Abstract
Background and Objectives
Exposure to repetitive head impacts (RHI) is linked to the development of chronic traumatic encephalopathy (CTE), which can only be diagnosed at post-mortem. The presence of a cavum septum pellucidum (CSP) is a common finding in post-mortem studies of confirmed CTE and in neuroimaging studies of individuals exposed to RHI. This study examines CSP in living former American football players, investigating its association with RHI exposure, traumatic encephalopathy syndrome (TES) diagnosis, and provisional levels of certainty for CTE pathology.
Methods
Data from the DIAGNOSE CTE Research Project were used to compare the presence and ratio of CSP in former American football players (n = 175), consisting of former college (n = 58) and former professional players (n = 117), and asymptomatic unexposed controls without RHI exposure (n = 55). We further evaluated potential associations between CSP measures and cumulative head impact index (CHII) measures (frequency, linear acceleration, and rotational force), a TES diagnosis (yes/no), and a provisional level of certainty for CTE pathology (suggestive, possible, and probable).
Results
Former American football players exhibited a higher CSP presence and ratio than unexposed asymptomatic controls. Among player subgroups, professional players showed a greater CSP ratio than former college players and unexposed asymptomatic controls. Among all football players, CHII rotational forces correlated with an increased CSP ratio. No significant associations were found between CSP measures and diagnosis of TES or provisional levels of certainty for CTE pathology.
Discussion
This study confirms previous findings, highlighting a greater prevalence of CSP and a greater CSP ratio in former American football players compared with unexposed asymptomatic controls. In addition, former professional players showed a greater CSP ratio than college players. Moreover, the relationship between estimates of CHII rotational forces and CSP measures suggests that cumulative frequency and strength of rotational forces experienced in football are associated with CSP. However, CSP does not directly correlate with TES diagnosis or provisional levels of certainty for CTE, indicating that it may be a consequence of RHI associated with rotational forces. Further research, especially longitudinal studies, is needed for confirmation and to explore changes over time.
Introduction
Chronic Traumatic Encephalopathy
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease characterized by the accumulation of hyperphosphorylated tau (p-tau) in perivascular spaces at the depths of the sulci and regional brain atrophy.1-4 The development of CTE is associated with exposure to repetitive head impacts (RHI), which are commonly observed in American football players.2,5,6 Currently, CTE is diagnosed only at post-mortem, which has led to a search for in vivo biomarkers that would make it possible to diagnose CTE during life.1,4,7,8
Traumatic Encephalopathy Syndrome
Data collected retrospectively from next of kin, in confirmed cases of CTE at post-mortem, suggest that clinical changes before death are associated with alterations in cognition (e.g., memory, executive function, and attention), mood (e.g., depression), and behavior (e.g., explosivity, impulsivity, rage, or violent outburst).9-11 These in vivo clinical changes associated with CTE have recently been used to develop the diagnosis of traumatic encephalopathy syndrome (TES).11,12 Using this approach, those diagnosed with the 2021 NINDS Consensus Diagnostic Criteria for TES must meet the following 4 criteria: (1) have extensive exposure to RHI, (2) present with core clinical features of cognitive impairment (in episodic memory and/or executive functioning) and/or neurobehavioral dysregulation, (3) show a progressive course in worsening of clinical features, and (4) have clinical symptoms that cannot be accounted for by other medical, neurologic, or psychiatric conditions. From the TES diagnosis, one can then also determine a provisional level of certainty for CTE pathology (suggestive, possible, probable, or definite CTE with TES). This is based on a stepwise assessment that includes an increased level of exposure to RHI, specific clinical features, and some supportive features (e.g., motor signs and psychiatric features).12 The diagnosis of TES, however, is based solely on clinical features, while the confirmation of CTE is based on neuropathologic confirmation. Importantly, the exclusion of biomarkers in the TES diagnostic criteria was intentionally decided by the consensus panelists as they agreed that biomarker development for CTE is not sufficiently mature. Thus, identifying biomarkers and/or supportive features for TES and the provisional levels of certainty for CTE pathology are needed.
Cavum Septum Pellucidum
The cavum septum pellucidum (CSP) represents a septal rupture observed between the lateral ventricles, formed by 2 leaflets originating from the corpus callosum and the fornix. One promising approach to understanding CTE involves studying anatomical occurrences, such as CSP, frequently observed at post-mortem, using structural MRI. Notably, research reveals a visible CSP in 69% of deceased athletes with neuropathologically confirmed CTE, with brain donors having CTE being 6.7 times more likely to exhibit CSP.13 In a more recent study that included young sports athletes, CSP was also more likely to be present in those with confirmed CTE than those with no CSP at post-mortem.3 Moreover, in vivo neuroimaging studies have detected CSP among fighters,14 retired elite rugby players,15 and former American football players.16-19 Despite CSP being considered a non-specific biomarker of CTE, investigating its prevalence in collision sports athletes with greater chances of being diagnosed with CTE at post-mortem may help validate its usefulness as a supportive metric.
Our study aims to unravel the factors contributing to or associated with a CSP in collision sport athletes with extensive exposure to RHI. It is important to note that CSP is not pathognomonic to RHI or CTE. Other studies have reported CSP in neuropsychiatric disorders, such as schizophrenia, anxiety disorders, and mood disorders.20-24 Furthermore, CSP has also been detected in healthy populations, but at a lower rate, and some may argue that the detection of a small CSP in brain imaging may be an incidental finding with no clinical relevance.25-27 Recent studies reporting CSP presence in healthy populations can also be attributed to advances in neuroimaging technology where novel high-resolution MRI with a spatial resolution of 1 mm3 or higher makes analysis more sensitive to detecting CSP.
In this study, our objective is to investigate the potential association between CSP and extensive RHI exposure, as well as its correlation with clinical features linked with CTE, assessed through TES diagnosis and provisional levels of certainty for CTE pathology. We aim to replicate prior research by categorizing morphometric features (presence and ratio) of CSP in a diverse cohort of former American football players (former college players and former professional players). We include former college players, a large sample of former professional American football players, and healthy controls who are asymptomatic and have no history of participation in contact sports, RHI exposure, or traumatic brain injury. Our overarching goal is to discern any anticipated associations with measures of head impact exposure, clinical diagnosis of TES, and provisional level of certainty for CTE pathology.
