Abstract
Habits are familiar behaviors triggered by cues, not outcome predictability, and are insensitive to changes in the environment. They are adaptive under many circumstances but can be considered antecedent to compulsions and intrusive thoughts that drive persistent, potentially maladaptive behavior. Whether compulsive-like and habitual behaviors share neural substrates is still being determined. Here, we investigated mice bred to display inflexible reward-seeking behaviors that are insensitive to action consequences. We found that these mice demonstrate habitual response biases and compulsive-like grooming behavior that was reversible by fluoxetine and ketamine. They also suffer dendritic spine attrition on excitatory neurons in the orbitofrontal cortex (OFC). Nevertheless, synaptic melanocortin 4 receptor (MC4R), a factor implicated in compulsive behavior, is preserved, leading to the hypothesis that Mc4r+ OFC neurons may drive aberrant behaviors. Repeated chemogenetic stimulation of Mc4r+ OFC neurons triggered compulsive and not inflexible or habitual response biases in otherwise typical mice. Thus, Mc4r+ neurons within the OFC appear to drive compulsive-like behavior that is dissociable from habitual behavior. Understanding which neuron populations trigger distinct behaviors may advance efforts to mitigate harmful compulsions.
Keywords: devaluation, habitual, orbital, reward, omission
Introduction
Obsessive compulsive disorder (OCD) has a lifetime prevalence of 2-3% in American adults [1, 2]. OCD can clinically manifest as intrusive recurring thoughts, known as obsessions, and compulsions, which are repetitive physical actions [3]. The orbitofrontal cortex (OFC) is hyperactive in patients with OCD, both at baseline and upon symptom provocation [4-6]. Further, one of the most consistent findings in structural imaging studies is smaller OFC volume in OCD [7, 8], consistent with synaptic maker attrition [9] and cognitive inflexibility in reversal learning tasks classically associated with the OFC [10, 11].
Habitual behavior is defined as routinized actions that are insensitive to changes in: 1) goal features and value, and 2) the consequences of one’s actions [12, 13]. Habitual behaviors are antithetical to goal-directed actions, which instead will flexibly change if reward values change, or if actions fail to result in desired consequences. Habits allow cognitive resources to be allocated to other activities while one completes behaviors that have been repeatedly reinforced in the past. However, prominent theories of compulsive disorders posit that compulsion involves a loss of control over habits, leading to persistent and maladaptive behaviors [12, 14, 15]. Stimulation of excitatory OFC neurons causes mice to favor inflexible and habitual action strategies at the expense of seeking goals [16-18] and also induces compulsive-like behavior [19]. Nevertheless, whether habit- and compulsive-like behavior have shared molecular/cellular substrates remains unclear.
A common strategy for investigating mechanisms of compulsive behavior involves mutating a high-confidence OCD risk gene in rodents and then examining behavior. Here, we inversed this strategy by instead first identifying genetically intact mice that favor inflexible reward-seeking action strategies and then investigating neurobehavioral correlates in their offspring, which also favored inflexible action strategies. We found that such experimentally bred mice are prone to using habitual action strategies, as assessed in the classical reinforcer devaluation task, and they demonstrate compulsive-like behavior that is ameliorated by fluoxetine and ketamine, first-line and experimental treatments for OCD [20-22], respectively. We next found that these mice suffer attrition of dendritic spines on excitatory OFC neurons, as well as synaptic marker loss. Humans suffering from OCD also suffer synaptic maker attrition in the OFC [9], and yet, also exhibit regional hyper-activity. This insight led us to next investigate melanocortin 4 receptor (MC4R), a G-protein coupled receptor for α-melanocyte stimulating hormone that is predominantly Gs-coupled [23], that can enhance long-term potentiation (depending on cell type [24-26]), and that contributes to drug seeking and compulsive-like behaviors in rodents [27-30]. For instance, elimination of MC4R signaling specifically within cortico-striatal circuits reduces grooming behavior in well-known genetic models for studying compulsive-like behavior [27]. And in experimentally bred mice, an initial analysis indicated that MC4R levels predicted the degree to which mice are biased towards inflexible choice behavior [31]. Synaptic MC4R was preserved in experimentally bred mice, and Mc4r+ OFC neurons triggered compulsive-like behavior, though not inflexible or habitual response biases. We thus establish a novel tool for studying co-morbid compulsive-like and habitual behavior and reveal dissociable control of these processes by a molecularly defined OFC neuron population.
Methods and Materials
Subjects.
Male and female mice were bred in-house from Jackson Laboratory stock. Experimentally bred mice expressed Thy1-driven yellow fluorescent protein (YFP)-H [32] (stock #003782) and originated on a C57BL/6 background. The breeding scheme is described below. Control mice were same age, same sex mice of the same strain. Other mice were Mc4rtm3.1(cre)Lowl/ mutants (Mc4r-2a-Cre) (stock #030759). These mice express viral 2A oligopeptide fused to Cre recombinase (Cre), which replaces the stop codon for the Mc4r gene. This results in the expression of Cre in Mc4r+ cells [33]. Mice were maintained on a mixed C57BL/6J-129S1/SvImJ background. Sex differences were not detected.
Mice were tested at adult postnatal day (P) 56+, except for the characterization of mice used for experimental breeding, which began at P28-31 and is described below. Mice were housed on a 14-h light cycle and provided food and water ad libitum, except during instrumental conditioning tests. During these tests, mice were food restricted to motivate responding for food reinforcement. Young mice were maintained at expected body weights according to Jackson Labs growth curves. Adult mice were maintained at 90-93% of their baseline body weight. All procedures were approved by Emory University IACUC.
Intracranial surgery and viral vectors.
Surgery was performed 3 weeks before behavioral testing to allow for adequate recovery and viral vector expression. Mc4r-2a-Cre mice were anesthetized with ketamine (100 mg/kg, i.p.) and dexmedetomidine (0.5 mg/kg, i.p.). Presurgical and post operative analgesic meloxicam was administered (5 mg/kg, s.c.). Mice were placed in a digitized stereotaxic frame (Stoelting), the scalp was incised with a midsagittal cut and the head was leveled to perform a craniotomy and infuse viral vectors. Infusions were performed using a microliter syringe (Hamilton). Viral vectors containing Cre-dependent excitatory Gq Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) (AAV5-hSyn-DIO-hM3D(Gq)-mCherry; Addgene, 44361) or Cre-dependent mCherry (AAV5-hSyn-DIO-mCherry; Addgene, 50459) were deposited into the OFC. Stereotaxic coordinates were AP+2.6, ML±1.2, DV-2.8; 0.5uL/site over 5 min. Needles were left in place for an additional 5 min following infusion. Once needles were withdrawn, the scalp was sutured and mice were revived with antisedan (3 mg/kg, i.p.).
Instrumental response training.
In illuminated Med-Associates conditioning chambers equipped with 3 nose poke ports and a food delivery magazine, mice were trained to nose poke at 2 of the ports to receive food reinforcers delivered in an independent food magazine. These ports are termed “active”. Interaction with the middle nose poke port was never reinforced; thus, this port is termed “inactive”. Mice were trained on a fixed ratio 1 (FR1) schedule of reinforcement. Mice were trained daily, with 30 pellets available for responding on each active port, for a total of 60 pellets/session. Once 60 pellets were attained, or total time reached 70 min, the session ended.
In initial experiments that identified mice for breeding, 20 mg purified sweet gain-based pellets served as the reinforcer (Bioserv). For subsequent tests to disentangle goal-directed vs. habit-based behavior, each response (left vs. right) resulted in a distinct reinforcer because a hallmark feature of goal-directed behavior is linking an action with the sensory properties of the associated reward. Here, 20 mg sweet grain- vs. chocolate-based pellets were used (Bioserv). These flavors were chosen because mice display no systematic preference for either pellet flavor.
Test of response flexibility.
This procedure is an adaptation of contingency degradation [34], which assesses the capacity of organisms to select actions based on the consequences of their behaviors or instead, utilize inflexible strategies that are insensitive to action consequences. Two 25 min sessions were conducted on 2 consecutive days: First, one active nose poke port was occluded. Pellets were delivered at a rate equivalent to the pellet delivery rate from the prior day, with no relation to responding on the available port. On the following day, the opposite nose poke was occluded and responding at the available port remained reinforced, as it was during training. The port designated to be reinforced vs. non-reinforced and the order of the sessions over the 2-day period was counter-balanced. Mice selected for breeding were trained in these conditions twice. On the final third day, both nose pokes were available during a 10 min choice test conducted in extinction. Flexible action is characterized by preferential responding on the reinforced port, while inflexible responding is reflected by no preference for the reinforced port.
Breeding strategy of experimentally bred mice.
Following behavioral testing, mice were left undisturbed until P56 or older. Mice were then paired for breeding if they had fulfilled 2/3 of the following criteria when tested in the above-described task: 1) >20% of total responses occurred on the inactive port during response training; 2) they failed to inhibit responding during the “non-reinforced” session relative to “reinforced” session; or 3) they failed to prefer the “reinforced” nose poke during the choice test. To maintain the line, every generation of breeding mice was behaviorally tested, and mice were selected for breeding based on these criteria. Mice used for other experiments (other behavioral tests, protein quantification, etc.) were behaviorally naïve. Control mice were age- and strain-matched mice bred in our colony.
Validation of the experimentally bred phenotype.
The offspring of experimentally bred mice and their same-age matched control counterparts were randomly assigned sequential identifiers upon weaning (i.e., 0, 1, 2, 3), and then all mice in a given litter were trained and tested in the test of response flexibility. In our initial report, the first mouse of each sex from each litter was selected, and their response rates were reported to establish the behavioral phenotype of these mice [31]. This practice – arbitrarily selecting one mouse per litter to contribute to a given dataset – avoids litter effects. In this manuscript, the second mouse of each sex from each litter was used for (re)-confirmation that response biases in the offspring of experimentally bred mice were detectable as early as the F2 generation. If both sexes were not available, then 2 mice of the same sex were included. In 1 litter, only 1 mouse was available for inclusion.
