Abstract
Purpose of Review
In this review, we provide an overview of what is currently known about the impacts of mechanical stimuli on metastatic tumor-induced bone disease (TIBD). Further, we focus on the role of the osteocyte, the skeleton’s primary mechanosensory cell, which is central to the skeleton’s mechanoresponse, sensing and integrating local mechanical stimuli, and then controlling the downstream remodeling balance as appropriate.
Recent Findings
Exercise and controlled mechanical loading have anabolic effects on bone tissue in models of bone metastasis. They also have anti-tumorigenic properties, in part due to offsetting the vicious cycle of osteolytic bone loss as well as regulating inflammatory signals. The impacts of metastatic cancer on the mechanosensory function of osteocytes remains unclear.
Summary
Increased mechanical stimuli are a potential method for mitigating TIBD.
Keywords: Osteocytes, Bone metastasis, Tumor-induced bone disease, Mechanical loading, Bone remodeling
Introduction
For several of the most common primary carcinomas (e.g., breast, prostate), the skeleton is the preferential metastatic site [1]. Tumor-induced bone disease (TIBD) resulting from metastasis is a major clinical problem that significantly harms patient outcomes. For breast and prostate cancers alone, which account for the greatest number of new cancer cases annually in the USA, approximately 3 in 4 patients with advanced disease will get skeletal metastases, which is incurable [1]. Further, breast and prostate cancers account for 30% (the highest among women) and 11%, respectively, of all cancer-related deaths each year in the USA [1]. The primary role of the skeleton is to provide structural support, enabling locomotion and protection for internal muscles, as well as a storage depot for minerals (e.g., calcium). Metastatic tumor cells dysregulate the bone tissue homeostasis, resulting in significant bone fragility. In the case of osteolytic lesions, such as those from breast cancer, accelerated bone loss occurs. In the case of osteoblastic (or sclerotic) lesions, such as those from prostate cancer, new bone formation is stimulated, though it is woven and mechanically weak. Thus, TIBD directly compromises the structural competence of the bone, thereby increasing patients’ risk for suffering a skeletal related event (SRE). SREs (e.g., severe pain, fracture), which are most numerous in breast cancer patients, increase the risk for subsequent SREs [as many as four per year [2]] and early death on the order of weeks [3]. The occurrence of a single SRE decreases patient quality of life and survival with greater risk for subsequent SREs. Taken together, management of skeletal health is integral to cancer patient care.
As the skeleton’s primary role is a mechanical one, bone homeostasis is primarily regulated by the local mechanical environment. Mechanical signals serve as the principal rheostat from which the skeleton actively optimizes for strength while minimizing metabolic cost, and remodels itself accordingly through the coordinated actions of bone cells. Osteocytes sense and integrate mechanical signals, and then activate osteoclasts to resorb (i.e., remove) old or damaged bone followed by deposition of new bone by osteoblasts. Currently, anti-resorptive osteoporosis drugs, such as bisphosphonates and denosumab, are the gold standard for managing TIBD. While they slow down bone resorption and have some anti-tumorigenic properties, they do not restore lost bone nor do they stimulate replacement of bone. Increased mechanical stimulation, typically in the form of physical activity, is a well-known anabolic therapy for bone. Exercise has been demonstrated to preserve or restore bone in multiple bone pathologies [4], and it also has documented anti-cancer effects [as reviewed elsewhere [5, 6]]. Exercise reduces the odds of getting cancer in the first place, improves quality of life, and numerous other measures in patients during and recovering from treatment, in part, due to reducing systemic inflammation. Its role is similarly beneficial in advanced disease [7–10], but the efficacy of exercise in addressing TIBD has been far less studied.
In recent years, multiple preclinical studies have pointed to the ability of increased mechanical stimulation to prevent or combat TIBD in vivo, particularly in lytic TIBD, and it may also have anti-tumorigenic properties [11••–19••]. However, much work remains to understand the cellular mechanisms underpinning its effects. In this review, we provide an overview of what is currently known about the impacts of mechanical stimuli on metastatic TIBD. Further, we focus on the role of the osteocyte, the skeleton’s primary mechanosensory cell as well as its most numerous. The osteocyte is central to the skeleton’s mechanoresponse, sensing and integrating local mechanical stimuli, and then controlling the downstream remodeling balance as appropriate [20], and has recently been discovered to have a role in TIBD [21]. Here, we also review its mechanosensory function in the context of TIBD.
Overview of Healthy Skeletal Remodeling and Tumor-Induced Bone Disease (TIBD)
Healthy Remodeling
The skeleton is a metabolically active, endocrine organ that undergoes continuous remodeling throughout life, whereby old or damaged bone is replaced with new bone [22] (Fig. 1). The stages of bone remodeling consist of activation, resorption, reversal, formation, and termination. First, activation of osteogenic cells, such as stem cells, osteoblasts, and osteocytes, attracts osteoclast precursors to the remodeling site. There, they fuse into mature, multi-nucleated osteoclasts, adhering to the bone surface and resorbing the matrix by dissolving and digesting the mineral content. Following osteoclast apoptosis or osteoclast secretion of osteogenic agents, mesenchymal stem cells (MSCs) proliferate and differentiate into mature osteoblasts, the cells that secrete nonmineralized osteoid, which mineralizes over time. Mature osteoblasts then become quiescent bone lining cells, undergo apoptosis, or terminally differentiate into osteocytes [20, 23].