Methods
Study Design and Participants
This study is part of the Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of Chronic Traumatic Encephalopathy (DIAGNOSE CTE) Research Project. This large multi-site study includes data collection from former college and professional American football players and an unexposed asymptomatic control group with no history of RHI (see below for numbers in each group). For further information regarding the study protocol, recruitment, and description of the sample, we refer the reader to a previous study.28 All baseline data were collected before the SARS-CoV-2 pandemic.
In the DIAGNOSE CTE Research Project, 240 participants were enrolled, comprising 180 former American football players (120 former professional players and 60 former college players) and 60 unexposed asymptomatic controls. Control participants had no history of contact sports, RHI exposure, or traumatic brain injury, and all participants were men aged 45–74. No specific eligibility criteria for cognitive or behavioral status were applied to former football players. Ten participants were excluded: 6 for incomplete structural MRI acquisition and 4 controls with pre-existing psychiatric conditions or exposure to RHI found at four-year follow-up. The final MRI study sample included 175 former football players (117 former professional players and 58 former college players) and 55 controls; see Table 1 for demographics.
Table 1.
Summary of Sample Characteristics and CSP and TES Parameters
| Former American football players (n = 175) | Former professional players (n = 117) | Former college players (n = 58) | Unexposed asymptomatic controls (n = 55) | |
| Demographics | ||||
| Age, mean (SD) | 57 (8) | 59 (8) | 54 (8) | 60 (8) |
| BMI, mean (SD), kg/m2 | 33 (5) | 32 (5) | 34 (5) | 31 (4) |
| Years of education, mean (SD) | 16.7 (1.5) | 16.6 (1.1) | 17.1 (1.9) | 17.3 (3.4) |
| Race, n (%) | ||||
| White | 112 (64) | 66 (56) | 46 (79) | 34 (61) |
| Black/African American | 60 (34) | 49 (41) | 11 (19) | 21 (38) |
| American Indian/Alaska Native | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Native Hawaiian/Other Pacific Islander | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Multiple races | 3 (2) | 2 (2) | 1 (2) | 0 (0) |
| Exposure to RHI | ||||
| Years of football play, mean (SD) | 15.9 (4.4) | 18.1 (3.4) | 11.6 (2.6) | |
| Age at first exposure, mean (SD) | 11.1 (2.8) | 11.5 (2.8) | 10.1 (2.6) | |
| Position Played, number (%) | ||||
| Offensive Linemen | 42 (24) | 22 (19) | 20 (34) | |
| Offensive Backs and Receivers | 50 (29) | 36 (31) | 14 (24) | |
| Defensive Linemen | 19 (11) | 14 (12) | 5 (9) | |
| Linebackers | 26 (15) | 19 (16) | 7 (12) | |
| Defensive Backs | 34 (19) | 22 (19) | 12 (21) | |
| Special Teams | 4 (2) | 4 (3) | 0 (0) | |
| CHII, mean (SD) | ||||
| Frequency (F) | 10,869 (4,689) | 12,014 (5,055) | 8,539 (2,613) | |
| Linear acceleration (G) | 228,034 (73,244) | 247,301 (70,792) | 188,813 (62,067) | |
| Rotational acceleration (R) | 18,285,483 (6,433,354) | 20,283,399 (6,117,024) | 14,218,296 (5,018,048) |
Mean (SD), [Range]. “Former American Football Players” consist of “Former Professional Players” and “Former College Players.”
Standard Protocol Approvals, Registrations, and Patient Consents
The study and its procedures were approved by the Boston University Medical Campus, Cleveland Clinic Lou Ruvo Center for Brain Health, Mayo Clinic, Banner Alzheimer's Institute, New York University (NYU) Langone Medical Center, and Partners Healthcare/Brigham and Women's Hospital Institutional Review Boards. All participants provided written informed consent before enrollment.
Evaluation of TES and Provisional Level of Certainty for CTE Pathology
All study participants were diagnosed through a multidisciplinary diagnostic consensus conference for TES (yes/no), and a provisional level of certainty for CTE pathology was given (suggestive of CTE, possible CTE, probable CTE, and definite CTE with TES) using the 2021 NINDS Consensus Diagnostic Criteria for TES.12 The diagnosis of TES, as noted previously, is contingent upon meeting 4 criteria: (1) extensive exposure to RHI; (2) clinical features of cognitive impairment and/or neurobehavioral dysregulation; (3) a progressive course of worsening symptoms; and (4) clinical features that cannot be accounted for by other neurologic, psychiatric, or medical conditions.12 Given the diagnostic criteria, unexposed asymptomatic controls did not meet the criteria for TES. To determine a TES diagnosis, all consensus conference panelists were presented with the participant's medical history, football history, other RHI exposure information, self- and informant-reported complaints of cognitive, mood and/or behavioral problems, functional dependence status, neurologic/motor findings, and standardized neuropsychological and neuropsychiatric test results. Of note, none of our participants had a provisional level of certainty for CTE pathology classified as definite CTE with TES as this requires post-mortem evaluation.
Exposure Variables
Each participant had a calculated measure of cumulative head impact index (CHII), which included the estimated number of impacts CHII-frequency (CHII-F), estimated total linear acceleration (g-force; CHII-G), and estimated total rotational acceleration sustained (radians/s2; CHII-R). Measures of CHII indicate an estimate of head impact exposure while participating in American football. CHII measures incorporate the position exposure matrix (PEM), a compilation of helmet sensor data from American football derived through a literature review, categorizing by player position and level of play. The mean and median impact frequencies, linear acceleration, and rotational acceleration within a season were weighted based on the respective study's sample size, providing weighted averages for impacts experienced per season at various levels of play (youth, high school, and college). The PEM was utilized to construct CHII measures by aggregating the weighted mean annual numbers and intensities of head impact exposures across all reported positions and levels of play. We refer the reader to a published study for full methods on calculations of the CHII metrics.29 Higher CHII scores reflect greater RHI exposure in each specific head impact domain (i.e., frequency, linear acceleration, and rotational acceleration).