We additionally compared response profiles to the F8 generation to establish phenotypic stability across generations. One mouse/sex/litter was selected, again based on random identification assignment at weaning.
Random interval training and reinforcer devaluation.
Satiety-specific devaluation was used to assess whether mice adapted their response strategies based on the value of anticipated outcomes or instead, utilized habit-based strategies that are insensitive to reinforcer value. Once mice acquired responding on an FR1 schedule of reinforcement, they were trained further using a random interval 30 s (RI30) schedule of reinforcement. RI training induces habit-based responding, particularly after extended experience [35]. The 4 additional days of RI30 training used here acted as a subthreshold training level that allows for the resolution to detect early habit formation [16, 17].
Following training and immediately before a choice test, mice were given unrestricted access to one pellet type used in training in a separate environment, thereby diminishing the value of that pellet by virtue of satiety ("devalued" condition). The value of the opposite pellet, with no pre-feeding access, was maintained (“valued” condition). After free feeding for 90 minutes, mice were reintroduced to the operant conditioning chambers for a 10-min choice test conducted under extinction conditions. Mice that adjust their response strategies according to outcome value inhibit responding for the devalued vs. valued pellet, while mice that use habitual response strategies do not [13].
As a control, on a separate day, mice were given unrestricted access to standard laboratory chow, maintaining pellet value, then tested again in a choice test. Response rates for the previously devalued outcome were compared between pellet (“devalued”) vs. chow (“valued”) pre-feeding conditions. Mice that are sensitive to reinforcer value will generate lower response rates following pellet vs. chow pre-feeding.
The pellet type and order of pellet vs. chow pre-feeding sessions were counterbalanced, and there were no differences between groups in food consumed during the pre-feeding phases.
Instrumental omission.
During the omission test, mice must inhibit a previously rewarded behavior in order to receive reward. Here, pellet delivery occurred every 20 s unless the mouse nose poked, which reset the count and delayed pellet delivery [36].
For EB mice, after reinforcer devaluation, responding on one port was reinstated using 2 sessions of additional training on an RI30 schedule of reinforcement (the same schedule they had experienced prior to omission). Next, the mice were tested in a 10 min omission test, and response rates were recorded.
For Mc4r-2a-Cre mice, after the test of response flexibility, responding on one port was reinstated via 3 sessions of additional training on an FR1 schedule of reinforcement (the same schedule they had experienced prior to omission). Next, mice were tested in an extended 30 min session, until extinction of responding.
Extinction.
Mice were trained using an FR1 schedule of reinforcement as above. Then, they were placed in the operant conditioning chambers for 30 mins, and responding was not reinforced. Responding was recorded.
Food intake.
We assessed food intake in Mc4r-2a-Cre mice. We adapted previously established methods [37]. Mice were single housed for 3 days prior to the experiment and given ad libitum standard chow and water. Baseline body weight was collected. Over the course of 5 days, body weight and food intake were measured 3 hr after lights on.
Behavioral testing battery.
The following tests were conducted 1/day on consecutive days. Mice in these experiments were naïve prior to testing and tested in the order indicated. Chambers were cleaned with water between mice. The experimenter was blinded to group throughout:
1. Open field test.
Exploration of an open field was tested for 5 min. In a large clean cage (18” x 9.5” x 8.5”), the central zone was demarcated with tape (5” x 5”) on the underside of the transparent floor. Mice were placed in the far corner of the cage. Time mice spent in the central zone of the open field, defined as crossing over the tape, was measured by a single rater.
2. Novelty-suppressed feeding.
Mice were food-restricted for 6 hr prior to testing and were then individually placed in a large clean cage (18” x 9.5” x 8.5”) in a dim room with a high-fat food pellet placed in the center. Mice were placed in the corner of the cage, and latency to approach the food, defined as nasal or oral contact, was recorded by a single rater.
3. Marble burying.
Mice were placed in a large clean cage (15.5” x 13” x 7.5”) with 3 inches of standard laboratory corncob bedding and 20 marbles arranged in a 5 x 4 grid under dim lighting. The number of marbles >50% buried was recorded at min 10, 15, 20 and 30.
4. Locomotor monitoring.
Mice were individually placed in a large clean cage (18” x 9.5” x 8.5”) positioned within customized locomotor monitoring frames equipped with 16 photobeams (Med-Associates). The total number of beam breaks was recorded during the 180-min test.
Stimulated grooming assay.
This assay was adapted from Xu et al. (2013) [27]. After injections described below, mice were quietly transported from the vivarium to the testing room and allowed to habituate for at least 1 hr. The experimenter then gently suspended each mouse momentarily by the tail, just long enough to rapidly spray the mouse 6 times with clean water using a standard laboratory spray bottle positioned roughly 6” from the mouse’s body. Mice were then immediately placed in a large clean cage (15.5” x 13” x 7.5”). Mice were observed every 30 s. for a total of 12 observations (6 min test), and instances of grooming and wet dog shakes were recorded by a blinded rater. A typical mouse will spend most of the time exploring the novel environment, punctuated by brief grooming bouts to remove the water. A mouse displaying compulsive-like behavior will groom more, at the expense of exploring the novel environment. After the test, each mouse was returned to the home cage. Then, the cage was cleaned with water and dried before the next mouse was tested.
Baseline grooming counts were collected by exactly replicating all steps of the procedure, except the trigger on the spray bottle was not depressed (thus, the mice were not sprayed). The two tests (baseline no spray vs. spray test) were conducted on the same day, morning and afternoon, in a counter-balanced fashion. Testing order and time of day did not have statistically detectable effects.
Drug administration and timing.
Drugs were administered in a volume of 1 mL/100 g. Fluoxetine (FLX) or vehicle (veh; saline) was injected daily at 5 mg/kg (i.p., Sigma) for one week. Behavioral testing occurred 24 hr following the final injection. Ketamine (KET) or veh (saline) was injected at 30 mg/kg (i.p., Dechra). Testing occurred 24 hr later. Setmelanotide (SET) or veh (2% DMSO and saline) was injected at 1 mg/kg (i.p., Creative Peptides). Testing occurred 30 min later. In this experiment, each mouse received both SET and veh injections in a counter-balanced order, with 3 days between injections.
Clozapine N-oxide (CNO) was administered at 1 mg/kg (i.p., Sigma) in 2% DMSO and saline daily for the durations indicated in the figure timelines. This dose does not produce detectable CNO metabolites in plasma (unlike higher doses) [38]. Nevertheless, all mice received CNO to equally expose all groups to any unintended consequences of the drug.
For experiments using both KET and CNO, mice were administered KET or veh 24 hr after the final injection of CNO. Testing occurred 24 hr following the KET injection. For investigation of repeated chemogenetic stimulation of Mc4r+ neurons, a preliminary grooming test was conducted before daily CNO injections occurring on days 3 and 4, again with baseline and spray tests counter-balanced. The grooming test was then repeated following the final CNO administration, again as depicted in the figure timelines.
Dendritic spine imaging and reconstruction.
Mice were treated with KET and tested in the spray test described above. After a 7-day washout period, mice were again administered veh or KET, with injections consistent with the original treatment. Twenty-four hr later, mice were sprayed with water – the stimulus that elicited grooming in the spray test – and allowed to rest for 1 hr. Mice were then deeply anesthetized with isoflurane and decapitated. Brains were post fixed in 4% paraformaldehyde (PFA) for 48 hr and then moved to 30% w/v sucrose. Brains were then sectioned into 50 μm coronal sections on a freezing microtome held at −17°C. Sections were mounted and cover slipped with Fluoromount-G™ mounting medium.
YFP in these mice labels layer V cortical pyramidal neurons. Secondary basilar dendritic branches on neurons in the OFC (anterior ventrolateral region) were imaged (6-8 independent segments per mouse) on a spinning disk confocal Leica microscope (VisiTech International). Z-stacks were collected with a 100X 1.4 numerical port objective using a 0.1 μm step size.
The FilamentTracer module in Imaris software (Oxford Instruments, version 8) was used to perform three-dimensional reconstructions of dendrites. Using semi-automated auto-depth function, dendritic spines were identified on segments ranging between 20-30 μm. Classification of mushroom-type spines had a head:neck diameter ratio ≥ 1.1 and head diameter ≥ 0.7 μm. Spines with a head:neck diameter ratio < 1.1 and a length:neck diameter ratio ≥ 2.5 were classified as thin-type or otherwise classified as stubby-type. Dendrites were imaged and reconstructed by a single rater blinded to group.
In situ RNA analysis.
Mice were sprayed with water as if in the spray test and allowed to rest for 1 hr. Mice were then deeply anesthetized with KET/xylazine (100 and 10 mg/kg, i.p.) and trans-cardially perfused with cold PBS and 4% PFA. Next, brains were moved through an increasing sucrose gradient over 3 days (24 hr per solution, 10%, 20%, 30%). Brains were flash frozen and stored at −80°C until sectioning into 12 μm sections using CryoStar NX70 cryostat and stored at −80°C on Superfrost Plus Slides.
Sections were treated as described in the ACD Technical Note (ACD #320535-TN) prior to RNA analysis. In situ RNA analysis was completed with the RNAScope Multiplex Fluorescent v2 kit (ACD #323100) according to the manufacturer’s protocol (ACD #323100-USM). Probes for Snap25 (ACD #516471; Lot 22283A), Mc4r (ACD #319181; Lot 22283C), and Cfos (ACD 316921; Lot 22263A) were used. Images were acquired at 40X magnification, with 0.8 μm step size, and 6 images per z-stack, on a Keyence BZ-X710 microscope. For quantification, analyses were performed using Cell profiler software. The analysis pipeline included background subtracting, intensity thresholding (Otsu method), parent object overlay, relating objects to one another, and automated cell counting. Snap25 was used to identify excitatory cell bodies – the parent objects. Mc4r and Cfos data were calculated as puncta per Snap25+ neuron – that is, how many Mc4r or Cfos transcripts were expressed in each excitatory neuron. Snap25+ cells were also divided by levels of Mc4r, in which case, over 21 puncta were considered “high” expression [39]. Cfos puncta were next calculated in cells expressing “low” vs. “high” levels of Mc4r to determine whether Cfos differed based on Mc4r content. Three to four images from separate sections were quantified. Counts/section were compared between groups. Also, counts on each section were averaged such that each mouse contributed a single value to analyses, and mouse means were compared between groups. These two analytic approaches yielded the same results. The experimenter was blinded to group throughout.