Fig. 1. Bone remodeling scenarios.
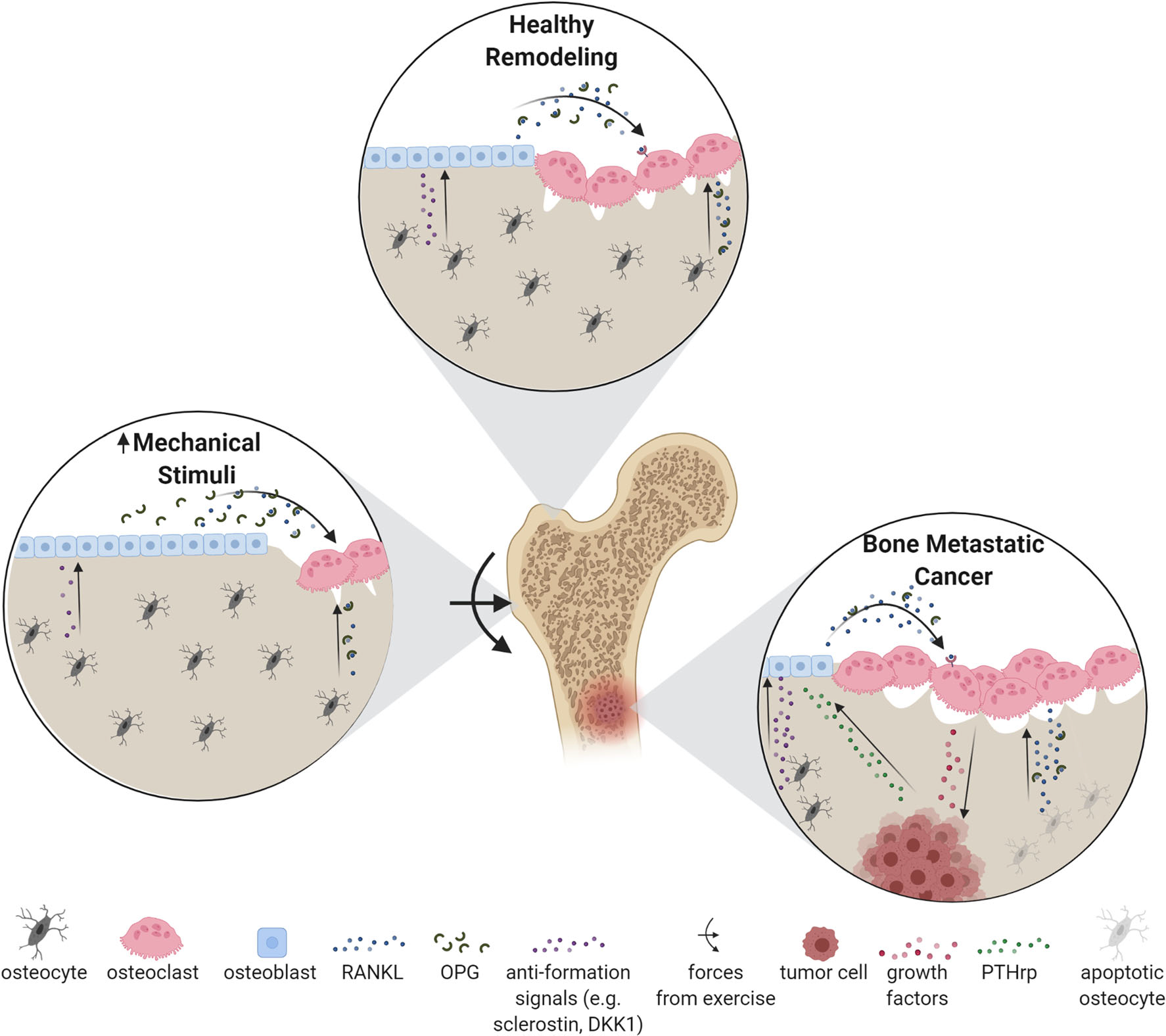
Healthy remodeling is characterized by balanced osteoclastic resorption and osteoblastic formation. When mechanical stimuli increases, for example due to exercise, the remodeling balance shifts towards formation. Increased loading promotes osteogenic differentiation of osteoblast progenitors (i.e. MSCs), osteoblast activity, and inhibits both osteoblast apoptosis and osteoclast activity. In contrast, during osteolytic bone metastatic cancer, tumor cells secrete PTHrP to stimulate osteoblast RANKL production, thereby stimulating osteoclastic resorption. In the process, growth factors are liberated from bone tissue that literally feed the tumor cells, thereby establishing a positive feedback loop known as the ‘vicious cycle’ [37]
Osteocytes, the most abundant cells in the skeleton, are terminally differentiated osteoblasts that have been buried in their own matrix. During this process, their morphology changes from cuboidal to stellate, facilitating the formation of an extensive interconnected network throughout the bone matrix called the lacunar-canalicular network (LCN). The widely connected LCN consists of the osteocyte cell body residing in a lacuna and its dendrites in canaliculi, all of which are bathed in fluid. Osteocytes connect both among themselves and all other cells in the skeleton via their extensive dendritic processes and gap junctions. Through the LCN, they are able to sense and integrate physiochemical signals, and appropriately control the balance between osteoclast and osteoblast activities via secretions of signaling proteins. During healthy remodeling, resorption and formation are balanced, maintaining bone mass. Healthy remodeling additionally includes shifting the balance towards net resorption or formation as needed in response to mechanical signals, described in detail in “Bone Functional Adaptation and TIBD” section.