MRI Acquisition
All participants underwent a head MRI at one of the 4 study neuroimaging sites (Brigham and Women's Hospital, NYU Langone Medical Center, Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, and Mayo Clinic Arizona). The imaging protocol included structural imaging, diffusion imaging, fMRI, and magnetic resonance spectroscopy. Here, we describe the structural data acquisition protocol. The protocol for the other imaging modalities can be found elsewhere.28 Images were acquired on a 3T scanner model (Siemens Magnetom Skyra, Erlangen, Germany; software version VE11) using a 20-channel head coil. The protocol included anatomical, diffusion, and fMRI scans. Relevant to this study is the high resolution (1 × 1 × 1 mm3) 3D T1-weighted magnetization-prepared-rapid-gradient-echo (MPRAGE) sequence (inversion time = 1100 ms, TR = 2530 ms, TE = 3.36 ms, 7-degree flip angle, 256 FOV).
CSP Analysis
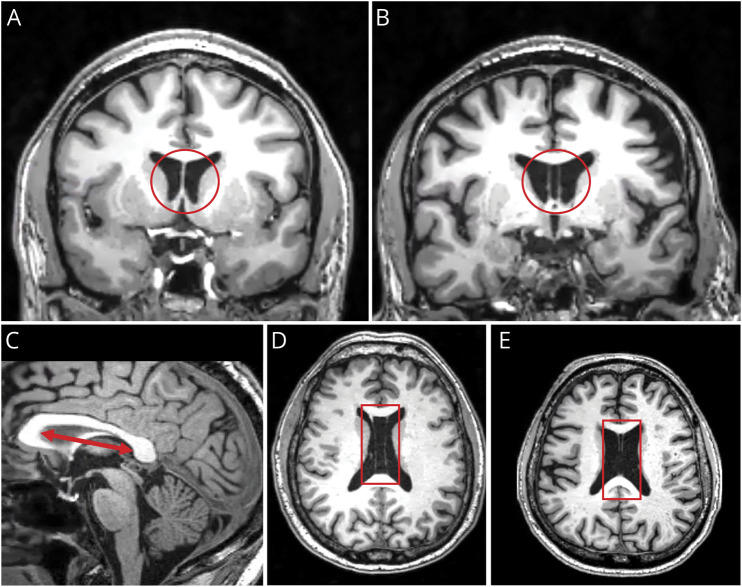
We adopted methods from our group's previous CSP findings in former symptomatic NFL players.16 Two raters (H.A. and L.B.J.) were trained by our expert rater (I.K.K.) in identifying CSP and, if present, measuring the length of CSP and the length of the septum; see Figure 1A-B. Both raters were blinded to the subject group (football player group and TES diagnosis) and the ratings of the other rater. First, we identified whether each participant had a visible CSP, defined by observing CSF between the 2 leaflets of the septum pellucidum on coronal slices of the T1-weighted MRI; see Figure 1B. Second, if present, we counted the number of coronal slices where CSP was visible and then multiplied the number of slices by the slice thickness (1 mm) to calculate the overall length of CSP. Based on previous methods established in a published study,16 a length ≥2 mm was classified as CSP being present. Third, to account for individual differences in head size, we measured the length of the entire septum pellucidum.16 We did this by defining the borders of the septum at the genu to the splenium of the corpus callosum on the midsagittal plane and measuring the longest distance between the 2; see Figure 1C. All measurements of CSP were done using 3D Slicer Version 4.10.2. Fourth, to adjust for individual differences in head size, we calculated the ratio of CSP (CSP length/septum length). Fifth, we noted whether participants had a visible CSP et vergae (Figure 1D) or cavum vergae (Figure 1E), which may be visible in posterior portions of the septum pellucidum. For these measures, we simply noted their presence.
Figure 1. Variations in the Septum Pellucidum.
(A) A visible septum pellucidum, which is the common observation. It is a thin triangular double membrane that separates the frontal horns of the right and left lateral ventricles of the brain. It extends between the anterior portion of the corpus callosum and the body of the fornix. (B) Visible (present) CSP is defined by cerebral spinal fluid being visible between 2 leaflets of the septum pellucidum as illustrated in a coronal slice. (C) The red double arrow line illustrates the widest area of the septum in the midsagittal plane. Measures were done from the genu to the splenium of the corpus callosum. (D) CSP et vergae where the 2 membranes are continuous. (E) CSP et cavum vergae detached at the splenium. For CSP et vergae and cavum vergae, we only report rate. CSP = cavum septum pellucidum.
Statistical Analysis
Reporting convention: throughout the results section, we report mean, standard deviation (SD), 95% CI, standardized test statistic z-score (Z), effect size (r), H-statistic (H), and p value (p) as appropriate. Importantly, when we use the term “former American football players,” we are referring to the combined group of former college and professional players. The dichotomized groups are always referred to as either former professional players or former college players, respectively. Throughout our analyses, we do not correct for demographic covariates as CSP has not shown to be influenced by demographical factors.
Inter-Rater Reliability of Cavum Septi Pellucidi Measures
First, we evaluated the reliability of CSP presence by calculating Cohen's kappa for binary outcomes. Next, we evaluated CSP length and ratio inter-rater reliability by conducting an intraclass correlation coefficient (ICC) for the 2 raters, a measure designed for continuous outcomes (two-way mixed model, consistency type).
Group Differences
Group-level analyses used mean rater values for all participants who had a visible CSP (≥2 mm). This allowed us to obtain a direct measure of CSP length in those with CSP presence. Chi-square tests were performed to evaluate differences in CSP presence between former American football players and unexposed asymptomatic control participants. All further analyses were conducted using the CSP ratio as it allows for correction of individual differences in head size. To test our hypothesis of group-level differences in the CSP ratio between our former American football players and unexposed asymptomatic controls, we used a Mann-Whitney-Wilcoxon test, given that our data were not normally distributed. Following this, we dichotomized the former American football players into former college and former professional players, in addition to the unexposed asymptomatic controls. Again, χ2 tests were performed to evaluate group-level differences in CSP presence. Group-level analyses were then conducted using a Kruskal-Wallis H test to compare the CSP ratio as a dependent variable. All further analyses were conducted in the full sample of former American football players.
Association Between CSP and CHII
To determine whether the CSP ratio was correlated with CHII-F, CHII-G, and CHII-R, we performed bivariate Pearson correlations in the complete sample of former American football players. This approach allowed us to independently identify correlations between CSP ratio and head impact indices. All unexposed asymptomatic controls were excluded from this analysis, given that they had no history of exposure to RHI.
