [PubMed] [Google Scholar]
- 33.De A., Jacobson B.A., Peterson M.S., Jay-Dixon J., Kratzke M.G., Sadiq A.A., et al. 4EGI-1 represses cap-dependent translation and regulates genome-wide translation in malignant pleural mesothelioma. Invest. New Drugs. 2018;36:217–229. doi: 10.1007/s10637-017-0535-z. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien J.B., Wilkinson J.C., Roman D.L. Regulator of G-protein signaling (RGS) proteins as drug targets: progress and future potentials. J. Biol. Chem. 2019;294:18571–18585. doi: 10.1074/jbc.REV119.007060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darmanis S., Sloan S.A., Zhang Y., Enge M., Caneda C., Shuer L.M., et al. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahlers-Dannen K.E., Spicer M.M., Fisher R.A. RGS proteins as critical regulators of motor function and their implications in Parkinson's disease. Mol. Pharmacol. 2020;98:730–738. doi: 10.1124/mol.119.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D., Zhang Y., Qiao R., Kong X., Zhong H., Wang X., et al. Integrated bioinformatics-based identification of diagnostic markers in Alzheimer disease. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.988143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazer L.L., Zhang H., Casey E.M., Husbands S.M., Neubig R.R. A nanomolar-potency small molecule inhibitor of regulator of G-protein signaling proteins. Biochemistry. 2011;50:3181–3192. doi: 10.1021/bi1019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu B.-L., Long Y., Luo W., Yan Z., Lai Y.-J., Zhao L.-G., et al. MMP13 inhibition rescues cognitive decline in Alzheimer transgenic mice via BACE1 regulation. Brain. 2019;142:176–192. doi: 10.1093/brain/awy305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y.C., Scotting P.J., Hsu L.S., Lin S.J., Shih H.Y., Hsieh F.Y., et al. Zebrafish rgs4 is essential for motility and axonogenesis mediated by Akt signaling. Cell Mol. Life Sci. 2013;70:935–950. doi: 10.1007/s00018-012-1178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue X.,W.L., Meng X., Jiao J., Dang N., Dang N. Regulator of G protein signaling 4 inhibits human melanoma cells proliferation and invasion through the PI3K/AKT signaling pathway. Oncotarget. 2017;11:78530–78544. doi: 10.18632/oncotarget.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risso G., Blaustein M., Pozzi B., Mammi P., Srebrow A. Akt/PKB: one kinase, many modifications. Biochem. J. 2015;468:203–214. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H., Fan D., Zhou G., Li X., Deng H. Phosphatidylinositol 3-kinase inhibitor(LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010;29:34. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preece P., Virley D.J., Costandi M., Coombes R., Moss S.J., Mudge A.W., et al. Beta-secretase (BACE) and GSK-3 mRNA levels in Alzheimer's disease. Brain Res. Mol. Brain Res. 2003;116:155–158. doi: 10.1016/s0169-328x(03)00233-x. [DOI] [PubMed] [Google Scholar]
- 45.Chen L., Wang L., Zhou G.F., Liu Y., Chen X., Xie X.Y., et al. TNIP2 inhibits amyloidogenesis by regulating the 3'UTR of BACE1: an in vitro study. Neurosci. Lett. 2023;808 doi: 10.1016/j.neulet.2023.137265. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Zhou G., Song L., Wen Q., Xie S., Chen L., et al. DEAD-box helicase 17 promotes amyloidogenesis by regulating BACE1 translation. Brain Sci. 2023;13:745. doi: 10.3390/brainsci13050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong B.-R., Zhou G.-F., Song L., Wen Q.-X., Deng X.-J., Ma Y.-L., et al. TUFM is involved in Alzheimer's disease-like pathologies that are associated with ROS. FASEB J. 2021;35 doi: 10.1096/fj.202002461R. [DOI] [PubMed] [Google Scholar]
- 48.Zhou G.-F., Tang J., Ma Y.-L., Fu X., Liu J.-Y., Yang R.-Z., et al. ARL6IP1 mediates small-molecule-induced alleviation of Alzheimer pathology through FXR1-dependent BACE1 translation initiation. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2220148120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longv. 2016;2016 doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jena A.B., Samal R.R., Bhol N.K., Duttaroy A.K. Cellular Red-Ox system in health and disease: the latest update. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114606. [DOI] [PubMed] [Google Scholar]