The cellular interactions during remodeling are regulated by a multitude of paracrine signals [22]. MSCs, osteoblasts, and osteocytes express signaling proteins that promote osteoclastogenesis, most notably macrophage colony-stimulating factor and receptor activator of nuclear factor kB Ligand (RANKL) [24–26]. They also express osteoprotegerin (OPG), a soluble RANKL inhibitor [27]. The local ratio of RANKL and OPG serves as an essential regulatory mechanism for directing the balance between osteoclastic resorption and osteoblastic formation. RANKL:OPG can also be altered by cytokines and growth factors, giving insight into how certain disease states can affect the remodeling process [22]. Several other signaling pathways are important for regulating bone homeostasis, such as Wnts and bone morphogenetic proteins [reviewed extensively elsewhere [28, 29]]. Osteocytes, however, have primary control over osteoclastogenesis because they express OPG and RANKL in larger quantities than osteoblasts or MSCs [25]. Osteocytes have additional control over the remodeling balance through constitutive expression of sclerostin and dickkopf-1 (DKK1), proteins that inhibit pro-osteoblastic Wnts and whose expressions are modulated by mechanical stimuli [30, 31]. Finally, apoptotic osteocytes recruit osteoclasts via RANKL release to initiate targeted bone resorption [32]. This results in the removal of “dead” bone and may improve the mechanical properties of the skeleton.
Other Cells in Remodeling
The bone marrow acts as the primary site in the body for hematopoiesis as well as plays a central role in supporting systemic immunity by housing immune progenitors (i.e., hematopoietic stem cells). Interestingly, bone itself also has a local immune environment with crosstalk between bone cells and immune cells. Relative to its surroundings, the bone has a low abundance of cytotoxic T cells and high proportion of regulatory T cells and myeloid-derived suppressor cells [33]. These immune cells play important roles in skeletal turnover production of OPG and RANKL, for instance. B and T cells are sources of OPG and RANKL and contribute to the overall RANKL:OPG ratio [34], which in-turn has an effect on the state of the overall bone remodeling process. These cells also have the ability to influence osteoclastogenesis, depending on the state of local inflammatory stimuli. For instance, B cells promote osteoclastogenesis when there is no inflammatory stimulus but switch to osteoclast inhibition under proinflammatory conditions [35]. Macrophages, neutrophils, and dendritic cells are also located in the bone marrow to alter bone remodeling by modulating bone cell differentiation and cytokine and chemokine secretion [36]. Conversely, bone cells also regulate the immune system in the bone by forming an “endosteal niche,” where osteoblasts regulate HSC populations and osteocytes are required for B and T cell lymphopoiesis [36–38]. Immune cells also modulate bone remodeling in disease, as inflammatory arthritis drives secretion of tumor necrosis factor alpha in the bone marrow of mice to promote osteoclastogenesis [39].
Metastatic Tumor-Induced Bone Disease (TIBD) and Remodeling
In breast cancer, TIBD typically manifests as osteolytic lesions (Fig. 1). Breast cancer cells hijack the bone remodeling process [40] by upregulating osteoclast activity by secreting parathyroid hormone-related protein (PTHrP), which increase RANKL production in osteoblasts with subsequent stimulation of osteoclasts. With a shift in remodeling towards net bone loss, bone-derived growth factors (e.g., TGFβ) are released that “feed” the tumor [41]. For this reason, anti-resorptive drugs such as bisphosphonates, which target osteoclasts, and denosumab, a human monoclonal antibody directed against RANKL, are used clinically to interfere with this “vicious cycle” [42, 43]. In the case of osteoblastic (or sclerotic) lesions, such as those from prostate cancer, new bone formation is stimulated, though it is woven and mechanically weak, thus prone to SREs. Anti-catabolic therapies are also used to treat metastatic prostate cancer bone lesions, typically to prevent bone loss from androgen deprivation therapy [44]. However, some patients do not respond to treatment, and recurrence is common, indicating that other cells have roles in the progression of cancer metastasis [45]. New anti-resorptive therapies, such as sclerostin inhibitors, have shown promise in reversing bone loss in mouse models of breast cancer [46]. Currently, bone therapies are limited to improving bone health and limiting the progression of bone metastases, and are unable to cure patients of TIBD [47]. The limitations of available therapies highlight the need to investigate the diverse cellular and mechanical players driving TIBD to improve treatment options for patients.