CSP and TES Measures
A χ2 test was done to explore the relationship between CSP presence (yes/no), TES diagnosis (yes/no), and provisional level of CTE pathology (suggestive of CTE, possible CTE, and probable CTE). We did not include unexposed asymptomatic controls in this analysis as exposure is a necessary condition for a TES diagnosis. As mentioned before, none of our participants had a provisional level of certainty for CTE pathology classified as definite CTE with TES, as this requires post-mortem evaluation; therefore, this category was not included in any of our analyses.
To test whether ratio differed in former American football players with a TES diagnosis, we characterized our groups in the following way: former American football players with a “no TES” diagnosis and former American football players with a “yes TES” diagnosis. Here, we performed a Mann-Whitney-Wilcoxon test. Following this, we performed a Kruskal-Wallis H test to test whether ratio differed between the provisional level of certainty for CTE pathology (suggestive CTE, possible CTE, and probable CTE).
To determine further the effects of CSP ratio on the odds/probability of a diagnosis of TES (yes/no), we conducted a binary logistic regression model. We also conducted an ordinal regression model to evaluate the association between CSP ratio and the probability of a provisional level of certainty for CTE pathology. In the ordinal regression, we included those with no TES and, therefore, no provisional level of certainty for CTE pathology. This allowed us to use CSP ratio as a continuous factor, including no TES, suggestive of CTE, possible CTE, and probable CTE. To increase power and understand better how RHI exposure may make CSP more prone to alterations, we used data from the complete sample of former American football players for both analyses. CSP ratio values were rescaled by multiplying them by 100 to get appropriate effect estimates.
Data Availability
Data from the DIAGNOSE CTE Research Project will be available to qualified investigators through the Federal Interagency Traumatic Brain Injury Research (FITBIR) Informatics System and through the NIH Center for Information Technology. DIAGNOSE CTE Research Project data, including those reported in this study, will also be available to qualified investigators through a project-specific data-sharing portal. Interested investigators should contact Dr. Robert A. Stern, bobstern@bu.edu.
Results
Demographic Data
Table 1 presents demographic data, highlighting significant group differences in age and BMI. Former American football players were younger (mean = 57.3, SD = 8) and had higher BMI (mean = 32, SD = 4) than unexposed asymptomatic controls (age mean = 60, SD = 8; BMI mean = 31, SD = 4), with no other demographic variables reaching statistical significance (p's > 0.06).
Dichotomizing the former American football player dataset into 2 groups, former professional players (mean = 59, SD = 8) were significantly older than former college players (mean = 54, SD = 8) (p < 0.001). Former college players (mean = 54, SD = 8) were also younger than unexposed asymptomatic controls (mean = 60, SD = 8) (p < 0.001). Former college players had a higher BMI (mean = 34, SD = 5) compared with former professional players (mean = 32, SD = 5) (p < 0.001) and unexposed asymptomatic controls (mean = 31, SD = 4) (p < 0.01). In addition, former professional players (mean = 16.6, SD = 1.1) had lower education levels than unexposed asymptomatic controls (mean = 17.3, SD = 3.4) (p = 0.03).
Inter-Rater Reliability of CSP Measures
Agreement between raters for CSP presence was calculated using Cohen's kappa coefficient = 1, p < 0.0001, which showed 100% agreement between the 2 raters in identifying CSP presence across participants. The measurements of the 2 raters were also highly correlated (CSP length, rho = 0.95, ICC(2,2) = 0.96, p < 0.001, 95% CI = 0.95–0.97; septum length, rho = 0.94, ICC(2,2) = 0.94, p < 0.001, 95% CI = 0.71–0.97; CSP ratio, rho = 0.94, ICC(2,2) = 0.96, p < 0.001, 95% CI = 0.95–0.97). As mentioned previously, all analyses that followed were calculated using the mean values of the 2 raters for ratio alone.
Group Differences
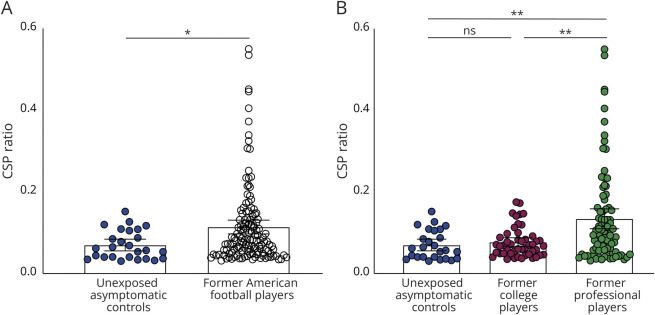
In the complete sample of participants, CSP was more visible (present) in 136 (77.7%) of the former American football players compared with 26 (47%) of the unexposed asymptomatic control participants (p < 0.001) (Table 2). CSP ratio was also significantly higher (Z = −2.5, p = 0.01, r = −0.2) in the former American football players (mean = 0.11 SD = 0.1, 95% CI = 0.1–0.13) than in the unexposed asymptomatic controls (mean = 0.07, SD = 0.03, 95% CI = 0.05–0.08); see Figure 2A. These results indicate that CSP presence and ratio are higher in former American football players than in unexposed asymptomatic controls.
Table 2.
Summary of Sample Characteristics and CSP and TES Parameters
| Former American football players (n = 175) | Former professional players (n = 117) | Former college players (n = 58) | Unexposed asymptomatic control (n = 55) | |
| Cavum septum pellucidum (CSP) | ||||
| CSP presence, n (%) | 136 (78) | 88 (75) | 48 (83) | 26 (47) |
| CSP length, mean (SD), mm | 6.2 (5.3) | 7.3 (6.1) | 4.2 (2.1) | 3.8 (2) |
| CSP ratio, mean (SD) | 0.11 (0.1) | 0.13 (0.11) | 0.08 (0.04) | 0.07 (0.03) |
| Traumatic encephalopathy syndrome (TES) | ||||
| Yes TES, n (%) | 111 (63) | 80 (68) | 31 (53) | 0 (0) |
| Provisional levels of certainty for CTE pathology | ||||
| TES suggestive of CTE, n (%) | 34 (20) | 21 (18) | 13 (22) | 0 (0) |
| TES possible CTE, n (%) | 20 (12) | 15 (13) | 5 (9) | 0 (0) |
| TES probable CTE, n (%) | 57 (33) | 44 (38) | 13 (22) | 0 (0) |
| Definite CTE with TES, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Mean (SD), [Range]. “Former American Football Players” consist of “Former Professional Players” and “Former College Players.”