Viral vector validation, c-Fos immunostaining, and quantification.
Mice were allowed to rest for one week with no behavioral analysis or injections. After this period, CNO was administered, and 1 hr later, mice were deeply anesthetized with KET/xylazine (100 and 10 mg/kg, i.p.) and trans-cardially perfused with cold PBS and 4% PFA. Brains were kept in 4% PFA for 24 hr and then moved to 30% w/v sucrose for an additional 24 hr. Brains were then sectioned into 50 μm coronal sections on a freezing microtome held at −20°C.
Sections were blocked for 1.5 hr at room temperature in a solution containing: 0.03% Triton X-100 (Sigma), 2% normal goat serum (NGS), and 1% bovine serum albumin (BSA). Then, sections were incubated at 4°C overnight with the primary antibody solution containing anti-c-Fos (1:1000; Abcam ab190289; Lot 1026805-1), 2% NGS, and 0.03% Triton X-100. The following day, sections were incubated at room temperature for 1 hr in a secondary antibody solution containing Alexa Fluor 488 (1:500; Life Technologies), 2% NGS, and 0.03% Triton X. Sections were mounted, cover slipped, and then imaged using a Keyence BZ-X710 microscope. Regions of the OFC with transduced virus were identified by fluorescence of the mCherry tag. Mice with mistargeted viral infusion sites were not included in analysis.
For c-Fos quantification, images were obtained at 40X magnification using an ROI of fixed size. Uniform exposure parameters were used throughout. Analyses were performed using Cell profiler software. The analysis pipeline included background subtracting, intensity thresholding (Otsu method), and automated cell counting. Three images from three separate sections were averaged to give each mouse a single value. The experimenter was blinded to group throughout.
Synaptoneurosome preparation and immunoblotting.
Mice were anesthetized with isoflurane and decapitated. Brains were flash-frozen, stored at −80°C, and sectioned into 1-mm coronal slices. Sections of the OFC were dissected using a 1-mm tissue core.
For whole-cell western blotting, tissue was homogenized by sonication in lysis buffer (200 μl: 137 mM NaCl, 20 mM tris-HCl (pH = 8), 1% NP-40, 10% glycerol, 1:1000 Protease Phosphatase Inhibitor Cocktail (Cell Signaling 5872S; Lot 19)). Protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). For each sample, 15 μg of total protein was separated by SDS-PAGE on 4-20% gradient Tris-glycine gels (Bio-Rad). Protein was transferred to a PVDF membrane (Bio-Rad), blocked with 5% non-fat dry milk for 1 hr, and incubated overnight at 4°C in PSD95 primary antibody (Cell Signaling 3450S; Lot 5). On the following day, PVDF membranes were incubated in horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:15,000, Cell Signaling 7074S; Lot 28) for 1 hr at room temperature. Pierce ECL chemiluminescence substrate (Thermo Fisher Scientific) was used to assess immunoreactivity, and the ChemiDoc XRS+ Imaging System (Bio-Rad) was used to acquire measurements.
For preparation of synaptoneurosomes and subsequent protein quantification, bilateral OFC punches from 5 mice were pooled to create a single sample with sufficient protein levels. Samples were homogenized using glass Dounce tissue homogenizers in Syn-PER Synaptic Protein Extraction Reagent (Thermo Scientific 87793; Lot WF320895) and 1:100 protease phosphatase inhibitor cocktail. Samples were then spun down to obtain synaptic fractions. Methods for protein quantification then proceeded as for whole-cell preparations, with the exception that total protein was separated by SDS-PAGE on 7.5% Tris-glycine gels (Bio-Rad). Additionally, membranes were incubated for 48 hr in MC4R primary antibody (Abcam ab150419; Lot GR105886-8).
Image Lab Software (Bio-Rad, version 5.0) was used to analyze densitometry values. Each sample was normalized to its respective total protein content. Protein levels were expressed as a fold change compared to control samples or fold change from the extra-synaptic fraction from each membrane replicate. Experimenters blinded to group processed and quantified all samples in triplicate.
Statistical analysis.
Statistical analyses were conducted with GraphPad Prism and SPSS. n values are reported in the figure captions.
For dendritic spine analyses, the spine type ratio for each dendrite was calculated as mushroom spines / thin spines. To examine variation in spine type ratios within a given group and the impact of KET treatment, the coefficient of variation (CE) of each group was calculated:
The mean absolute deviation (MAD), which is the absolute difference between each individual dendrite’s spine type ratio and the average of the group, was calculated by:
Lastly, we calculated the spine type ratio percent change from veh treatment:
Response rates, marbles buried, time in the center of the open field, grooming counts, grooming ratios (after / before repeated treatment), dendritic spine densities, dendritic spine type ratios, dendritic spine ratio MAD, food intake, and Cfos puncta per Mc4r bin were analyzed by ANOVA, with repeated measures when appropriate. In the case of significant interactions, posthoc Tukey’s or paired t-tests were applied, with results indicated graphically. Fig 1C-D, 2F, and S1B depict experiments with several post-hoc comparisons, which were subject to the Benjamini-Hochberg Procedure for correcting for multiple comparisons, with a false discovery rate of 5%. Grooming counts were also compared by linear regression against the preference ratios, referring to the response rate on the reinforced/non-reinforced ports.
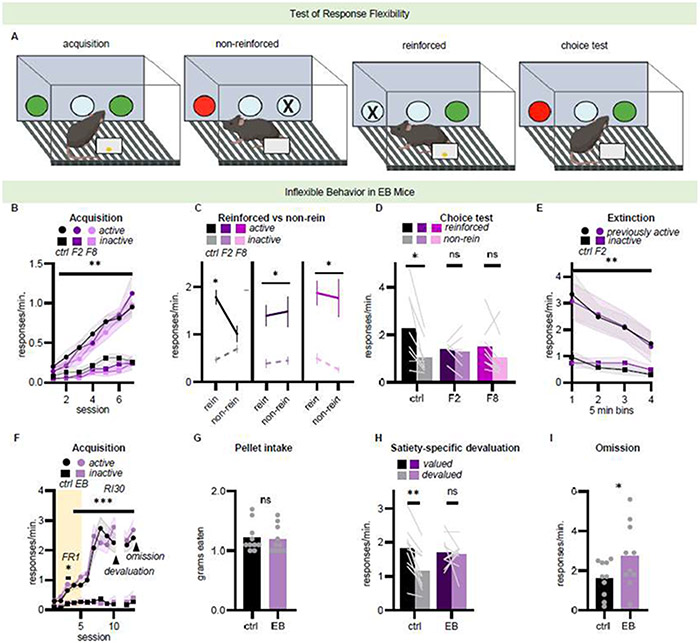
Fig 1. Mice bred to favor inflexible behavior exhibit habitual behavior.
A, Mice are trained to nose poke at two ports for food reinforcement, while one middle port is never reinforced. Next, one port is occluded. Responding is not reinforced, and instead, pellets are delivered noncontingently (non-reinforced). Meanwhile, nose poking on the other port results in food reinforcement, as during training (reinforced). A brief choice test measures the mouse’s ability to update expectancies regarding the consequences of their actions. B, Mice were trained to nose poke, with no group differences. C, When one nose poke was unexpectedly not reinforced, control mice inhibited that action, with response levels similar to the always-inactive port. In contrast, mice from both F2 and F8 generations did not modify responding. D, EB mice were also not able to update action strategies during a subsequent choice test, indicated by no response preference. See supplement for choice test data with the center nose poke. n=7-8 mice/group. E, By contrast, separate EB mice reduced responding when pellets were withheld entirely (extinction). n=10/group. F, Separate mice were trained according to an FR1 schedule of reinforcement (shading), followed by an RI30 schedule. Arrows indicate the timing of pre-feeding devaluation and omission tests. G, Groups did not differ in food consumption during a pre-feeding period prior to the devaluation test. H, Nevertheless, only control mice inhibited responding for the devalued food, while EB mice do not. I, EB mice also generate higher response rates when tested on an omission schedule of reinforcement. n=10/group. Dots and connected symbols represent group means +/− SEMs. Light grey lines and symbols represent individual mice. *p<0.05, **p<0.01, ***p<0.0001, ns non-significant. Asterisks above response curves signify difference from the inactive port. “EB” refers to mice experimentally bred to favor inflexible behavior. “FR” refers to fixed ratio, and “RI30” refers to random interval 30 s.