- 51.Wei J.R., Dong J., Li L. Cancer-associated fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor growth and drug resistance in lung adenocarcinoma. Aging. 2020;12:13220–13233. doi: 10.18632/aging.103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue W., Wang T., Tian W.J., Pang S.Q., Zhang H.F., Jia W.D. NQO1 mediates lenvatinib resistance by regulating ROS-induced apoptosis in hepatocellular carcinoma. Curr. Med. Sci. 2024;44:168–179. doi: 10.1007/s11596-023-2804-8. [DOI] [PubMed] [Google Scholar]
- 53.Tsai C.H., Lii C.K., Wang T.S., Liu K.L., Chen H.W., Huang C.S., et al. Docosahexaenoic acid promotes the formation of autophagosomes in MCF-7 breast cancer cells through oxidative stress-induced growth inhibitor 1 mediated activation of AMPK/mTOR pathway. Food Chem. Toxicol. 2021;154 doi: 10.1016/j.fct.2021.112318. [DOI] [PubMed] [Google Scholar]
- 54.Park H., Kim C.H., Jeong J.H., Park M., Kim K.S. GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7:9634–9644. doi: 10.18632/oncotarget.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Chang J.T., Lee L.Y., Fan K.H., Lu Y.C., Li Y.C., et al. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget. 2017;8:1508–1528. doi: 10.18632/oncotarget.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin H., Luo Y., Gong T., Fang H., Li H., Ye G., et al. GDF15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with Nrf2 to maintain redox homeostasis in colorectal cancer. Cell Oncol. (Dordr) 2024 doi: 10.1007/s13402-024-00918-w. [DOI] [PubMed] [Google Scholar]
- 57.Gorman A.M., Healy S.J., Jäger R., Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Gulbins E., Dreschers S., Bock J. Role of mitochondria in apoptosis. Exp. Physiol. 2003;88:85–90. doi: 10.1113/eph8802503. [DOI] [PubMed] [Google Scholar]
- 59.Jakobsson P.J., Mancini J.A., Riendeau D., Ford-Hutchinson A.W. Identification and characterization of a novel microsomal enzyme with glutathione-dependent transferase and peroxidase activities. J. Biol. Chem. 1997;272:22934–22939. doi: 10.1074/jbc.272.36.22934. [DOI] [PubMed] [Google Scholar]
- 60.Schröder O., Sjöström M., Qiu H., Stein J., Jakobsson P.J., Haeggström J.Z. Molecular and catalytic properties of three rat leukotriene C(4) synthase homologs. Biochem. Biophys. Res. Commun. 2003;312:271–276. doi: 10.1016/j.bbrc.2003.10.115. [DOI] [PubMed] [Google Scholar]
- 61.Chen C.H., Zhou W., Liu S., Deng Y., Cai F., Tone M., et al. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer's disease. Int. J. Neuropsychopharmacol. 2012;15:77–90. doi: 10.1017/S1461145711000149. [DOI] [PubMed] [Google Scholar]
- 62.Kang E.L., Cameron A.N., Piazza F., Walker K.R., Tesco G. Ubiquitin regulates GGA3-mediated degradation of BACE1. J. Biol. Chem. 2010;285:24108–24119. doi: 10.1074/jbc.M109.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vassar R. Caspase-3 cleavage of GGA3 stabilizes BACE: implications for Alzheimer's disease. Neuron. 2007;54:671–673. doi: 10.1016/j.neuron.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 64.McDade E., Bateman R.J. Stop Alzheimer's before it starts. Nature. 2017;547:153–155. doi: 10.1038/547153a. [DOI] [PubMed] [Google Scholar]
- 65.Schechter I., Ziv E. Kinetic properties of cathepsin D and BACE 1 indicate the need to search for additional beta-secretase candidate(s) Biol. Chem. 2008;389:313–320. doi: 10.1515/BC.2008.025. [DOI] [PubMed] [Google Scholar]