Figure 2. Group-Level Differences in CSP Ratio.
(A) Data from former American football players and unexposed asymptomatic controls indicate a greater CSP ratio in former American football players. (B) The dichotomized dataset illustrates a significantly greater CSP ratio in former professional players compared with unexposed asymptomatic controls and former college players. Note: n.s. indicates no significance, * indicates p < 0.05, ** indicates p < 0.01, and error bars represent 95% confidence intervals. CSP = cavum septum pellucidum.
Dichotomizing the former American football player dataset into 2 groups, former professional football players and former college players, we found that CSP was present in 88 former professional players (75%) and 48 former college players (83%), although this was not statistically significant (p = 0.259). However, CSP presence significantly differed among the unexposed asymptomatic control group, former professional football players (p < 0.001), and former college players (p < 0.001). These results indicate that CSP presence is greater in former college and professional players when compared to unexposed asymptomatic controls; however, we did not observe differences between the 2 football player groups.
CSP ratio also showed a significant difference among the 3 groups (H(2) = 13.23, p = 0.001) (Figure 2B). More specifically, CSP ratio was higher in former professional football players (mean = 0.13, SD = 0.11, 95% CI = 0.12–0.16) than in former college players (mean = 0.08, SD = 0.04, 95% CI = 0.06–0.09) or unexposed asymptomatic controls (mean = 0.07, SD = 0.03, 95% CI = 0.05–0.08). Post hoc analysis indicated a significant difference between unexposed asymptomatic controls and former professional players (Z = −3, p < 0.01, r = −0.24), with a higher CSP ratio in former professional football players. There was also a significant difference between the former professional players and the former college players (Z = −2.71, p < 0.01, r = −0.2), but no difference between the former college players and the unexposed asymptomatic controls was observed (p > 0.3; Figure 2B).
Finally, 29 former American football players had either a CSP et vergae or cavum vergae. No unexposed asymptomatic controls showed signs of either.
Association Between CSP and CHII
In the former American football players (N = 175), there was a significant association between CHII-R and CSP ratio (Pearson's r = 0.18; p = 0.02) (Figure 3). No other correlations reached statistical significance (all p's > 0.056).
Figure 3. Correlations With CHII Rotational Acceleration.
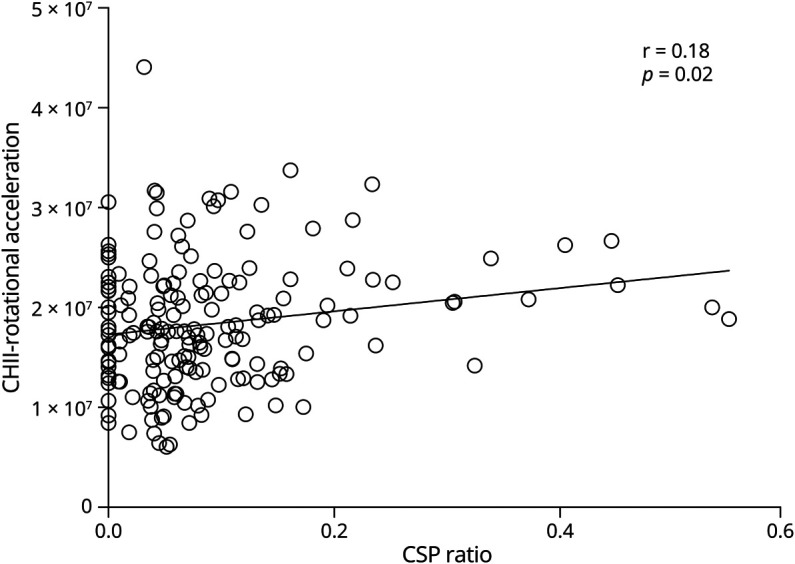
Bivariate Pearson correlation between CSP ratio and CHII-R. Results indicate that CSP ratio is correlated with increased CHII-R. CHII-R = cumulative head impact index-rotational acceleration; CSP = cavum septum pellucidum.
CSP and TES Measures
As mentioned previously, all analyses evaluating TES measures were performed on former American football players only because, by definition, no control could meet TES criteria. CSP presence (yes/no) and a TES diagnosis (yes/no) did not show a statistically significant difference between the 2 groups of players (X2(1) = 1.4, p = 0.24). In addition, CSP presence (yes/no) and the probability of a provisional level of certainty for CTE pathology groups showed no significant difference between the 2 groups of players (X2(3) = 1.5, p = 0.7). A secondary analysis, only including participants over the age of 60, who show greater signs of cognitive impairments and neurobehavioral dysregulation, also showed no significant difference between the 2 groups (all p's > 0.8).
There were also no statistically significant differences between the 2 groups of players with or without a diagnosis of TES and CSP ratio (Z = −0.5, p = 0.9, r = −0.036). Moreover, we did not observe CSP ratio differences between the provisional levels of certainty for CTE pathology (suggestive of CTE, possible CTE, and probable CTE), all p's > 0.86.
Next, the logistic regressions analyzing the relationship between CSP ratio on the likelihood of a diagnosis of TES did not show any associations (CSP ratio, OR = 7.1 (95% CI = 0.26–303, p = 0.2). Similarly, logistic regressions between CSP ratio and the provisional level of certainty for CTE pathology did not indicate any significant relationships (CSP ratio OR = 2.3 [95% CI 0.16–33.3, p = 0.52]). Overall, we found no significant associations between CSP ratio and the likelihood of diagnosing TES or the provisional level of certainty for CTE pathology.
Discussion
This study revealed a notable CSP presence in former American football players (77.7%) compared with unexposed asymptomatic control participants (47%). In dividing the football players into 2 groups (former college and former professional), both groups showed significantly higher CSP presence compared with unexposed asymptomatic controls. There were, however, no differences observed between former college players and former professional players in CSP presence. In addition, when assessing CSP ratio, former American football players exhibited a higher ratio than both the unexposed asymptomatic control group and former college players.
In evaluating CHII, we observed a positive association between CSP ratio and estimated cumulative rotational acceleration (CHII-R) measures in former American football players. This finding suggests that the intensity of rotational forces experienced by American football players is associated with CSP ratio. However, we reported no associations between CSP measurements and a diagnosis of TES or provisional levels of certainty for CTE pathology. These findings lead us to postulate that CSP is linked to the rotational forces encountered during RHI in football.