Osteocytes were definitively associated with TIBD in multiple myeloma patients, whereby elevated circulating sclerostin [48] as well as increased osteocyte death [49] was correlated with TIBD and abnormal bone remodeling. In the ensuing years, much work has helped identify their role in bone metastases. In vivo, osteocyte connexin 43 hemichannel activity is an important mediator of breast cancer suppression. Mice lacking connexin 43 have increased breast tumor growth that is resistant to bisphosphonate treatment [50]. Furthermore, metastatic cancer may exert a deleterious effect on the ordering of osteocyte LCN. Specifically, the LCN became largely disorganized and misshaped in an in vivo model of bone metastatic melanoma [51], an effect that may potentially disrupting osteocyte communication and overall mechanosensitivity [52]. Many studies in recent years have focused on the interplay between osteocytes and metastatic cancers in the context of mechanics due to the fact that the bone remodeling process is often driven by mechanics.
As their progenitors are housed in marrow tissue, immune cells play a vital role in the pathology and progression of TIBD, supporting the tumor microenvironment to drive bone resorption and tumor progression. Tumor-associated macrophages (TAMs) and CD4+ T cells, including Th17 and Tregs, support osteoclastogenesis and bone metastasis progression by secretion of both pro- and anti-inflammatory cytokines and chemokines [53, 54]. Immature dendritic cells promote multiple myeloma disease progression and contribute to pathological bone destruction by recruiting osteoclasts and also transdifferentiating into osteoclast-like cells [55]. Plasmacytoid dendritic cells have been shown to promote lytic TIBD and metastatic progression in a mouse model of breast cancer bone metastasis by inducing a Th2 immune response characterized by elevated Tregs and myeloid-derived suppressor cells (MDSCs) [56]. MDSCs drive an immunosuppressive and tumorigenic microenvironment in the bone marrow, and can also differentiate into osteoclasts in myelomas, further supporting TIBD [54]. Interestingly, bisphosphonate treatment has been shown to reduce the MDSC population in the bone marrow of a mouse spontaneous breast cancer tumor-bearing mice [57]. The bone marrow acts as a reservoir for another myeloid population, neutrophils, which play an important role in TIBD. Neutrophils can carry out proinflammatory N1 or pro-tumorigenic N2 functions, primarily dependent on tumor type [58]. In metastatic prostate cancer patient bone biopsies, neutrophils were recruited to the tumor lesions in the bone, yet the neutrophils were cytotoxic and restricted bone metastasis progression in mice [59]. In breast cancer, abundant TAMs in primary tumors correlate with metastasis and a poor prognosis, and bone metastases have significantly higher TAMs than primary tumors [60]. M2 protumorigenic and anti-inflammatory macrophages carry out efferocytosis of apoptotic prostate cancer cells in the bone to promote an immunosuppressive tumor microenvironment, and promote metastatic tumor growth [61]. Yet, macrophage efferocytosis has also been shown to acquire a proinflammatory profile to support prostate cancer growth in the bone marrow, reflecting that immune cells distinctly regulate the tumor microenvironment in the bone to promote metastatic cancer progression [62].
Bone Functional Adaptation and TIBD
Mechanostat Overview
In the skeleton, dynamic biomechanical forces comprise a crucial biological rheostat that maintains a skeleton sufficiently strong to withstand daily physical activity while minimizing metabolic cost. The “mechanostat” is the feedback process by which this functional adaptation is governed [63]. Physical activity is well-documented to shift remodeling to net formation in adolescents [64], athletes in high-impact sports [e.g., tennis [65]], and pre-menopausal women [66] (Fig. 1). It imparts external forces applied to the whole bone, which causes mechanical strains to arise in the bone tissue that are ultimately transmitted to the cells within, osteocytes in particular (Fig. 2) [22]. It is also protective against bone loss during pathologies, such as type I osteoporosis [67], and can even restore lost bone [68]. Its efficacy against TIBD, as discussed below, is much less established.
Fig. 2. Generation of loading-induced fluid flow within the LCN.
