Abstract
Patient:Male, 55-year-old
Final Diagnosis: Diabetes mellitus type 2 • diabetic foot ulcer wagner grade I
Symptoms: Foot ulcer
Clinical Procedure: Low level laser therapy (LLLT)
Specialty: Rehabilitation
Objective:
Unusual clinical course
Background:
Diabetes mellitus (DM) is one of the most prevalent diseases worldwide and is associated with increased morbidity and mortality. One of the microvascular complications of DM is diabetic foot ulcer (DFU), which is associated with increased mortality from serious infections and decreased functional capacity of the patient due to amputation. Uncontrolled diabetes is a significant risk factor for poor wound healing. There is a need for alternative treatments that can promote wound healing in these patients. Several studies have shown the effect of low-level laser therapy (LLLT) on wound healing in patients with DFU. LLLT is a potential therapeutic approach in patients with DFU.
Case Report:
A 55-year-old male patient presented with a history of DM, diabetic neuropathy, and diabetic foot. The patient had uncontrolled blood sugar levels, with an HbA1C of 9.3%. The patient received therapy in the form of wound care with normal saline, topical antibiotics, and LLLT, with a dose of 10 J/cm2 with a frequency of therapy 3 times per week. After 12 weeks of therapy, there was improvement, characterized by wound tissue growth and no significant adverse effects during therapy.
Conclusions:
LLLT can provide benefits in patients with DFU and uncontrolled diabetes. The wound showed improvement after 12 weeks of therapy, and there were no significant adverse effects during therapy. LLLT is a minimally invasive, easy-to-use, and inexpensive therapeutic option to induce wound healing in patients with DFU and uncontrolled diabetes.
Keywords: Low-Level Light Therapy; Diabetic Foot; Diabetes Complications; Diabetes Mellitus, Type 2
Introduction
Diabetes mellitus (DM), a chronic metabolic condition, is characterized by high plasma glucose levels [1]. One of the most common illnesses worldwide, DM is linked to higher rates of morbidity and mortality [2]. According to the World Health Organization (WHO), there were more than 422 million adults with DM worldwide in 2014 [2]. This prevalence continues to increase and is expected to reach 642 million by 2040 [3]. One of the microvascular complications of DM is diabetic foot ulcer (DFU), which is associated with increased mortality from serious infections [4]. Epidemiological studies show that 20% of patients with DM have DFU, which, if left untreated, leads to amputation, reducing the functional status of patients [4].
Low-level laser therapy (LLLT) is one of the modalities that uses photons at non-thermal temperatures to alter biological activity. Currently, the main uses of LLLT are to reduce pain and inflammation, trigger tissue healing and regeneration, and prevent tissue damage [5]. Numerous studies have demonstrated how LLLT helps patients with DFU wounds heal [6]. LLLT is a minimally invasive, easy-to-use, and inexpensive therapeutic option for patients with DFU. In patients with DM, there is an imbalance in metalloprotease levels that can degrade the extracellular matrix, reduce tissue extensibility, and ultimately slow down wound healing [7]. LLLT is thought to trigger DFU wound healing by increasing the stability of extracellular structures and stimulating growth factors needed to facilitate wound healing [7].
The use of LLLT in patients with uncontrolled diabetes with chronic wounds has not been extensively studied. The current management of DFUs is debridement, wound dressing, and antibiotics [8]. Patients with uncontrolled diabetes may not respond to traditional treatments, which is a substantial risk factor for poor wound healing [9]. There is a need for alternative treatments that can promote wound healing in these patients, and LLLT can have a role to play in this regard.
This case study intends to investigate the function of LLLT in the treatment of diabetic wounds in a patient with uncontrolled diabetes. A patient with uncontrolled type 2 diabetes who appeared with a right foot ulcer that had been persistent for a long time is described in the case study. Despite treatment with antibiotics and wound dressings, the ulcer did not improve, and the patient was referred for LLLT treatment. The case report describes the treatment protocol, the patient’s response to treatment, and the follow-up outcomes.