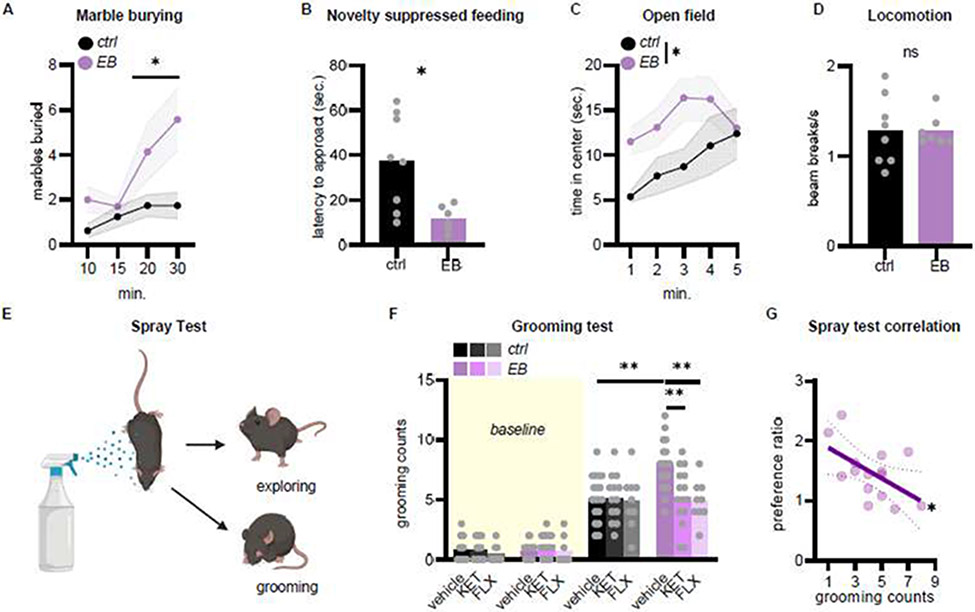
Fig 2. Mice bred to favor inflexible behavior exhibit compulsive-like behavior.
A, EB mice buried more marbles in the marble burying task and B, took less time to approach food in the novelty suppressed feeding task. C, EB mice also spent more time in the center of an open field. D, Meanwhile, there were no differences in locomotion. n=5-8 mice/group. E, In a stimulus-elicited spray test, F, groups did not differ in baseline grooming counts, but water spray elicited grooming to a greater degree in EB mice. FLX and KET normalized grooming scores. n=9-18 mice/group. G, In separate mice, the response preference score (reinforced nose poke / non-reinforced nose poke) negatively correlated with stimulus-elicited grooming, such that lower preference ratios predicted higher grooming counts. n=15. Bars and connected symbols represent group means +/− SEMs. Light grey lines and symbols represent individual mice. *p<0.05, **p<0.01, ns non-significant. “EB” refers to mice experimentally bred to favor inflexible behavior.
Unpaired t-tests or Mann-Whitney U tests were used to compare: latency to approach novel food, beam breaks, western blot density values, percent of Cfos+ and Cfos+/Mc4r+ cells, c-Fos+ cell counts, and % change in spine type ratios. One-sample t-tests were also used to analyze % change in spine type ratios of KET-treated mice relative to veh-treated counterparts, such that scores of 0 refer to no change (here, 1-sample t-tests compared group means to 0). In our final analysis of grooming ratios, planned comparisons were applied based on a priori hypotheses regarding the consequences of repeatedly stimulating Mc4r+ OFC neurons.
Throughout, alpha was set at 0.05 and comparisons were 2-tailed, except in the experiment in which omission followed devaluation, given the hypothesis that mice insensitive to devaluation will also be insensitive to omission schedules. Values falling >2 standard deviations outside the mean were considered outliers and excluded. Based on these parameters, one mouse from the experimentally bred group was excluded from the analysis of novelty suppressed feeding, one mouse from the mCherry veh group was excluded from the analysis of grooming ratios, and one dendrite from the Ctrl group was removed from the analysis of % change upon KET treatment.
Results
Breeding mice for action inflexibility results in habitual and compulsive-like behavior
We capitalized on mice that had been experimentally bred to favor inflexible action strategies when seeking reward [31]. This line of mice was established by first identifying mice that spontaneously failed to modify familiar behaviors when adaptive – that is, they failed to inhibit a food-reinforced action when that action was not rewarded. When bred, their offspring [filial generation (F) 1] generated the same profile, as did their offspring (F2) and so forth. These experimentally bred (EB) mice were originally described in Allen et al [31].
Here, we first further validated the effects of experimental breeding by comparing mice from the F2 and F8 generations to measure phenotypic stability across generations. Mice were trained to nose poke at two recessed ports for food reinforcers. Once they proficiently acquired the responses, one response then unexpectedly ceased to be reinforced and instead, pellets associated with that response were delivered noncontingently (Fig 1A). Thus, the response-outcome contingency associated with one action was violated, which should trigger response inhibition.
We found that all mice, regardless of group, could nose poke for food reinforcers during training and neglect an inactive, never-reinforced port (main effect of day F(6,120)=45.147 p<0.001, main effect of port F(1,120)=149.875 p<0.001, no main effect of group F(2,20)=1.433 p=0.2620, interaction day X port F(6,120)=25.869 p<0.001, no interaction group X day F<1, no interaction group X port F<1; Fig 1B). When one nose poke was then unexpectedly not reinforced, control mice inhibited that response, with response rates comparable to those on the never reinforced port, while the F2 and F8 generations did not (no main effect of session F(1,20)=1.298 p=0.268, main effect of port F(1,20)=77.296 p<0.0001, no main effect of group F(2,20)=0.578 p=0.5699, no interaction session X port F(2,20)=2.513 p=0.129, no interaction group X port F(2,20)=2.292 p=0.127, no interaction group X session F(2,20)=0.899 p=0.423, interaction session X port X group F(2,20)=4.130 p=0.032; Fig 1C). Indeed, the only significant posthoc comparison was between response conditions in the control group; response rates generated by the F2 and F8 generations did not differ from either the reinforced or non-reinforced rate generated by the control group or by each other. This was despite equivalent numbers of pellets delivered across groups (no main effect of group F<1, main effect of session F(1,20)=11.390 p=0.003, no interaction F(2,20)=1.248 p=0.3084; Fig S1A).
Mice responded nearly identically during a subsequent choice test, again with the F2 and F8 groups not modifying their choice behavior based on action contingency (main effect of port F(1,20)=12.490 p=0.0021, no main effect of group F<1, interaction F(2,20)=3.995 p=0.0347; Fig 1D). Again, control mice not only differentiated between the reinforced and non-reinforced response, but they responded on the non-reinforced port at rates comparable to the never active center port; meanwhile, EB mice made no such distinctions (main effect of port F(2,40)=23.466 p<0.0001, no main effect of group F<1, interaction group X port F(4,40)=3.066 p=0.0279; Fig S1B). This was despite intact extinction conditioning – meaning, response suppression when pellets were entirely unavailable (main effect of time bin F(3,54)=6.088 p=0.001, main effect of port F(1,54)=35.492 p<0.0001, no main effect of group F<1, no interaction group X port F<1, no interaction group X bin F<1, interaction port X bin F(3,54)=4.366 p=0.008; Fig 1E). Thus, experimental breeding produced behavioral response biases, such that when rewards are present, mice persist in familiar routines even when they are not explicitly reinforced.
Goal-directed action and habitual behavior differ in two key ways: Goal-directed actions are sensitive to changes in the causal relationship between actions and their outcomes, and they are sensitive to changes in the value of given outcomes. Meanwhile, habitual behavior is insensitive to action consequences and reward value [13]. Poor action flexibility in EB mice resembles insensitivity to action-reward contingencies when organisms execute habits. We thus next tested mice in the most common test for disambiguating goal-directed vs. habitual behavior: the reinforcer devaluation task. Mice were first trained to respond for distinct pellets that were equally preferred (no main effect of pellet F(1,162)=0.622 p=0.441, no interaction group X pellet F<1, Fig S1C). Mice acquired the nose poke responses, without differences between groups (main effect of day F(9,162)=36.932 p<0.001, main effect of port F(1,162)=202.201 p<0.001, no main effect of group F<1, no interaction group X port F<1, no interaction group X day F(1,9)=1.296 p=0.2430, interaction day X port F(9,162)=40.3666 p<0.001; Fig 1F).
Next, one pellet was devalued by allowing mice ad libitum access to the same food prior to test, thus decreasing its value. Groups did not differ in food consumption (t18=0.2839 p=0.7797; Fig 1G). Nevertheless, only control mice responded less for that pellet, while the EB mice did not modify their actions (no main effect group F(1,18)=0.7440 p=0.3997, main effect port F(1,18)=9.356 p=0.0068, interaction F(1,18)=6.906 p=0.017; Fig 1H).
As a control, mice were given unrestricted access to standard laboratory chow, maintaining pellet value. Response rates for the previously devalued outcome were compared between chow (valued) vs. pellet (devalued) pre-feeding conditions. Again, chow intake did not differ between groups (t18=1.204 p=0.2442; Fig S1D). And again, control mice responded less in the devalued condition, while EB mice responded similarly across conditions (no main effect of group F<1, no main effect of port F(1,18)=1.836 p=0.1922, interaction F(1,18)=5.396 p=0.0321; Fig S1E).
We lastly tested mice in an omission task, another classical assay for detecting habitual behavior. Here, mice must refrain from responding to receive food. Nevertheless, EB mice maintain robust response rates, insensitive to contingency (t18=1.940 p=0.0341; Fig 1I). Overall, then, EB mice behave in ways that are consistent with habitual behavior [13].
We next hypothesized that habitual response biases would be accompanied by compulsive-like behavior, referring to repeatedly completing a given behavior, often to an atypical degree. In a marble burying task, EB mice indeed buried more marbles (main effect of group F(1,13)=6.456 p=0.0246, main effect of time F(3,39)=7.854 p=0.0003, interaction F(3,39)=3.129 p=0.0365; Fig 2A), suggesting that they engaged in compulsive-like behavior. However, marble burying can also be considered an anxiety-like behavior. To clarify whether EB mice displayed anxiety-like behavior, we tested them in a novelty-suppressed feeding assay and open field test. In both cases, anxiety-like behavior is inferred when mice resist entering central zones. Instead, EB mice more readily approached food in the novelty-suppressed feeding test (t12=2.854 p=0.0145; Fig 2B) and spent more time in the center of an open field (main effect of group F(1,13)=4.939 p=0.0446, main effect of time F(4,52)=2.781 p=0.0361, no interaction F(4,52)=1.086 p=0.3729; Fig 2C). These behaviors could not obviously be attributed to aberrant locomotor activity (t13=0.00476 p=0.9960; Fig 2D).