Our findings are consistent with previous results from our group showing that symptomatic former professional American football players evince a higher prevalence of CSP.16 It is important to note, however, that even in previous studies, healthy controls also show a presence of CSP. As mentioned before, a small CSP is likely an incidental finding with no clinical relevance.25-27 We speculate that it could also be due to high-resolution advances in neuroimaging technology. Using 1 mm slice thickness makes it more likely (more sensitive) to detect the presence of CSP than in previous imaging studies that used thicker slices.16,20 Importantly, as noted below, we believe that CSP ratio, rather than CSP presence, is a more sensitive measure associated with exposure to RHI as noted by our findings.
Our findings align with research done in confirmed CTE pathology cases, which shows that 69% of cases show a CSP.30 Of note, athletes exposed to RHI and later diagnosed with CTE at post-mortem demonstrate a 6.7-fold higher likelihood of exhibiting a CSP.13 CSP has not only been identified in former American football players13,16,31 but also in other contact sports athletes, such as boxers, both in vivo and at post-mortem.14,32 Our study enhances the understanding of prolonged exposure to RHI, indicating its potential to cause tears in septal leaflets. Notably, we find that CSP ratio offers greater sensitivity than CSP presence, with higher ratios observed in former professional players compared with former college players. Differences in presence alone were noted only between controls and former professional players. These results suggest that prolonged RHI exposure may lead to more significant alterations in the septum pellucidum among former professional players. Overall, our findings support the theory that RHI exposure, particularly relating to rotational forces, is a good indicator of CTE at post-mortem. We recommend that future studies incorporate CSP length or ratio for a more precise assessment of RHI exposure.
To explore further the relationship between CSP and exposure to RHI, we correlated the CSP ratio with CHII, including CHII-F (frequency), CHII-G (linear acceleration), and CHII-R (rotational force). We found that higher CSP ratios correlated with increased CHII-R measures. This further supports our hypothesis that exposure to RHI increases CSP abnormality measures and that the severity of these abnormalities is associated with rotational forces encountered during football play. These findings are supported by previous studies suggesting that CSP may result from forces associated with RHI,14 as well as studies indicating that rotational kinematics experienced during head impacts are important predictors of traumatic brain injury risk.33,34 Our findings contribute to the literature by revealing that prolonged exposure to RHI can lead to tears in the septal leaflets. However, the exact amount of exposure to RHI needed to create these tears is not exactly known. Others have found that boxers who initially did not exhibit a CSP later developed one or those who did have a CSP showed evidence of greater CSP length at follow-up.14,35 We note that longitudinal studies are needed to explore changes over time.
Furthermore, we investigated the presence and ratio of CSP among former American football players with and without a TES diagnosis, as well as across provisional levels of certainty for CTE pathology. We did not identify any significant associations between CSP measures and TES diagnosis or the provisional levels of certainty for CTE pathology. One plausible interpretation of these null findings is that, while CSP measures may be linked to exposure, they might lack the sensitivity required to reveal brain morphometric associations with the fundamental clinical features of TES, such as cognitive impairment and/or neurobehavioral dysregulation. It is essential to note that TES is intended to capture clinical symptomology and is not a direct diagnostic indicator of CTE. Presently, the sole confirmed predictor of CTE pathology at post-mortem is exposure to RHI.36,37 Therefore, the validation of TES and its correlation with CTE remains an area yet to be firmly established. Overall, our observations suggest that CSP alone may not serve as a robust predictor of TES diagnosis or the provisional level of certainty for CTE pathology. However, it can be utilized as a supplementary measure to assess the extent of exposure to RHI. We encourage further research to explore the connection between TES diagnosis and identifiable morphometric changes associated with CTE pathology observed at post-mortem, in living individuals at higher risk of developing CTE. Such investigations would contribute to the validation of TES as a clinical diagnostic measure for CTE.
First, it involves only male participants, limiting generalizability to women exposed to RHI. Second, the sample of former American football players spans play from 1952 to 2007, encompassing various historical changes in the sport. Third, CHII scores are estimates derived from helmet accelerometer data of college players. Fourth, we lack post-mortem data, preventing a direct link between CSP measures and CTE pathology. Fifth, the study is specific to former American football players, limiting generalizability to other sports. Sixth, the results are not generalizable to active athletes. Finally, the lack of longitudinal data hinders capturing CSP changes across the lifespan.
This study reveals increased CSP presence and ratios in former American football players compared with unexposed controls. Post hoc analysis shows higher CSP ratios in former professional players, indicating prolonged RHI exposure's potential impact. A positive correlation between CSP ratio and CHII-R suggests that rotational kinematics contribute to CSP. While no associations with TES or CTE pathology certainty levels were found, the study supports a link between extensive RHI exposure, particularly rotational forces, and septal leaflet tears. CSP emerges as an indicator of heightened RHI exposure, potentially aiding risk assessment for CTE development.
TAKE-HOME POINTS
→ Increased presence and ratio of cavum septum pellucidum (CSP) in former American football players compared with unexposed asymptomatic controls.
→ Positive correlation between CSP ratio and estimates of rotational acceleration in former American football players.
→ A connection between extensive repetitive head impact exposure and tears in the septal leaflets.