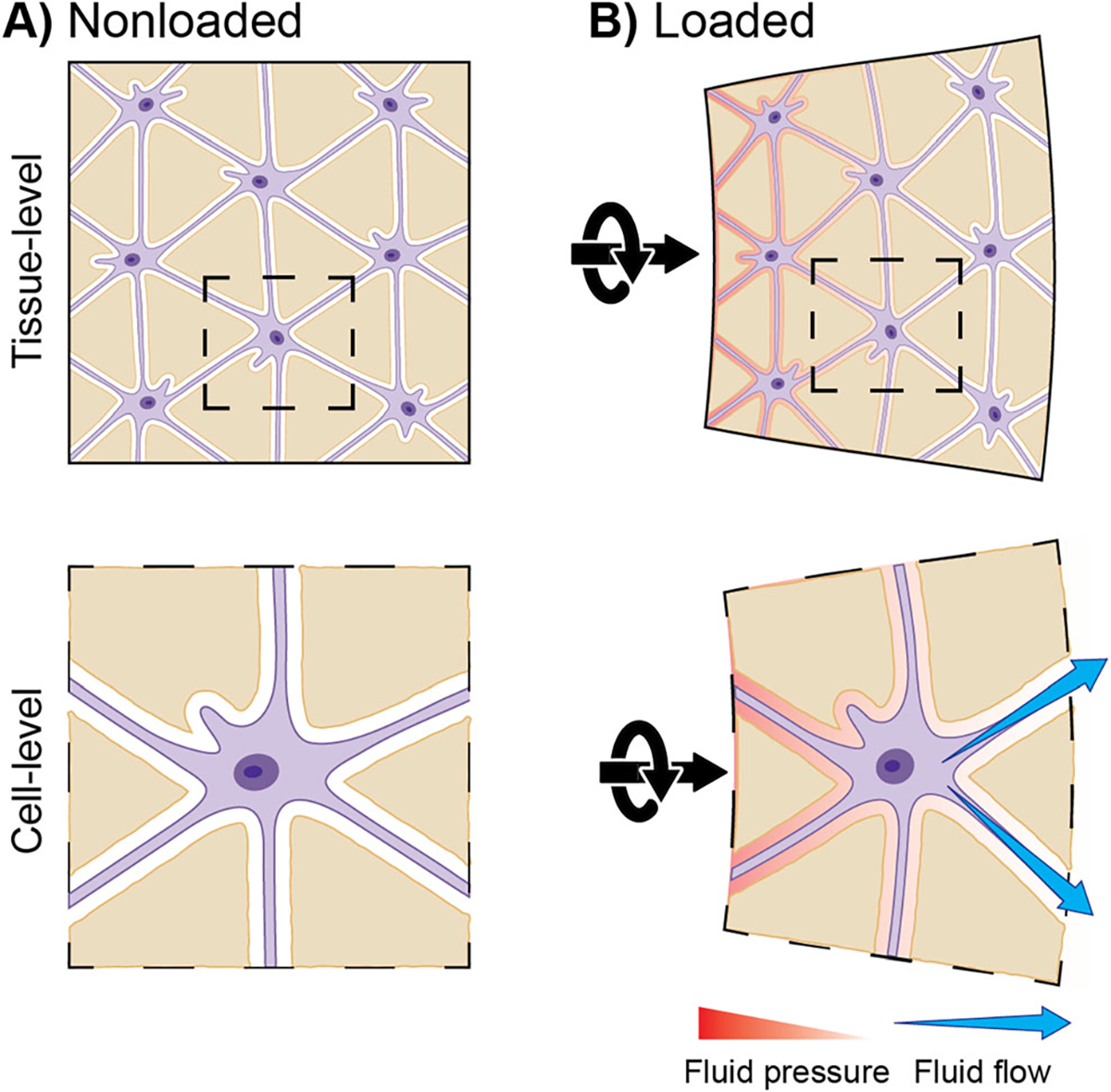
A) Osteocytes are situated within the lacunocanalicular network (LCN), which is filled with extracellular fluid. B) Upon loading, the hydrated tissue deforms, pressurizing the fluid within. Fluid flows over osteocytes, from zones of high pressure to low pressure
Osteocytes: Mechanosensors of the Mechanostat
Osteocytes are considered to be “master orchestrators” [23] of the bone remodeling process because they are the primary mechanosensory cell in the skeleton. As such, they are the principal actors in governing the overall architecture and mass of bone in order to provide a structure that resists habitual loads. In conditions of reduced or insufficient mechanical loading (e.g., bedrest, microgravity), osteocytes increase their secretion of catabolic factors, such as sclerostin and DKK1, thereby inhibiting osteoblast differentiation and function [69]. In contrast, increased mechanical loading downregulates osteocyte expression of sclerostin and RANKL, leading to a proformation remodeling environment [70] (Fig. 1). Physical activity-induced fluid flow and matrix deformations are critical signals that osteocytes sense and integrate [as reviewed extensively elsewhere [20, 23]] to appropriately coordinate downstream activities of osteoblasts and osteoclasts. Without mechanical loading, osteocytes undergo cell death, and without osteocytes, mechanotransduction is severely impaired [23, 32].