Grooming assays have promising translational value in rodent models for studying compulsion [40]. For instance, mice may be sprayed with water and placed in a novel chamber. Mice will engage in bouts of grooming, interspersed by periods of exploration. Mice engaged in compulsive-like behavior will groom beyond a typical duration at the expense of exploration (Fig 2E). We tested EB mice in this assay. As a positive control, some mice were treated with FLX, a primary treatment for obsessive-compulsive disorder in humans, or subanesthetic KET, an experimental treatment with promise for ameliorating compulsive behaviors. We reasoned that any compulsive-like behavior should be resolved by FLX or KET.
Groups did not differ in baseline grooming scores, prior to water spray (no main effect of group F<1, no main effect of treatment F(2,78)=1.598 p=0.2088, no interaction F<1; Fig 2F). Following water spray, the EB mice groomed more than control mice, and excessive grooming was normalized by FLX and KET (no main effect of group F(1,78)=3.803 p=0.0547, main effect of treatment F(2,78)=4.963 p=0.0094, interaction F(2,78)=4.060 p=0.0210; Fig 2F).
Finally, a separate group of EB mice was tested in both instrumental conditioning and grooming tasks. Grooming counts negatively correlated with preference ratios (responses on the reinforced port/non-reinforced port), such that mice with poor response flexibility exhibited more stimulus-elicited grooming (r2=0.2955 p=0.0362; Fig 2G). Thus, experimental breeding for inflexible action strategies produced mice that are also prone to compulsive-like behavior.
Experimental breeding alters OFC neurobiology
We next examined dendritic spines on pyramidal neurons within the OFC of EB mice following a stimulus spray, which elicited compulsive-like behavior. Half of the mice were treated with KET, which ameliorated compulsive-like behavior (Fig 3A-B). EB mice had lower spine densities than control mice (main effect of group F(1,198)=13.940 p=0.0002, no main effect of treatment F<1; Fig 3C-D). KET appeared to mitigate this effect, with an interaction between group and treatment (F(1,198)=5.126 p=0.0247). And while both EB groups had lower densities than control mice, the post-hoc p value between control vs. EB + KET was 0.0136, while the p value was far smaller (p=0.0002) in the absence of KET. This observation led to further analyses.
Fig 3. Aberrant dendritic spine densities and morphologies in mice displaying compulsive-like behavior.
A, Representative coronal section from YFP+ mouse. Arrows: YFP+ neurons in the OFC. B, Experiment timeline. C, Representative dendrites on excitatory OFC neurons and corresponding 3D reconstructions. Scale bar = 2 μm. D, EB mice exhibit lower spine densities than control counterparts. E, Spine type ratios do not differ between EB and control mice; F, however, large variations in spine type ratios in EB mice are exemplified by elevated MAD of spine type ratio, which is normalized by KET. G, KET especially affects spine type ratios in EB mice compared to control mice and compared to 0, no change. n=6-9 mice/group. Bars represent group means, gray dots represent individual dendrites, and black dots represent the mean of each mouse’s dendrites. *p<0.05, ***p<0.001, #p<0.0001 in 1-sample t-test. “EB” refers to mice experimentally bred to favor inflexible behavior.
Dendritic spines can be classified as mushroom (mature) vs. thin (immature) types. Ratios of mushroom:thin spines were typical across groups, with a minority of spine types being mature (mushroom shaped) (no main effect of group F<1, no main effect of treatment F(1,198)=1.969 p=0.1622, no interaction F(1,198)=2.041 p=0.1547; Fig 3E). However, EB mice displayed considerable dendrite-to-dendrite variation in spine profiles, as indicated by the coefficient of variation, and again, this was mitigated by KET (ctrl VEH=70.73%, ctrl KET=88.72%, EB VEH=122.3%, EB KET=75.15%). In other words, dendrites of EB mice were highly variable in terms of whether they contained rich densities of mature or immature spines, and KET normalized dendrite-to-dendrite variation.
This phenomenon of increased variability can be exemplified by a larger difference between each dendrite’s spine type ratio and the mean of the group, the mean absolute deviation (MAD). EB mice had an increased MAD of spine type ratios, indicating greater variability, which was rescued with KET treatment (main effect of treatment F(1,198)=6.417 p=0.0121, no main effect of group F(1,198)=1.881 p=0.1718, interaction F(1,198)=7.631 p=0.0063; Fig 3F).
Given that KET altered grooming behavior in EB mice and not control mice, we next hypothesized that KET would impact spine types in EB mice relative to control mice. We calculated the percent change in spine type ratios in EB mice treated with KET relative to their veh-treated counterparts. KET elicited spine type plasticity (t53=4.910 p<0.0001 compared to 0, no change; Fig 2G). When the same calculation was performed for control mice, KET produced no change in spine type ratios (t46=0.8779 p=0.3846 compared to 0; Fig 3G). Accordingly, the groups also differed when compared with each other (t99=2.168 p=0.0326; Fig 3G), with KET triggering structural plasticity in EB mice.
Altogether, EB mice suffer spine attrition on excitatory neurons in the OFC and considerable variation in dendrite-to-dendrite morphological profiles. KET induced plasticity on OFC dendrites of EB mice, corresponding with the amelioration of compulsive-like grooming – pointing to the OFC as a potential locus driving compulsive-like behavior.
Preservation of MC4R systems in mice displaying compulsive-like behavior
Humans with OCD suffer synaptic marker loss in the OFC [9]. We therefore measured the synaptic marker PSD-95, revealing lower levels in EB mice (consistent with spine loss, t10=2.181 p=0.05; Fig 4A). And yet, humans with OCD also commonly display hyper-activity in the OFC. This insight led us to next investigate MC4R, which is expressed throughout the cortex, including on glutamatergic neurons in the OFC [41], potentiates long-term potentiation, and is implicated in compulsive-like behavior. We measured synaptic MC4R content using synaptoneurosomes, composite particles that contain post-synaptic cell membranes (Fig 4B; Fig S2A). Each sample contained tissues pooled across several mice to ensure sufficient protein content; therefore, this experiment represents 15-20 mice/group. Interestingly, we found no differences between groups (Mann-Whitney U=5.0 p=0.8571; Fig 4C). Altogether, EB mice have lower excitatory synaptic marker and spine presence in the OFC but preserved levels of synaptic MC4R.
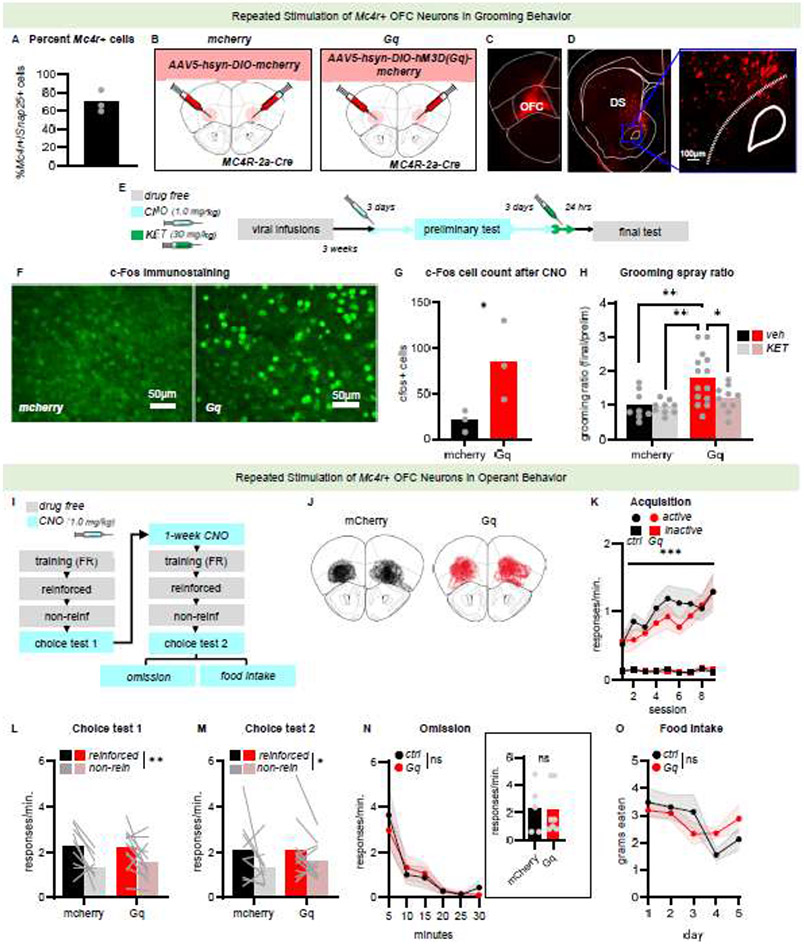
Fig 4. Preservation of MC4R systems in mice displaying compulsive-like behavior.
A, EB mice have decreased levels of PSD95 in the OFC. n=6 mice/group. B, Synaptoneurosomes are composite particles containing the pre- and post-synaptic compartments. C, Despite decreased levels of PSD-95, EB and control mice have equal levels of MC4R in synaptoneurosome fractions (n=3-4 pooled samples totaling 15-20 mice/group). D-E, We next visualized Mc4r and Cfos in excitatory OFC neurons (Snap25+ cells) after a stimulus spray. F, The percent of neurons expressing Cfos did not differ between groups. G, Of the Cfos+ excitatory neurons, the majority also expressed Mc4r. H, When Mc4r+ cells were divided based on low or high numbers of transcripts, the number of Cfos puncta corresponded with expression levels, such that cells with more Mc4r also had more Cfos. n=11-14/group. Bars represent group means. Large grey circles represent individual pooled samples in C, and individual mice in all other graphs. Small, light grey circles represent individual sections. *p<0.05, ns non-significant. “EB” refers to mice experimentally bred to favor inflexible behavior.