The results of this case report imply that LLLT can be a successful choice for treating chronic wounds in people with uncontrolled diabetes. The case report also highlights the need for further research to explore the potential of LLLT in improving wound healing and glycemic control in diabetic patients with chronic wounds.
Case Report
A 55-year-old male patient was referred to the physical medicine and rehabilitation clinic in Makassar, Indonesia with a history of type 2 DM, diabetic neuropathy, and DFU. The patient had DM for 2 years and was taking metformin 1500 mg/day. The patient had a grade II diabetic ulcer (Wagner grading system) on the first toe of the right foot for the last 3 months. The ulcer had been treated with conventional wound care, with non-adherent saline gauze dressing, and there was no improvement. At the time of the initial visit, the patient’s HbA1C level was 7.3%, fasting blood sugar level was 104 mg/dL, and 2-h postprandial blood sugar 247 mg/dL.
On physical examination, the patient’s weight was 65 kg, height was 167 cm, and body mass index was 23.31 kg/cm2, which is a normal value. There was an ulcer on the first toe of the right foot, measuring approximately 6×2 cm, with a depth of 3 mm. There were no signs of venous insufficiency (CRT <2 s) and no signs of severe infection. The joint range of motion of the right toe was full, and the intrinsic muscle strength of the left and right feet was 5 out of 5. There was a decrease in tactile sensation in the left and right feet by approximately 30%.
The patient was then scheduled for routine wound care with non-adherent saline gauze dressing and administration of LLLT 3 times a week. Wound care was performed by cleaning the wound with normal saline (NaCl 0.9%) and topical antibiotics (framicetyne sulfate 1%). Then, 660 nm wavelength, continuous mode, and 4 J/cm2 dosage of LLLT therapy was used. One centimeter was left between the LLLT administration and the wound, in a perpendicular posture. Wound care was performed by nurses, and LLLT administration was performed by a physiatrist. After that, the wound was closed with gauze and a bandage. Also, the patient received therapy from an endocrinologist in the form of metformin 1500 mg/day.
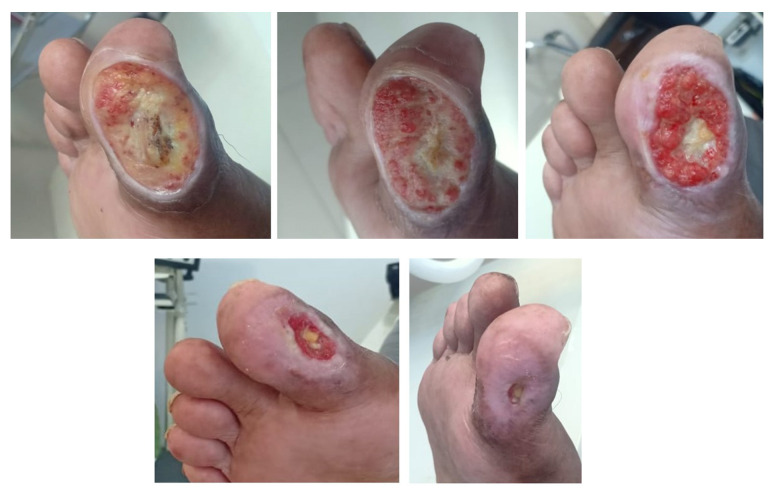
During the course of therapy, tissue growth was observed from time to time. After 12 weeks of therapy, the wound appeared to close, and there were no significant adverse effects during the therapy process (Figure 1). After treatment, the patient’s HbA1C level was 9.3%, fasting blood sugar level was 189 mg/dL, and 2-h postprandial blood sugar level was 259 mg/dL.
Figure 1.
Wound repair over time.