Immune Cells, Osteocytes, and the Mechanostat
Cells residing in the bone marrow tissue—MSCs, HSCs, immune cells, etc.—are also mechanosensitive, and contribute to the canonical bone remodeling process during loading. For example, mechanical loading applied to megakaryocytes promoted their differentiation into platelets [71] as well as augmented their inhibition of osteoblast differentiation [72]. While osteoimmunology (the study of the cross-regulation between bone cells and the immune system) is an ever-growing field, much work is needed to unravel the intersection among it, the mechanostat, and TIBD. Osteocytes, though, are likely to play pivotal roles. Osteocytes secrete and are influenced by inflammatory cytokines related to remodeling typically in a positive feedback loop exacerbating bone loss [73]. Many of these cytokines are featured in bone cancers (see “Metastatic Tumor-Induced Bone Disease (TIBD) and Remodeling” section), are mechanoresponsive, and have overlapping roles in remodeling. For example, TNF-α is a proinflammatory cytokine that stimulates osteoclastogenesis directly as well as indirectly via increased osteocytic RANKL secretion [74]. Moreover, the mechanoresponse of osteocytes was blunted when they were treated with TNF-α [75], while osteocyte secretion of TNF-α was reduced with loading [76], highlighting the reciprocal role of signaling proteins in bone and immune functions. To the authors’ knowledge, no studies have investigated how mechanical loading applied to a model of bone metastatic cancer influences the immune system, or vice versa. A recent study, though, demonstrated that osteocytes responded to disuse (via hindlimb unloading) by upregulating their secretion of proinflammatory cytokines (i.e., TNF-α, IL-6) and that exogenous irisin, an exercise-induced cytokine, abrogated this response [77], suggesting that an anabolic signal such as loading may similarly stifle pro-tumorigenic immune signals.
Mechanical Loading in Bone Metastatic Cancer
Physical Activity/Exercise in Cancer Patients
Physical activity has been widely documented to reduce the risk of many types of cancer, including breast [6, 78, 79]. Research to date suggests that physical activity may actually decrease the risk of recurrence and prolong overall survival in women with primary breast cancer. For example, Friedenreich et al. reported a significant association between prediagnosis and postdiagnosis physical activity and survival [5]. The intensity of the physical activity is also important [6], where running had a much greater reduction in mortality (41%) than did walking (4.6%) [80]. In the context of bone health, exercise has similar protective effects as identified previously. One year of Football Fitness training improved L1-L4 BMD, leg muscle strength, and postural balance in women treated for early-stage breast cancer [81], although the effects may not persist indefinitely [82], similar to what has been seen in other bone pathologies. Some of the additional benefits include reduction in inflammation, fatigue, and body weight alongside improved quality of life and physical condition [10].
In the context of advanced disease, exercise-containing interventions result in improvements in overall physical function and quality of life/fatigue [7–9] as well as overall survival [10]. For patients with bone metastasis specifically, though, more work is needed to tailor interventions for improved bone measures in a particularly at-risk patient population [83]. To highlight this point, while exercise interventions that combined resistance and aerobic had positive effects on lumbar BMD in breast cancer survivors [84, 85], bone metastasis patients are at the highest risk for serious complications from exercise, with spinal cord compression being one of the most severe. To this end, the International Bone Metastases Exercise Working Group is currently working to establish the first exercise guidelines for adults with bone metastases based upon widespread surveys of physicians, physical therapists, and exercise physiologists with clinical or research expertise in providing medical and/or exercise advice to individuals with bone metastases [86].
Impacts of Exercise on TIBD in Preclinical Models
Preclinical studies of exercise include forced treadmill running or swimming, and voluntary wheel running of rodents. Many have been conducted in an effort to understand mechanisms of and capitalize upon the benefits of exercise in preventing primary cancer incidence and progression as well as improving patient outcomes [reviewed in [87]]. Even though the totality of preclinical studies is highly heterogeneous, many support clinical findings and offer insight into mechanisms. For example, treadmill running may cause a shift in the metabolism of orthotopic mammary tumors, resulting in tumor cell starvation [88]. Further, swimming promoted immune system polarization toward an anti-tumor response via modulation of T cells, MDSCs, and dendritic cells [89–91].
Until recently, however, no exercise studies had been conducted to study metastatic bone disease. An excellent study from Wang et al. showed that moderate treadmill running in mice also suppressed TIBD [19••]. Importantly, this study utilized a syngeneic mouse model and utilized mouse ages much older than are typically used (i.e., 14 weeks versus 6 weeks of age), better reflecting patient physiology. Hence, this study and the models used therein provide a strong case for the utility of mechanical stimulation to combat TIBD in a clinical setting.
Impacts of Exercise Analogues (Controlled Loading) on TIBD in Preclinical Models
More work researching the impacts of loading on TIBD has been conducted using in vivo exercise analogues. In such models, controlled dynamic forces are applied to rodent limbs to mimic physical activity. To data, only three model systems that are known to stimulate bone formation—tibial compression, joint compression, and low-intensity vibration—have been used to study the interplay between mechanical stimuli and TIBD. Though only less than 10 studies have been published to date, mostly using breast cancer bone metastasis models, they have all generally shown increased mechanical stimulation to be protective against TIBD and inhibit tumor progression in both immunocompromised and immunocompetent (i.e., syngeneic) mice.