Next, we assessed whether Mc4r+ neurons were stimulated by water spray, which spurs compulsive-like grooming (Fig 4D-E). First, we found that the majority of excitatory OFC neurons expressed Cfos upon stimulus spray, with no group differences (t24=0.2523 p=0.8030; Fig 4F). Next, we found that a majority of these Cfos+ cells expressed Mc4r, again with no differences between groups (t24=0.2025 p=0.8412; Fig 4G). Finally, cells with higher expression of Mc4r transcripts also had more Cfos, with no differences between groups (main effect of Mc4r puncta bin F(1,22)=52.842 p<0.0001, no main effect of group F<1, no interaction F<1; Fig 4H). Groups were compared by considering each slide an independent sample. An additional analysis considered each mouse an independent sample, generating the same outcomes (Supplementary Table 1). Thus, Mc4r+ excitatory neurons in the OFC appear responsive to spray in EB mice, despite alterations in other systems.
Control of compulsive-like behavior by molecularly defined OFC neurons
Altogether, EB mice have lower excitatory synaptic marker and spine presence, while immediate-early gene expression in OFC neurons containing Mc4r appears robust. This pattern led us to question whether Mc4r-containing OFC neurons might be responsible for compulsive-like behavior, inflexible choice behavior, and/or habitual behavior. As a goal of using animal models is to reveal biological principles applicable to healthy organisms, we turned to typical mice (i.e., mice not subjected to experimental breeding). We first found that a majority of excitatory OFC neurons contain Mc4r (Fig 5A). We next transduced Mc4r+ OFC neurons with either a control mCherry label or Gq-coupled (stimulatory) chemogenetic receptors (Fig 5B; Fig S2B). We found that labeled Mc4r+ neurons project to the dorsal striatum, important given that cortico-striatal connections are considered central hubs for driving compulsion (Fig 5C-D). They were most prominent in the dorsal and central zones of the striatum, consistent with OFC-to-striatum projection patterns in general [42-44].
Fig 5. Stimulation of Mc4r+ OFC neurons induces compulsive-like behavior but not inflexible or habitual behavior.
A, Of excitatory OFC neurons (labeled by Snap25), the majority also expressed Mc4r. B, Mc4r-2a-Cre mice received bilateral infusions of either a control Cre-dependent mCherry viral vector or a viral vector containing Gq DREADD. C, Representative viral vector infusion in the OFC. D, Terminals from Mc4r+ OFC neurons containing the mCherry reporter were observed in the striatum, as expected. E, Experiment timeline. F, Representative c-Fos after CNO. G, CNO + Gq DREADD increased c-Fos, as expected. n=3/group. H, Repeated chemogenetic stimulation of Mc4r+ neurons potentiated grooming, which was mitigated by KET. Data are represented as fold change from a preliminary test, when groups did not differ (see supplement). n=9-14/group. I, Experiment timeline. J, Spread of viral vector infusion across all mice/group. K, Responses did not differ between groups during training. L, Choice test responses following unexpected non-reinforcement also did not differ between groups. M, Repeated CNO also had no effect. n=9-12. N, Response rates during instrumental omission also did not differ between groups. n=5-7. O, Groups did not differ in ad libitum food intake. n=4-5. Bars and connected symbols represent group means +/− SEMs. Light grey lines or symbols represent individual mice. *p<0.05, **p<0.01, ns non-significant. Asterisks in K signify difference from the inactive port.
Gq-coupled chemogenetic receptors in Mc4r+ OFC neurons induced immediate-early gene expression in the presence of the DREADD ligand CNO, as expected (Mann-Whitney U=0.00, p=0.05; Fig 5E-G). With our DREADD thus validated, mice in behavioral studies were treated daily with CNO for a week, given that repeated stimulation of excitatory OFC neuron terminals induced compulsive-like behavior in a prior report [19]. On the third and fourth day, mice were tested in a preliminary spray test, with or without CNO. Then, mice were tested again after treatment. Grooming counts on the final spray test were divided by those generated during the initial preliminary test to assess the potentiation of grooming with stimulation of Mcr4+ neurons. Scores of 1 refer to no change, whereas scores >1 reflect a potentiation. As a positive control, half of the mice were treated with KET, with the hypothesis that KET would mitigate compulsive-like grooming.
Chemogenetic stimulation and KET both impacted grooming scores (main effect of group F(1,37)=9.8442 p=0.0033, main effect of treatment F(1,37)=3.9092 p=0.0555, interaction F(1,37)=2.7854 p=0.1036; Fig 5H). Planned comparisons indicated that stimulating Mc4r+ OFC neurons increased grooming scores, which was normalized by KET. Raw grooming counts did not differ between groups at either test, yet, mice with chemogenetic stimulation demonstrate elevated grooming counts in the final test compared to their respective grooming counts observed in the preliminary test (main effect of group F<1, main effect of test F(1,37)=3.331 p=0.761, interaction group X test F(3,37)=3.946 p=0.0154; Fig S2C). There were no effects on baseline grooming in the absence of water spray, indicating that effects were specific to stimulus-elicited grooming (all Fs<1; Fig S2D). MC4R is largely Gs-coupled in excitatory neurons [45], suggesting that ligand binding should have similar consequences relative to chemogenetic stimulation of these cells, as previously reported in both mice [27] and rats [30].
To then investigate whether the same cell population controlled reward-seeking behavior, mice expressing Gq-DREADDs in Mc4r+ neurons were trained to respond in operant conditioning chambers for food. Training occurred in the absence of CNO and without group differences (main effect of day F(8,152)=7.892 p<0.001, main effect of port F(1,152)=67.757 p<0.001, interaction day X port F(8,152)=8.605 p<0.001, no main effect of group F(1,19)=0.461 p=0.5050, no interaction group X day F(8,152)=1.009 p=0.4320, no interaction group X port F<1; Fig 5I-K). Stimulating Mc4r+ neurons when one behavior was unexpectedly not reinforced had no impact – mice flexibly preferred the reinforced over non-reinforced response (choice test 1: main effect of port F(1,19)=13.010 p=0.0019, no main effect of group F<1, no interaction F<1; Fig 5L). Mice were then repeatedly stimulated, as in our stimulus-elicited grooming task, but again, they responded flexibly when expectations were violated (choice test 2: main effect of port F(1,19)=4.925 p=0.0388, no main effect of group F<1, no interaction F<1; Fig 5M).
We next tested mice in an omission task. Response rates after 10 min did not differ between groups (t10=0.4224 p=0.6817; inset Fig 5N), unlike with EB mice in Fig 1. With more time, responding nearly extinguished, again with no group differences (main effect of time F(5,50)=12.70 p<0.001, no main effect of group F<1, no interaction F<1; Fig 5N). We also confirmed that food intake was unaffected by stimulating Mc4r+ neurons across multiple days (main effect of day F(4,28)=3.684 p=0.0157, no main effect of group F<1, no interaction F(4,28)=1.466 p=0.2392; Fig 5O). Thus, chemogenetically stimulating Mc4r+ neurons in the OFC potentiates stimulus-elicited grooming, but not obviously inflexible or habitual choice or ad libitum feeding.
Discussion
This investigation was motivated by theories that habitual behaviors are antecedent to compulsions [12, 14, 15]. Implicit in this notion is that habitual and compulsive-like behaviors might have shared neurobiological substrates. To investigate this possibility, we utilized a line of mice bred to exhibit inflexible choice strategies, revealing that these mice demonstrate habitual and compulsive-like behavior and neurobiological features similar to individuals suffering from OCD. Although this mouse line exhibits both habitual and compulsive-like behaviors, our investigation ultimately revealed dissociable control of habitual and compulsive-like behavior by Mc4r+ neurons in the OFC, with this molecularly defined cell population controlling compulsive-like behavior.
Mice experimentally bred for action inflexibility exhibit habitual and compulsive-like behavior
We utilized mice bred to favor inflexible reward-seeking strategies. Mice were trained to nose poke for food at two nose poke ports in operant conditioning chambers. We then violated the relationship between the action of nose poking on one port and receiving a food reward by delivering pellets noncontingently. Typical mice will update their action strategies and decrease non-reinforced responding. EB mice were generated by breeding mice that spontaneously failed to update action strategies, creating offspring with the same behavioral propensities [31]. Here, we first confirmed that this behavioral phenotype is consistent across generations.
Insensitivity to action-consequence relationships is a key feature of habitual behavior [13]. We thus next tested mice in a reinforcer devaluation task, the most common assay by which to disambiguate goal seeking vs. habitual behavior in rodents. In this case, food reward is devalued by allowing mice ad libitum access to the same food prior to test, thus decreasing its value. Control mice responded less for that reward, relative to a different reward, and relative to a condition when they were pre-fed with another food. Meanwhile, EB responded similarly across conditions. Overall, then, EB mice behave in ways that are consistent with habitual behavior: insensitive to action contingency and outcome value [13].
We next found that EB mice display excessive marble burying and grooming – behaviors consistent with compulsive-like behavior. And while marble burying can be interpreted as anxiety-like behavior [46], EB mice rapidly approached a novel food in the novelty-suppressed feeding task and spent excessive time in the center of an open field, antithetical to anxiety-like behavior. Instead, we view these behaviors as potentially risky or compulsive-like – exploratory actions that are atypical, above “normal” levels [47]. To further investigate the notion that excessive grooming reflected compulsive-like behavior, we confirmed that it could be normalized by FLX and subanesthetic KET, first-line and experimental treatments for OCD, respectively. Altogether, mice experimentally bred to favor inflexible modes of response and habitual response strategies may offer face, construct, and predictive validity for investigating compulsive-like behavior.
OFC neurobiology associates with compulsive-like behavior
We investigated neurobiological consequences of experimental breeding, focusing on the OFC, first imaging layer V neurons – those that send and receive the bulk of long-range connections in the cortex. Dendritic spines were lost in habit-prone EB mice. Interestingly, we did not detect spine loss in a prior study conducted in behaviorally naïve EB mice [31], whereas images here were collected following water spray, a mild stressor used to elicit compulsive-like grooming. Acute stressors cause modest spine loss on layer V OFC neurons [48]; potentially, EB mice are more prone to stressor-related neurosequelae, which could be investigated in the future.