Discussion
DFU is among the major causes of morbidity and mortality in patients with DM. The risk of a person with DM to experience DFU is estimated at 19% to 34% [1]. Peripheral vascular disease (PVD) and diabetic neuropathy are the 2 main risk factors for DFU [10]. Diabetic neuropathy can affect almost 50% of patients with DM [11]. Symptoms can affect sensory, motor, or autonomic functions [11]. Diabetic neuropathy can lead to DFU because there is increased skin atrophy, excessively dry or excessively moist skin, decreased sensation, and decreased balance [10].
PVD in patients with DM can result in decreased blood flow function in the lower extremities, resulting in ischemia [10]. Unrecognized trauma due to neuropathy will result in wounds that are difficult to heal, due to ischemia [12]. This is exacerbated by immunosuppression due to DM, which can increase the risk of infection [10].
There are 3 stages to the wound-healing process: inflammation, proliferation, and remodeling [13]. Growth factors and cytokines, as well as inter- and intracellular signaling, are necessary for cell proliferation, differentiation, migration, and optimal protein synthesis throughout the healing phase of a wound [14]. In patients with DM, this process can be disrupted. Wounds can experience healing failure, which will usually remain in the inflammatory phase and will eventually become chronic wounds [15]. In patients with DM, there is an impairment of PI3K/AKT signaling that will increase the risk of complications in DM [16]. PI3K/AKT signaling has a crucial role in the process of tissue healing, regeneration, remodeling, and re-epithelialization. Therefore, the disturbance of PI3K/AKT regulation can interfere with the wound healing process [16].
Current DFU therapy includes blood sugar control, debridement, antibiotics, dressing, and offloading [17]. This series of therapies can give unfavorable results in patients with uncontrolled blood sugar [18]. Patients with uncontrolled blood sugar levels can be immunocompromised due to the negative effects of the hyperglycemic state [19]. This will result in immune system dysfunction due to impaired neutrophil function, impaired antioxidant system, and humoral immunity [20].
In addition to the above management strategies, rehabilitation management in the form of exercise is also considered to have a positive effect on patients with DFU [21]. Although the literature is still sparse, non-weight-bearing exercise has shown a positive effect on reducing wound size in DFU and did not show negative effects during the intervention [21]. In addition, some literature also shows that other modalities can be considered as DFU therapies. LLLT, a therapeutic modality that uses photon energy, is considered to have good results in wound recovery [14]. In LLLT, also referred to as photobio-modulation, biological tissues are exposed to low-energy laser irradiation, with only minor thermal consequences [22]. Although the exact mechanism of action of LLLT was initially unknown, recent research suggests that chromophores and signaling pathways can play a role in how well the treatment works [14,23]. LLLT is one of the non-invasive therapies commonly used in medical rehabilitation practice. LLLT has almost no adverse effects and seems to be one of the promising therapeutic options in DFU. LLLT can trigger cell proliferation, fibroblast migration, re-epithelialization, and improve micro-circulation [5–7,22]. LLLT also has anti-inflammatory and antibacterial effects on the wound [24].
Photons from LLLT are absorbed by chromopores at the molecular and cellular level, which will lead to increased recruitment of growth factors and cytokines [7,25]. In addition, it can also cause activation of cellular signalling, thus triggering protein synthesis, cell proliferation, differentiation, and cellular migration [5]. LLLT can increase the production of adenosine tri-phosphate, cyclic adenosine monophosphate, reactive oxygen species, nitric oxide, and calcium, all of which have important roles in triggering cellular signaling [16,23,24].
The potential benefits of LLLT for treating DFU may stem from its impact on multiple cellular processes and molecular routes. When used to treat DFU, LLLT can change the extracellular matrix and collagen formation, attract growth factors and cytokines, and affect the migration, proliferation, and differentiation of several cell types. Chronic hyperglycemia in patients with diabetes causes an imbalance in the levels of metalloproteases, which in turn causes the extracellular matrix to be excessively broken down, the skin’s tensile strength to decrease, and wound healing to be delayed [6].