Tibial compression applies forces to the tibia in the axial direction, and is well-documented to stimulate bone formation [92] and protect against pathological bone loss [93]. Studies applying tibial compression to mouse models of bone metastatic breast cancer [11••, 12, 14, 19••] and multiple myeloma [13] demonstrated that increased mechanical stimuli generally suppresses TIBD as well as tumor growth in the injected limbs [11••–14, 19••]. While the benefits of increased loading surpassed those from a therapeutic anti-osteoclast drug (i.e., spebrutinib), even when the two were combined [14], the skeletal benefits may be reversed at higher, damage-inducing magnitudes [11••, 19••]. Using tibial compression, several potential mechanisms for the protective benefits of loading have been found in osteocytes specifically, including through altered TGF-β signaling and osteopontin secretion in osteocytes [11••], or by reducing the fraction of apoptotic, hypoxic, and HIF+ osteocytes [19••].
In joint loading models, forces are applied laterally across the joint. These models have provided similar anti-tumorigenic and bone-protective results in the context of metastatic breast cancer. Knee loading reduced tumor burden and was protective against TIBD in limbs intratibially injected with breast cancer cells [18•]. Interestingly, in the same study, knee loading also appeared to reduce the growth of tumors in the mammary fat pad via systemic alterations in metabolism, as evidenced by decreased cholesterol and volatile organic compounds in urine. Similarly, ankle loading was also protective against breast cancer cell-induced osteolysis and growth in injected tibiae [15]. Importantly, these results also held for obese mice [16]. Obesity is a predisposition to a chronic, proinflammatory state via increased inflammatory mediators (e.g., TNF-α), and is a known correlate of breast cancer patient morbidity [94], highlighting that anabolic signals in the bone mechanical environment, even in the absence of aerobic exercise, may stifle pro-tumorigenic immune signals.
Finally, low-intensity vibration (LIV) applies very low forces (<10 με) at high frequencies (20–100 Hz). LIV successfully mitigated tumor growth while protecting bone integrity in an in vivo model of multiple myeloma [17] as well as in aged mouse model of spontaneous granulosa cell ovarian cancer [95]. LIV may be a safe anabolic method for patients with very fragile skeletons [96], particularly those with overt bone disease from metastatic tumors, but this question remains outstanding to date.
Direct Loading of Cancer Cells In Vitro
Mechanical signals are well-recognized to modulate the behavior of cancer cells [97], typically studied in the context of their primary site. However, the mechanical environment of the skeleton has a very different mechanical environment than most of the body. Thus, recapitulating skeletal signals, for example, cyclic ones, is crucial for understanding how metastatic tumor cells function in a bone microenvironment. To this end, a number of in vitro studies have utilized loading that mimics skeletal signals, such as oscillatory fluid flow or dynamic compression. A recent meta-analysis showed that, generally, applied loading significantly reduced breast cancer cell viability, proliferation, and tumorigenic potential [98]. This analysis included reports of direct and controlled loading of cancer cells [e.g., cyclic compression applied to a bone-mimetic scaffold laden with MDA-MB-231 cells [12]] as well as culturing cancer cells with serum collected from exercise intervention participants [e.g., cycling [99]]. Interestingly, sera collected following an acute bout of high-intensity exercise (i.e., a single, high-intensity endurance cycling session) decreased tumorigenic measures in breast cancer cells far more than did sera following a 9-week period of regular (3–4 sessions/week) exercise [99], highlighting a need to determine the impacts of specific types and volumes of exercise.
Impacts of Mechanically Loaded Osteocytes on Cancer Cells In Vitro
In vivo, metastatic tumor cells and resident bone cells interact, with skeletal mechanical signals modulating their interactions. Osteocytes, being the primary mechanosensor and mechanotransducer cell in bone, would intuitively have downstream effects on tumor cells dependent upon the mechanical environment. Research to date, however, is inconsistent. For example, conditioned media collected from mechanically loaded osteocytes has increased [100] and decreased breast cancer cell proliferation [11••, 50] as well as increased [11••, 100] and decreased tumor migration [50, 101•]. Some of the discrepancies may be attributed to a variety of differing experimental parameters: loading regimes [i.e., steady [50] versus oscillatory flow [11••, 50, 100, 101•]], and the use of osteocyte cell lines at varying stages [i.e., early [11••, 50, 100, 101] versus middle [11••]]. The Yokota lab, though, demonstrated that conditioned media from both early- and late-stage osteocytes consistently promoted migration and inhibited proliferation across six types of mammary tumor cells (mouse and human) [11••]. A further consideration is indirect osteocyte mechanotransduction to metastatic tumor cells via resident cells. Specifically, several studies from the You lab have revealed that the anti-metastatic potential of flow-stimulated osteocytes is mediated by osteoclasts and endothelial cells. Specifically, applying conditioned media from flowed osteocytes directly to MDA-MB-231 cells increased their migration while reducing their apoptosis [100]. In contrast, conditioned media from osteoclasts or endothelial cells cultured in conditioned media from flowed osteocytes reduced breast cancer cells’ migration while increasing their apoptosis. They went on to further show that flowed osteocytes reduced endothelial permeability, reduced breast cancer cell adhesion to endothelial monolayers, and reduced extravasation distance, thereby downregulating bone-metastatic potential by indirect signaling through endothelial cells [101•, 102].