Spines can be categorized as long and thin (functionally variable and immature) or mushroom in shape (mature). Typically, the majority of spines on a given dendrite are thin, but this profile was quite variable in EB mice, which could disorganize input fidelity on local neuron ensembles that are necessary for flexible action [17]. For instance, inputs from the amygdala and ventral hippocampus are necessary for flexible action; in their absence, mice instead defer to routinized behaviors [17] or fail to adhere to flexible, goal-sensitive action strategies, respectively [49].
We included in this investigation KET-treated mice, given intense interest in the capacity of subanesthetic KET to confer therapeutic-like effects in OCD and other psychiatric illnesses. In experiments with mice, KET restored spine densities on excitatory neurons in the medial prefrontal cortex following glucocorticoid excess, and these modifications were necessary for sustained remission of depressive-like symptoms [50]. Here, KET normalized variability in spine type compositions in EB mice, concordant with the amelioration of compulsive-like grooming. Notably, FLX, which also ameliorated compulsive-like behavior here, also impacts dendritic spine structure and densities, and these actions are thought to contribute to antidepressant and anti-compulsion properties [51]. Thus, actions on spine morphologies may explain why chemically distinct therapeutics can have common behavior outcomes.
Dendritic spine attrition in mice displaying compulsive-like behavior appears consistent with structural imaging literature revealing smaller OFC volume and loss of synaptic markers in OCD [9]. A challenge, though, is integrating these patterns with evidence of OFC hyperactivity in OCD [6]. This apparent discrepancy could be explained in several ways; for instance, some but not all cell types may account for neural activity that ultimately controls behavior. One candidate cell type is those expressing MC4R, a high-affinity receptor for α-melanocyte-stimulating hormone. MC4R is classically studied for its role in hypothalamic control of feeding and metabolism, and mutations in MC4R remain the most common monogenic cause of obesity in humans [52, 53]. Early observations of grooming upon MC4R stimulation [30, 54] led to the recent discovery that deletion of Mc4r within OFC-striatal circuits ameliorates excessive grooming in Sapap3−/− mice, a common model for studying OCD, and reduces synaptic strength within this circuit [27]. And in EB mice, striatal MC4R levels predict the degree to which mice are biased towards inflexible choice behavior, with more MC4R associated with inflexible choice [31].
Despite loss of dendritic spine densities and excitatory synapse markers in the OFC, levels of MC4R within synaptic compartments of EB mice were preserved. Further, after a water spray, the majority of excitatory Mc4r+ neurons within the OFC expressed Cfos, suggesting that MC4R-containing excitatory neurons are stimulated by the spray. Finally, we found that Mc4r+ OFC neurons terminate in the striatum, relevant because activity within cortico-striatal circuits is overwhelmingly implicated in OCD symptomatology [55, 56]. These insights ultimately led to the discovery that chemogenetic stimulation of Mc4r+ OFC neurons triggered excessive grooming in otherwise normal mice. Optogenetic stimulation of striatal projecting OFC neurons – in general – similarly induces compulsive-like grooming [19]; our findings suggest that Mc4r+ OFC neurons are sufficient to drive this phenomenon.
Interestingly, neither acute nor repeated stimulation of Mc4r+ neurons induced inflexible choice or habitual behavior, despite impact in the water spray test. Why might this be? Water spray is an acute stressor in rodents, and grooming is a common response to aversion in rodents [57]. MC4R signaling is intertwined with the stress response [58, 59]. Intracerebroventricular injection with melanocortin agonists increases plasma corticosterone, which is attenuated by selective MC4R antagonism [60]. Moreover, exposure to stressors, such as forced swimming or restraint, induces markers of cell activation in the arcuate nucleus, containing the pro-opiomelanocortin (POMC) neurons that release the MC4R ligand α-melanocyte stimulating hormone [61]. Potentially, Mc4r+ neurons are involved in reacting to external (aversive) stimuli, in which case, this cell population may not necessarily be expected to impact action flexibility, which does not involve aversive stimuli, per se.
Our findings do not negate evidence that excitatory plasticity in the OFC can induce action inflexibility or habitual behavior. Chemogenetic stimulation of excitatory OFC neurons using both Gq- and Gs-coupled DREADD constructs induces inflexible choice in the same task used here – when mice must select actions based on their consequences [16, 17]. Further, high-fat diet increases the excitability of OFC neurons, inducing biases towards habit-based behaviors [18, 62, 63]. And in reversal tasks requiring slow, incremental responding that resembles habitual behavior, lesions of the dorsolateral striatum, which controls habitual behavior, and the OFC produce nearly identical deficits [64]. Finally, continuous theta burst stimulation of the OFC, which reduces local activity, has beneficial effects for compulsive behaviors in OCD [65] and improves goal planning – countering habit bias [66], again suggesting that local hyper-activation contributes to habitual behavior. One possibility is that stimulating Mc4r+ cells here did not induce inflexible choice or habitual behavior simply due to an insufficient number of stimulated neurons. However, a majority of excitatory OFC neurons express Mc4r (see Fig 5A), meaning, a good number of cells were transduced. Further, Li et al. chemogenetically stimulated only OFC neurons that project to the BLA, this small population sufficient to induce inflexible choice [17]. Potentially, Mc4r+ cortico-striatal neurons control compulsive-like behavior, while Mc4r-null OFC-to-BLA populations control choice behavior, though this possibility would have to be empirically tested.
Another important point is that here we focused on Mc4r+ neurons in the control of grooming, rather than the actions of MC4R itself because MC4R-induced grooming has already been reported several times [27-30, 54, 67-69]. MC4R-mediated signaling is depleted during periods of starvation, leading to a model suggesting that MC4R suppression mitigates compulsive-like behavior when organisms should prioritize flexible food-seeking behaviors; meanwhile, activity of MC4R drives compulsive-like behavior [27].
Conclusions
Here, mice experimentally bred for action inflexibility also exhibited habitual and compulsive-like behavior. These mice suffered dendritic spine attrition on excitatory OFC neurons and abnormalities in dendritic spine morphologies. These changes seem likely associated with inflexible behavior, given prior experiments indicating that dendritic spine plasticity is necessary for action flexibility in this task – when mice must select actions based on their consequences [70, 71]. And in a separate line of investigation, obesity-inducing diet also caused spine loss on excitatory OFC neurons, concurrent with biases towards habit-based behavior [18, 72].
Meanwhile, we found that Mc4r+ OFC neuron controlled compulsive- and not inflexible choice or habitual behavior. Such dissociations may account for why genetic mutants for studying compulsive-like behavior have generated mixed results when examined in tests of habitual behavior, with some mutants rapidly developing or adhering to habits [73, 74], while others demonstrate no such propensities or even resilience to habit formation [75]. Determining how risk genes like Sapap3 intersect with MC4R systems may provide new insight into the neurobiology of habit-based and compulsive-like behaviors.
Supplementary Material
Highlights.
A common way to investigate compulsive behavior is to mutate risk genes in rodents.
Here, we instead investigated intact mice bred to favor inflexible action strategies.
We found that they are prone to habitual and compulsive-like behavior.
Melanocortin 4 receptor in the orbital cortex is intact, despite other alterations.
Mc4r+ neurons induce compulsive-like behavior but unexpectedly spare action strategy.
Acknowledgments.
Images were created with BioRender.com. We thank Dr. Elizabeth Heaton for technical assistance and Dr. Christina Gremel for helpful feedback.
Funding statement.
This research project was supported in part by the Emory University Integrated Cellular Imaging Core and Children’s Healthcare of Atlanta. This work was also supported in part by NIH MH117103 and DA044297 and Graduate Research Fellowship Program under Grant No. 1937971. The Emory National Primate Research Center is supported by NIH OD011132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. The authors declare no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability.