LLLT has been demonstrated to promote collagen synthesis in a number of research models, such as mouse diabetic injured fibroblasts and mouse surgical wounds. It is feasible that LLLT will help treat DFU by stabilizing the extracellular structural support needed to let diabetic patients’ wounds heal. Important cytokines and growth factors may be attracted by LLLT in order to promote wound healing. LLLT promotes the expression of factors that control cell division, migration, survival, and wound healing, including platelet-derived growth factor, transforming growth factor-beta, basic fibroblast growth factor, interleukin 1 and interleukin 8, and macrophage phagocytic activity. The recruitment of these essential growth factors and cytokines can play a significant role in the repair of DFU [6,14].
In the patient presented in this case report, wound improvement was observed, with a significant reduction in wound size. The patient received LLLT therapy with routine wound care for 12 weeks. With uncontrolled glucose levels, the photobiomodulation effect of LLLT was able to show good results in this patient. There are many therapeutic options that can be given to patients with diabetic foot ulcer. We chose LLLT, due to various considerations. starting from internal factors as well as external factors from the patient. Patient preference becomes very important, and psychological considerations and patient costs are also taken into consideration. When compared with other therapies, LLLT is classified as an inexpensive therapy that is less invasive, is easy to perform, has low adverse effects, and does not cause pain during therapy. Based on these considerations, we chose LLLT for this patient.
During therapy, our patient was under the supervision of an endocrinologist and remained on diabetes medication. However, at the end of therapy, the patient’s blood sugar level was still not controlled. This can be due to many factors, including diet, lifestyle, and patient adherence to medication. Identification and approach to these factors can also help to improve the patient’s blood sugar levels.
A viable therapy alternative for patients with uncontrolled diabetes appears to be LLLT. Sixteen patients with uncontrolled diabetes and DFU were split into 2 groups in a randomized controlled trial study conducted by Feitosa et al in 2015. Both groups received standard wound care management, while 1 group additionally received LLLT therapy, with a 632.8 nm wavelength and a 4 J/cm2 fluence. For 4 weeks, 3 treatments per week totaling 80 s each were administered. Compared with that of the control group, the LLLT group’s wound size and pain levels significantly decreased (P<0.05), according to the data [14].
Another study by Ruh et al examined the possible effects of LLLT on a case series including 8 patients with DM who had pressure ulcers of grades II and IV. The authors used a 100 mW laser at 660 nm and a total fluence of 2 J/cm2 to administer LLLT. According to their findings, the wound area treated by LLLT significantly improved, and pro-healing factors, including vascular endothelial growth factor (angiogenesis) and TGF increased, while TNF (a pro-inflammatory factor) dropped. These findings imply that the improvement brought about by LLLT can be linked to elements that speed up the healing of wounds [26].
Conclusions
DFU is one of the complications that can affect the quality of life of a person with DM, with increased amputation potential that can reduce functional capacity. DFUs in patients with uncontrolled blood sugar levels usually do not respond well to conventional wound care therapies. The patient in this case report had a DFU with uncontrolled blood sugar levels, with an HbA1C of 7.3%. After receiving LLLT and wound care with normal saline dressing for 12 weeks, the patient’s wound showed significant improvement in size and depth. In patients with uncontrolled blood sugar levels, LLLT can be a promising therapeutic option through specific biological mechanisms that accelerate the wound healing process. Further research is needed on whether the administration of LLLT can affect hyperglycemic conditions in patients with DM.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Lovic D, Piperidou A, Zografou I, et al. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):104–9. doi: 10.2174/1570161117666190405165911. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO diabetes country profiles 2016: Explanatory notes. Geneva, Switzerland: World Health Organization; 2016. Accessed February 18, 2023. Available from: https://www.who.int/diabetes/countryprofiles/diabetes_profiles_explanatory_notes.pdf?ua=1. [Google Scholar]