Appendix 1. Authors
| Name | Location | Contribution |
| Hector Arciniega, PhD | Department of Rehabilitation Medicine, New York University Grossman School of Medicine; NYU Concussion Center, NYU Langone Health; Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Leonard B. Jung | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA; cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, University Hospital, Ludwig-Maximilians-Universität, Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Fatima Tuz-Zahra, MS | Department of Biostatistics, Boston University School of Public Health, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Yorghos Tripodis, PhD | Department of Biostatistics, Boston University School of Public Health, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Omar John, BS | Department of Rehabilitation Medicine, NYU Grossman School of Medicine; Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Nicholas Kim, BS | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Holly W. Carrington, BA | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Evdokiya E. Knyazhanskaya, BA | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Arushi Chamaria | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Katherine Breedlove, PhD | Center for Clinical Spectroscopy, Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Tim L.T. Wiegand | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA; cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, University Hospital, Ludwig-Maximilians-Universität, Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Daniel Daneshvar, MD, PhD | Department of Physical Medicine and Rehabilitation, Harvard Medical School; Department of Physical Medicine and Rehabilitation, Massachusetts General Hospital, Boston; Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Tashrif Billah, MS | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Ofer Pasternak, PhD | Psychiatry Neuroimaging Laboratory, Department of Psychiatry; Department of Radiology, Brigham and Women's Hospital, and Harvard Medical School; Department of Psychiatry, Massachusetts General Hospital, Boston | Drafting/revision of the manuscript for content, including medical writing for content |
| Michael J. Coleman, MS | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Charles H. Adler, MD, PhD | Department of Neurology, Mayo Clinic College of Medicine, Mayo Clinic Arizona, Scottsdale | Drafting/revision of the manuscript for content, including medical writing for content |
| Charles Bernick, MD, MPH | Cleveland Clinic Lou Ruvo Center for Brain Health; Department of Neurology, University of Washington, Seattle | Drafting/revision of the manuscript for content, including medical writing for content |
| Laura J. Balcer, MD, MSCA | Department of Neurology; Department of Population Health; Department of Ophthalmology, New York University Grossman School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content |
| Michael L. Alosco, PhD | Department of Neurology, Boston University Alzheimer's Disease Research Center and CTE Center, Boston University Chobanian & Avedisian School of Medicine, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Alexander P. Lin, PhD | Center for Clinical Spectroscopy, Department of Radiology; Department of Radiology, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Inga K. Koerte, MD, PhD | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA; cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, University Hospital, Ludwig-Maximilians-Universität, Munich, Germany; Department of Psychiatry, Massachusetts General Hospital, Boston, MA; Graduate School of Systemic Neurosciences, Ludwig-Maximilians-Universität; Graduate School of Systemic Neurosciences, Ludwig-Maximilians-Universität, Munich, Bavaria, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Jeffrey L. Cummings, MD, ScD | Chambers-Grundy Center for Transformative Neuroscience, Pam Quirk Brain Health and Biomarker Laboratory, Department of Brain Health, School of Integrated Health Sciences, University of Nevada Las Vegas | Drafting/revision of the manuscript for content, including medical writing for content |
| Eric M. Reiman, MD | Banner Alzheimer's Institute and Arizona Alzheimer's Consortium; Department of Psychiatry, University of Arizona; Department of Psychiatry, Arizona State University; Neurogenomics Division, Translational Genomics Research Institute and Alzheimer's Consortium | Drafting/revision of the manuscript for content, including medical writing for content |
| Robert A. Stern, PhD | Department of Neurology, Boston University Alzheimer's Disease Research Center and CTE Center; Department of Anatomy and Neurobiology; Department of Neurosurgery, Boston University Chobanian & Avedisian School of Medicine, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Sylvain Bouix, PhD | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School, Boston; Department of Software Engineering and Information Technology, École de technologie supérieure, Université du Québec, Montreal, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Martha E. Shenton, PhD | Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women's Hospital, and Harvard Medical School; Department of Psychiatry, Massachusetts General Hospital; Department of Radiology, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Appendix 2. Coinvestigators
| Coinvestigators are listed at Neurology.org/cp. |
Study Funding
This study was supported by NINDS U01NS093334 (JLC, EMR, MES, and RAS) and K00 NS113419 (HA), NIH/NIMHD Loan Repayment Program L32 MD016519 (HA), Harvard's Mind Brain and Behavior Young Investigator Award (HA), Rainwater Charitable Foundation Tau Leadership Fellows Award (HA), Burroughs Wellcome Fund Postdoctoral Diversity Enrichment Program (HA), Black Men's Brain Health Emerging Scholars Fellowship (HA), the Grass Foundations Henry Grass, M.D. Rising Star in Neuroscience Award (HA), and NINDS R01NS100952 (IKK).
Disclosure
C.H. Adler consulted for Avion, CND Life Sciences, Jazz, and PreCon Health; LJB is Editor-in-Chief of the Journal of Neuro-Ophthalmology and is a paid consultant to Biogen (Cambridge, MA, USA); C. Bernick receives research support from the Ultimate Fighting Championship, Top Rank promotions, Haymon Boxing, Las Vegas Raiders, and Professional Bull Riders. He is a paid consultant for Aurora Concussion Therapy Systems, Inc. (St. Paul, MN); A.P. Lin consulted for Agios, BioMarin, and Moncton MRI. He is a co-founder of BrainSpec, Inc; J.L. Cummings has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Biohaven, Cassava, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI pharmaceutical, assessment, and investment companies; E.M. Reiman is a compensated scientific advisor for Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, Retromer Therapeutics, and Vaxxinity and is a cofounder of ALZPath; R.A. Stern is a paid consultant to Biogen (Cambridge, MA, USA) and Lundbeck (Copenhagen, Denmark). He is a member of the Board of Directors of King-Devick Technologies, Inc. (Chicago, IL, USA), and he receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA). He has been a member of the Medical Science Committee for the National Collegiate Athletic Association Student-Athlete Concussion Injury Litigation; I.K. Koerte receives funding for a collaborative project from Abbott Inc. She receives royalties for book chapters. Her spouse is an employee at Siemens AG and a stockholder of Siemens AG and Siemens Healthineers. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.McKee AC, Cairns NJ, Dickson DW, et al. . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathologica. 2016;131(1):75-86. doi: 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AC, Abdolmohammadi B, Stein TD. The neuropathology of chronic traumatic encephalopathy. Handb Clin Neurol. 2018;158:297-307. doi: 10.1016/B978-0-444-63954-7.00028-8 [DOI] [PubMed] [Google Scholar]