Data will be made available through the Emory University Dataverse.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, and Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, and Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, Miguel EC, Rauch SL, Goodman WK, Phillips KA, et al. (2010). Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety 27, 507–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena S, Brody AL, Schwartz JM, and Baxter LR (1998). Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl, 26–37. [PubMed] [Google Scholar]
- 5.Rauch SLB, L.R. (1998). Neuroimaging of OCD and related disorders In: Jenike MA, Baer L, Minichiello WE. In Obsessive Compulsive Disorders. Theory and Management. . (Boston MA: Mosby; ), pp. 289–317. [Google Scholar]
- 6.Nakao T, Okada K, and Kanba S (2014). Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin Neurosci 68, 587–605. [DOI] [PubMed] [Google Scholar]
- 7.Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, and Bogerts B (1999). Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry 56, 913–919. [DOI] [PubMed] [Google Scholar]
- 8.Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, Han MH, Chang KH, and Kwon JS (2004). Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 16, 342–349. [DOI] [PubMed] [Google Scholar]
- 9.Piantadosi SC, Chamberlain BL, Glausier JR, Lewis DA, and Ahmari SE (2021). Lower excitatory synaptic gene expression in orbitofrontal cortex and striatum in an initial study of subjects with obsessive compulsive disorder. Molecular Psychiatry 26, 986–998. [DOI] [PubMed] [Google Scholar]
- 10.Veale DM, Sahakian BJ, Owen AM, and Marks IM (1996). Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med 26, 1261–1269. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, and Sahakian BJ (2007). Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 164, 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burguière E, Monteiro P, Mallet L, Feng G, and Graybiel AM (2015). Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol 30, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balleine BW, and O'Doherty JP (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillan CM, Robbins TW, Sahakian BJ, van den Heuvel OA, and van Wingen G (2016). The role of habit in compulsivity. Eur Neuropsychopharmacol 26, 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torregrossa MM, Quinn JJ, and Taylor JR (2008). Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry 63, 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinton EA, Li DC, Allen AG, and Gourley SL (2019). Social Isolation in Adolescence Disrupts Cortical Development and Goal-Dependent Decision-Making in Adulthood, Despite Social Reintegration. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li DC, Dighe NM, Barbee BR, Pitts EG, Kochoian B, Blumenthal SA, Figueroa J, Leong T, and Gourley SL (2022). A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory. Nat Neurosci 25, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seabrook LT, Naef L, Baimel C, Judge AK, Kenney T, Ellis M, Tayyab T, Armstrong M, Qiao M, Floresco SB, et al. (2023). Disinhibition of the orbitofrontal cortex biases decision-making in obesity. Nat Neurosci 26, 92–106. [DOI] [PubMed] [Google Scholar]
- 19.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, and Hen R (2013). Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340, 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez CI, Kegeles LS, Flood P, and Simpson HB (2011). Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 72, 567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, and Simpson HB (2013). Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 38, 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams TG, Bloch MH, and Pittenger C (2017). Intranasal Ketamine and Cognitive-Behavioral Therapy for Treatment-Refractory Obsessive-Compulsive Disorder. J Clin Psychopharmacol 37, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao YX (2010). The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31, 506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruso V, Lagerström MC, Olszewski PK, Fredriksson R, and Schiöth HB (2014). Synaptic changes induced by melanocortin signalling. Nat Rev Neurosci 15, 98–110. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, Fu WY, Cheng EY, Fu AK, and Ip NY (2013). Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci 33, 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim BK, Huang KW, Grueter BA, Rothwell PE, and Malenka RC (2012). Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu P, Grueter BA, Britt JK, McDaniel L, Huntington PJ, Hodge R, Tran S, Mason BL, Lee C, Vong L, et al. (2013). Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc Natl Acad Sci U S A 110, 10759–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, and Duman RS (2005). Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci 21, 2233–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spruijt BM, De Graan PNE, Eberle AN, and Gispen WH (1985). Comparison of structural requirements of α-MSH and ACTH for inducing excessive grooming and pigment dispersion. Peptides 6, 1185–1189. [DOI] [PubMed] [Google Scholar]
- 30.Alvaro JD, Taylor JR, and Duman RS (2003). Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther 304, 391–399. [DOI] [PubMed] [Google Scholar]
- 31.Allen AT, Heaton EC, Shapiro LP, Butkovich LM, Yount ST, Davies RA, Li DC, Swanson AM, and Gourley SL (2022). Inter-individual variability amplified through breeding reveals control of reward-related action strategies by Melanocortin-4 Receptor in the dorsomedial striatum. Commun Biol 5, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, and Sanes JR (2000). Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51. [DOI] [PubMed] [Google Scholar]
- 33.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. (2015). A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond LJ (1980). The effect of contingency upon the appetitive conditioning of free-operant behavior. J Exp Anal Behav 34, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson A, Nicholas DJ, and Adams CD (1983). The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. The Quarterly Journal of Experimental Psychology Section B 35, 35–51. [Google Scholar]
- 36.Rossi MA, and Yin HH (2012). Methods for studying habitual behavior in mice. Curr Protoc Neurosci Chapter 8, Unit 8.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellacott KL, Morton GJ, Woods SC, Tso P, and Schwartz MW (2010). Assessment of feeding behavior in laboratory mice. Cell Metab 12, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, and Weinshenker D (2018). The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 8, 3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woon EP, Butkovich LM, Peluso AA, Elbasheir A, Taylor K, and Gourley SL (2022). Medial orbitofrontal neurotrophin systems integrate hippocampal input into outcome-specific value representations. Cell Rep 40, 111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, and Fentress JC (2016). Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross RA, Kim A, Das P, Li Y, Choi YK, Thompson AT, Douglas E, Subramanian S, Ramos K, Callahan K, et al. (2023). Prefrontal cortex melanocortin 4 receptors (MC4R) mediate food intake behavior in male mice. Physiol Behav 269, 114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philippe M, Verena A, Henk JG, Suzanne NH, and Jean-Michel D (2013). The Rat Prefrontostriatal System Analyzed in 3D: Evidence for Multiple Interacting Functional Units. The Journal of Neuroscience 33, 5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, and Groenewegen HJ (2008). The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett 432, 40–45. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann KS, Yamin JA, Rainnie DG, Ressler KJ, and Gourley SL (2017). Connections of the Mouse Orbitofrontal Cortex and Regulation of Goal-Directed Action Selection by Brain-Derived Neurotrophic Factor. Biol Psychiatry 81, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao YX (2014). Constitutive activity in melanocortin-4 receptor: biased signaling of inverse agonists. Adv Pharmacol 70, 135–154. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar D. (2020). A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin Psychopharmacol Neurosci 18, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, and Paylor R (2009). Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 204, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barfield ET, Sequeira MK, Parsons RG, and Gourley SL (2020). Morphological Responses of Excitatory Prelimbic and Orbitofrontal Cortical Neurons to Excess Corticosterone in Adolescence and Acute Stress in Adulthood. Front Neuroanat 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barfield ET, and Gourley SL (2019). Glucocorticoid-sensitive ventral hippocampal-orbitofrontal cortical connections support goal-directed action - Curt Richter Award Paper 2019. Psychoneuroendocrinology 110, 104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, et al. (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duman CH, and Duman RS (2015). Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, et al. (2008). Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40, 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, et al. (2009). Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41, 18–24. [DOI] [PubMed] [Google Scholar]
- 54.Gispen WH, Wiegant VM, Greven HM, and de Wied D (1975). The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure-activity studies. Life Sci 17, 645–652. [DOI] [PubMed] [Google Scholar]
- 55.Fettes P, Schulze L, and Downar J (2017). Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Front Syst Neurosci 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J, Zhou P, Yuan S, Wu Y, Wang C, Zhang N, Li CR, and Liu N (2022). Symptom provocation in obsessive-compulsive disorder: A voxel-based meta-analysis and meta-analytic connectivity modeling. J Psychiatr Res 146, 125–134. [DOI] [PubMed] [Google Scholar]
- 57.Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, and Bains JS (2016). Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7, 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaki S, and Okubo T (2007). Melanocortin-4 receptor antagonists for the treatment of depression and anxiety disorders. Curr Top Med Chem 7, 1145–1151. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Garza JC, Truong HV, Henschel J, Zhang W, and Lu XY (2007). The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148, 5531–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu XY, Barsh GS, Akil H, and Watson SJ (2003). Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23, 7863–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, Garza JC, Li W, and Lu XY (2013). Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol 16, 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seabrook LT, Peterson CS, Noble D, Sobey M, Tayyab T, Kenney T, Judge AK, Armstrong M, Lin S, and Borgland SL (2023). Short- and Long-Term High-Fat Diet Exposure Differentially Alters Phasic and Tonic GABAergic Signaling onto Lateral Orbitofrontal Pyramidal Neurons. J Neurosci 43, 8582–8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau BK, Murphy-Royal C, Kaur M, Qiao M, Bains JS, Gordon GR, and Borgland SL (2021). Obesity-induced astrocyte dysfunction impairs heterosynaptic plasticity in the orbitofrontal cortex. Cell Rep 36, 109563. [DOI] [PubMed] [Google Scholar]
- 64.Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, and Holmes A (2011). Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci 14, 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price RB, Gillan CM, Hanlon C, Ferrarelli F, Kim T, Karim HT, Renard M, Kaskie R, Degutis M, Wears A, et al. (2021). Effect of Experimental Manipulation of the Orbitofrontal Cortex on Short-Term Markers of Compulsive Behavior: A Theta Burst Stimulation Study. Am J Psychiatry 178, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown VM, Gillan CM, Renard M, Kaskie R, Degutis M, Wears A, Siegle GJ, Ferrarelli F, Ahmari SE, and Price RB (2022). A double-blind study assessing the impact of orbitofrontal theta burst stimulation on goal-directed behavior. J Psychopathol Clin Sci 131, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adan RA, Szklarczyk AW, Oosterom J, Brakkee JH, Nijenhuis WA, Schaaper WM, Meloen RH, and Gispen WH (1999). Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. Eur J Pharmacol 378, 249–258. [DOI] [PubMed] [Google Scholar]
- 68.Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, Garner KM, Adan RA, and Cuppen E (2012). Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 20, 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nijenhuis WA, Kruijtzer JA, Wanders N, Vrinten DH, Garner KM, Schaaper WM, Meloen RH, Gispen WH, Liskamp RM, and Adan RA (2003). Discovery and in vivo evaluation of new melanocortin-4 receptor-selective peptides. Peptides 24, 271–280. [DOI] [PubMed] [Google Scholar]
- 70.Whyte AJ, Kietzman HW, Swanson AM, Butkovich LM, Barbee BR, Bassell GJ, Gross C, and Gourley SL (2019). Reward-Related Expectations Trigger Dendritic Spine Plasticity in the Mouse Ventrolateral Orbitofrontal Cortex. J Neurosci 39, 4595–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DePoy LM, Zimmermann KS, Marvar PJ, and Gourley SL (2017). Induction and Blockade of Adolescent Cocaine-Induced Habits. Biol Psychiatry 81, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson JL, Drysdale M, Baimel C, Kaur M, MacGowan T, Pitman KA, and Borgland SL (2017). Obesity-Induced Structural and Neuronal Plasticity in the Lateral Orbitofrontal Cortex. Neuropsychopharmacology 42, 1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hadjas LC, Lüscher C, and Simmler LD (2019). Aberrant habit formation in the Sapap3-knockout mouse model of obsessive-compulsive disorder. Sci Rep 9, 12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Boom BJG, Mooij AH, Misevičiūtė I, Denys D, and Willuhn I (2019). Behavioral flexibility in a mouse model for obsessive-compulsive disorder: Impaired Pavlovian reversal learning in SAPAP3 mutants. Genes Brain Behav 18, e12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehmer I, Feenstra M, Willuhn I, and Denys D (2020). Instrumental learning in a mouse model for obsessive-compulsive disorder: Impaired habit formation in Sapap3 mutants. Neurobiol Learn Mem 168, 107162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available through the Emory University Dataverse.