- 66.Pulina M.V., Hopkins M., Haroutunian V., Greengard P., Bustos V. C99 selectively accumulates in vulnerable neurons in Alzheimer's disease. Alzheimers Dement. 2020;16:273–282. doi: 10.1016/j.jalz.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Geetanjali Bansal K.M.D., Xie Z., Xie Z. R4 RGS proteins regulation of G protein signaling and beyond. Pharmacol. Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neitzel K.L., Hepler J.R. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin. Cell Dev. Biol. 2006;17:383–389. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Gerber K.J., Squires K.E., Hepler J.R. Roles for regulator of G protein signaling proteins in synaptic signaling and plasticity. Mol. Pharmacol. 2016;89:273–286. doi: 10.1124/mol.115.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding J., Guzman J.N., Tkatch T., Chen S., Goldberg J.A., Ebert P.J., et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 71.Xu F.L., Yao J., Wang B.J. Association between RGS4 gene polymorphisms and schizophrenia: a protocol for systematic review and meta-analysis. Medicine. 2021;100 doi: 10.1097/MD.0000000000027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang M.W., Lin Y.J., Chang C.W., Lei F.J., Ho E.P., Liu R.S., et al. RGS4 deficit in prefrontal cortex contributes to the behaviors related to schizophrenia via system x(c)(-)-mediated glutamatergic dysfunction in mice. Theranostics. 2018;8:4781–4794. doi: 10.7150/thno.25189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manning B.D., Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hyun S.A., Ko M.Y., Jang S., Lee B.S., Rho J., Kim K.K., et al. Bisphenol-A impairs synaptic formation and function by RGS4-mediated regulation of BDNF signaling in the cerebral cortex. Dis. Models Mech. 2022;15:dmm049177. doi: 10.1242/dmm.049177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bommakanti R.K., Vinayak S., Simonds W.F. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J. Biol. Chem. 2000;275:38870–38876. doi: 10.1074/jbc.M007403200. [DOI] [PubMed] [Google Scholar]
- 76.Autry A.E., Monteggia L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leone A.M., Errico M., Lin S.L., Cowen D.S., Lione A.M. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J. Neurochem. 2000;75:934–938. doi: 10.1046/j.1471-4159.2000.0750934.x. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Ye Q., She Q.B. New insights into 4E-BP1-regulated translation in cancer progression and metastasis. Cancer Cell Microenviron. 2014;1:e331. doi: 10.14800/ccm.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raught B.,G.A., Gingras A.C. eIF4E activity is regulated at multiple levels. Int. J. Biochem. Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 80.Haugabook S.J., T L., Yager D., Zenk B., Healy B.M., Eckman E.A., et al. Reduction of Abeta accumulation in the Tg2576 animal model of Alzheimer's disease after oral administration of the phosphatidyl-inositol kinase inhibitor wortmannin. FASEB J. 2001;15:16–18. doi: 10.1096/fj.00-0528fje. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Hong R., Du P., Yang D., He M., Wu Q., et al. The metabolic genomic atlas reveals potential drivers and clinically relevant insights into the etiology of esophageal squamous cell carcinoma. Theranostics. 2022;12:6160–6178. doi: 10.7150/thno.70814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patella F., Cutler D.F. RGS4 controls secretion of von Willebrand factor to the subendothelial matrix. J. Cell Sci. 2020;133:jcs247312. doi: 10.1242/jcs.247312. [DOI] [PubMed] [Google Scholar]
- 83.Motoki K., Kume H., Oda A., Tamaoka A., Hosaka A., Kametani F., et al. Neuronal beta-amyloid generation is independent of lipid raft association of beta-secretase BACE1: analysis with a palmitoylation-deficient mutant. Brain Behav. 2012;2:270–282. doi: 10.1002/brb3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez A.E., Munoz V.C., Cavieres V.A., Bustamante H.A., Cornejo V.H., Januario Y.C., et al. Autophagosomes cooperate in the degradation of intracellular C-terminal fragments of the amyloid precursor protein via the MVB/lysosomal pathway. FASEB J. 2017;31:2446–2459. doi: 10.1096/fj.201600713R. [DOI] [PubMed] [Google Scholar]
- 85.Thameem F., Yang X., Permana P.A., Wolford J.K., Bogardus C., Prochazka M. Evaluation of the microsomal glutathione S-transferase 3 (MGST3) locus on 1q23 as a Type 2 diabetes susceptibility gene in Pima Indians. Hum. Genet. 2003;113:353–358. doi: 10.1007/s00439-003-0980-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are included within the article and its supporting information.