- 3.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Bandyk DF. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31(2–4):43–48. doi: 10.1053/j.semvascsurg.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Tchanque-Fossuo CN, Ho D, Dahle SE, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen. 2016;24(2):418–26. doi: 10.1111/wrr.12399. [DOI] [PubMed] [Google Scholar]
- 7.Leyane TS, Jere SW, Houreld NN. Cellular signalling and photobiomodulation in chronic wound repair. Int J Mol Sci. 2021;22(20):11223. doi: 10.3390/ijms222011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Panero AJ, Ruiz-Muñoz M, Cuesta-Vargas AI, Gónzalez-Sánchez M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: A systematic review. Medicine (Baltimore) 2019;98(35):e16877. doi: 10.1097/MD.0000000000016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 10.Reardon R, Simring D, Kim B, et al. The diabetic foot ulcer. Aust J Gen Pract. 2020;49(5):250–55. doi: 10.31128/AJGP-11-19-5161. [DOI] [PubMed] [Google Scholar]
- 11.Dawane JS, Pandit VA, Bhosale MS, Khatavkar PS. Evaluation of effect of Nishamalaki on STZ and HFHF diet induced diabetic neuropathy in wistarrats. J Clin Diagn Res. 2016;10(10):FF01–FF05. doi: 10.7860/JCDR/2016/21011.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, et al. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J Vasc Surg. 2013;58(3):766–75. doi: 10.1016/j.jvs.2012.11.095. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Ayadi A, Jay JW, Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int J Mol Sci. 2020;21(3):1105. doi: 10.3390/ijms21031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feitosa MC, Carvalho AF, Feitosa VC, et al. Effects of the low-level laser therapy (LLLT) in the process of healing diabetic foot ulcers. Acta Cir Bras. 2015;30(12):852–857. doi: 10.1590/S0102-865020150120000010. [DOI] [PubMed] [Google Scholar]
- 15.Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11(18):2301–9. doi: 10.2174/1381612054367328. [DOI] [PubMed] [Google Scholar]
- 16.Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52–59. doi: 10.1016/j.cytogfr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Garwood CS, Steinberg JS, Kim PJ. Bioengineered alternative tissues in diabetic wound healing. Clin Podiatr Med Surg. 2015;32(1):121–33. doi: 10.1016/j.cpm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110(3):104–9. doi: 10.1177/0141076816688346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol. 2009;6(1):36–41. doi: 10.1080/15476910802604564. [DOI] [PubMed] [Google Scholar]
- 20.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442–49. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran MM, Haley MN. Does exercise improve healing of diabetic foot ulcers? A systematic review. J Foot Ankle Res. 2021;14(1):19. doi: 10.1186/s13047-021-00456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosca RC, Ong AA, Albasha O, et al. Photobiomodulation therapy for wound care: A potent, noninvasive, photoceutical approach. Adv Skin Wound Care. 2019;32(4):157–67. doi: 10.1097/01.ASW.0000553600.97572.d2. [DOI] [PubMed] [Google Scholar]
- 23.Glass GE. Photobiomodulation: The clinical applications of low-level light therapy [published correction appears in Aesthet Surg J 2022;42(5):566] Aesthet Surg J. 2021;41(6):723–38. doi: 10.1093/asj/sjab025. [DOI] [PubMed] [Google Scholar]
- 24.Sutton E, Ganie S, Chan C, et al. Photobiomodulation and diabetic foot and lower leg ulcer healing: A narrative synthesis. Foot (Edinb) 2021;48:101847. doi: 10.1016/j.foot.2021.101847. [DOI] [PubMed] [Google Scholar]
- 25.Chittoria RK, Kumar SH. Low-level laser therapy (LLLT) in wound healing. In: Shiffman M, Low M, editors. Chronic wounds, wound dressings and wound healing. Recent clinical techniques, results, and research in wounds. Vol 6. Cham: Springer; 2018. [Google Scholar]
- 26.Ruh AC, Frigo L, Cavalcanti MFXB, et al. Laser photobiomodulation in pressure ulcer healing of human diabetic patients: Gene expression analysis of inflammatory biochemical markers. Lasers Med Sci. 2018;33(1):165–71. doi: 10.1007/s10103-017-2384-6. [DOI] [PubMed] [Google Scholar]