- 3.McKee AC, Mez J, Abdolmohammadi B, et al. . Neuropathologic and clinical findings in young contact sport athletes exposed to repetitive head impacts. JAMA Neurol. 2023;80(10):1037-1050. doi: 10.1001/jamaneurol.2023.2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Stern RA, Nowinski CJ, et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43-64. doi: 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fesharaki-Zadeh A. Chronic traumatic encephalopathy: a brief overview. Front Neurol. 2019;10:713. doi: 10.3389/fneur.2019.00713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koerte IK, Hufschmidt J, Muehlmann M, Lin AP, Shenton ME. Advanced neuroimaging of mild traumatic brain injury. In: Laskowitz D, Grant G, eds. Translational Research in Traumatic Brain Injury; 2016. [PubMed] [Google Scholar]
- 7.McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg Clin N Am. 2016;27(4):529-535. doi: 10.1016/j.nec.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25(3):350-364. doi: 10.1111/bpa.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of american football. JAMA. 2017;318(4):360-370. doi: 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern RA, Daneshvar DH, Baugh CM, et al. . Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129. doi: 10.1212/WNL.0b013e3182a55f7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montenigro PH, Baugh CM, Daneshvar DH, et al. . Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. doi: 10.1186/s13195-014-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz DI, Bernick C, Dodick DW, et al. . National Institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology. 2021;96(18):848-863. doi: 10.1212/WNL.0000000000011850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alosco ML, Mian AZ, Buch K, et al. . Structural MRI profiles and tau correlates of atrophy in autopsy-confirmed CTE. Alzheimers Res Ther. 2021;13(1):193. doi: 10.1186/s13195-021-00928-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, Wu J, Bullen J, et al. . Association of cavum septum pellucidum and cavum vergae with cognition, mood, and brain volumes in professional fighters. JAMA Neurol. 2020;77(1):35-42. doi: 10.1001/jamaneurol.2019.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanwell P, Iverson GL, Van Patten R, Castellani RJ, McCrory P, Gardner AJ. Examining for cavum septum pellucidum and Ventricular Enlargement in retired elite-level rugby league players. Front Neurol. 2022;13:817709. doi: 10.3389/fneur.2022.817709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koerte IK, Hufschmidt J, Muehlmann M, et al. . Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma. 2016;33(4):346-353. doi: 10.1089/neu.2015.3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner RC, Hess CP, Brus-Ramer M, et al. . Cavum septum pellucidum in retired american pro-football players. J Neurotrauma. 2016;33(1):157-161. doi: 10.1089/neu.2014.3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn AW, Zuckerman SL, Solomon GS, Casson IR, Viano DC. Interrelationships among neuroimaging biomarkers, neuropsychological test data, and symptom reporting in a cohort of retired national football league players. Sports Health. 2017;9(1):30-40. doi: 10.1177/1941738116674006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAllister D, Akers C, Boldt B, et al. . Neuroradiologic evaluation of MRI in high-contact sports. Front Neurol. 2021;12:701948. doi: 10.3389/fneur.2021.701948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon JS, Shenton ME, Hirayasu Y, et al. . MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry. 1998;155(4):509-515. doi: 10.1176/ajp.155.4.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1-2):1-52. doi: 10.1016/s0920-9964(01)00163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landin-Romero R, Amann BL, Sarró S, et al. . Midline brain abnormalities across psychotic and mood disorders. Schizophr Bull. 2016;42(1):229-238. doi: 10.1093/schbul/sbv097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degreef G, Bogerts B, Falkai P, et al. . Increased prevalence of the cavum septum pellucidum in magnetic resonance scans and post-mortem brains of schizophrenic patients. Psychiatry Res. 1992;45:1-13. doi: 10.1016/0925-4927(92)90009-s [DOI] [PubMed] [Google Scholar]
- 24.Beraldi GH, Prado KS, Amann BL, Radua J, Friedman L, Elkis H. Meta-analyses of cavum septum pellucidum in mood disorders in comparison with healthy controls or schizophrenia. Eur Neuropsychopharmacol. 2018;28(12):1325-1338. doi: 10.1016/j.euroneuro.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 25.Trzesniak C, Oliveira IR, Kempton MJ, et al. . Are cavum septum pellucidum abnormalities more common in schizophrenia spectrum disorders? A systematic review and meta-analysis. Schizophrenia Res. 2011;125:1-12. doi: 10.1016/j.schres.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 26.Born CM, Meisenzahl EM, Frodl T, et al. . The septum pellucidum and its variants. An MRI study. Eur Arch Psychiatry Clin Neurosci. 2004;254(5):295-302. doi: 10.1007/s00406-004-0496-z [DOI] [PubMed] [Google Scholar]
- 27.Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WTC, Andreasen NC. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry. 1997;41(11):1102-1108. doi: 10.1016/S0006-3223(96)00209-0 [DOI] [PubMed] [Google Scholar]
- 28.Alosco ML, Mariani ML, Adler CH, et al. . Developing methods to detect and diagnose chronic traumatic encephalopathy during life: rationale, design, and methodology for the DIAGNOSE CTE Research Project. Alzheimers Res Ther. 2021;13(1):136. doi: 10.1186/s13195-021-00872-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daneshvar DH, Nair ES, Baucom ZH, et al. . Leveraging football accelerometer data to quantify associations between repetitive head impacts and chronic traumatic encephalopathy in males. Nat Commun. 2023;14(1):3470. doi: 10.1038/s41467-023-39183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKee AC, Cantu RC, Nowinski CJ, et al. . Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709-735. doi: 10.1097/NEN.0b013e3181a9d503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9(4):211-221. doi: 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet. 1963;2(7312):795-801. doi: 10.1016/s0140-6736(63)90498-7 [DOI] [PubMed] [Google Scholar]
- 33.Meaney DF, Morrison B, Dale Bass C. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J Biomech Eng. 2014;136(2):021008. doi: 10.1115/1.4026364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holbourn AHS. Mechanics of head injuries. Lancet. 1943;242(6267):438-441. doi: 10.1016/s0140-6736(00)87453-x [DOI] [Google Scholar]
- 35.Aviv RI, Tomlinson G, Kendall B, Thakkar C, Valentine A. Cavum septi pellucidi in boxers. Can Assoc Radiologists J. 2010;61(1):29-32. doi: 10.1016/j.carj.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 36.McKee AC, Stein TD, Huber BR, et al. . Chronic traumatic encephalopathy (CTE): criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol. 2023;145(4):371-394. doi: 10.1007/s00401-023-02540-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowinski CJ, Bureau SC, Buckland ME, et al. . Applying the Bradford Hill criteria for causation to repetitive head impacts and chronic traumatic encephalopathy. Front Neurol. 2022;13:938163. doi: 10.3389/fneur.2022.938163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the DIAGNOSE CTE Research Project will be available to qualified investigators through the Federal Interagency Traumatic Brain Injury Research (FITBIR) Informatics System and through the NIH Center for Information Technology. DIAGNOSE CTE Research Project data, including those reported in this study, will also be available to qualified investigators through a project-specific data-sharing portal. Interested investigators should contact Dr. Robert A. Stern, bobstern@bu.edu.