Impacts of Cancer Cells on Osteocytes
Osteocyte dysfunction, particularly in their ability to mechanosense, contributes to skeletal disease in multiple pathologies (e.g., chronic kidney disease [103], hyperparathyroidism [104]). However, while tumors in the skeletal clearly impact osteocytes, much work remains to fully elucidate the full extent. Only ~10 years ago, OCys were definitively implicated in TIBD. Increased lesions, osteoclast formation, and bone loss in late-stage multiple myeloma patients were correlated with increased osteocyte apoptosis and circulating levels of DKK1 and sclerostin [48, 49]. In a preclinical multiple myeloma model without any loading treatment, osteocytes and tumor cells physically interacted, reciprocally signaling via Notch, ultimately increasing osteocyte apoptosis and RANKL and sclerostin expression [105]. In separate studies, the osteocyte network was markedly disorganized in cancer-bearing bone tissues in both multiple myeloma [106] and bone metastatic melanoma [51] preclinical models, suggestive of a concomitant dysfunctional mechanoresponse. Finally, the Bonewald lab recently demonstrated that even non-bone metastatic cancers (i.e., colon, ovarian, and lung) altered the osteocyte LCN and dramatically increased osteocyte death [107•].
Far fewer studies have incorporated mechanical loading. Applying perfusion via rocking, we have shown that early-stage osteocytes could still undergo loading-induced dendrite formation despite the presence of breast cancer conditioned media [108•]. However, when conditioned media was collected from MDA-MB-231 cells that under rocking perfusion and subsequently used to culture osteocytes during loading, osteocyte OPG expression was inhibited while RANKL expression was unaffected. Thus, the osteocyte RANKL:OPG ratio increased upon culture with conditioned media from loaded breast cancer cells, a more physiological scenario, highlighting that loading impacts both cell types and their reciprocal interactions.
Conclusions and Future Directions
Increased mechanical stimulation in the skeletal microenvironment is now understood to play a major role in metastatic TIBD, and much work is ongoing to identify and study the underlying mechanisms. Research approaches include both controlled applied loading and exercise models, and in vivo and in vitro model systems. Central to the interaction between loading and TIBD is the osteocyte, the “master orchestrator” of bone homeostasis and the central mechanosensory cell.
Osteocytes and their progenitors span a long lineage from MSCs to osteoblasts to osteocytes. Osteogenic cells at each step of the differentiation process are mechanoresponsive and also interact with tumor cells. During the transition from osteoblast to osteocyte, the cell undergoes a dramatic change in morphology (i.e., cuboidal to stellate) and function. Osteocyte differentiation is typically classified to one of three phases: embedding/osteoid, mineralizing osteocytes, and mature osteocytes. Whether the role of osteocytes in TIBD is conserved across each phase is unknown. The majority of in vitro studies utilize the early-stage osteocyte cell line, MLO-Y4s [11••, 50, 100–102, 108•–110], though cell lines that mimic mature osteocytes [e.g., IDG-SW3s [111], OCY454s [112]], specifically by expressing sclerostin, now exist and are only beginning to be utilized. Recently, the Bonewald group utilized the IDG-SW3 line in conjunction with mouse models to demonstrate that even non-bone metastatic cancers (e.g., ovarian) impact osteocytes and the LCN [107•] with likely impacts on mechanosensing and transducing. Future work understanding the intersection among the osteocyte lineage, loading, and TIBD will help produce more targeted therapies.
Metastatic TIBD can present as osteolytic, sclerotic (osteoblastic), or mixed. The vast majority of studies investigating the impacts of loading on TIBD have focused on osteolytic cancers (e.g., breast, prostate) because of the strong anabolic signal increased loading provides. Sclerotic bone is characterized by poorly organized bone tissue, reminiscent of woven bone, and is also mechanically weak. Thus, patients with sclerotic TIBD, such as the majority of those with advanced prostate cancer, are also highly susceptible to SREs, such as fracture. Modulating the mechanical environment during fracture healing, which includes remodeling of woven bone, improves mechanical outcomes [113]. Determining whether loading is a potential avenue for stimulating the replacement of sclerotic bone with mechanically competent bone should be investigated.
Primary cancers often encompass multiple molecular subtypes. For example, breast cancer is classified based on four clinical subtypes, which are categorized according to gene expression of hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status: HR+/HER2−, HR+/HER2+, HR−/HER+, triple negative [114]. Despite clinical differences in disease presentation (i.e., lytic, mixed, blastic), time of incidence (i.e. dormancy), and other distinguishing tumor features, the skeleton is the predominant distant metastatic site for all subtypes. Understanding the impacts of mechanical loading on the tumor subtypes is necessary for translation to the clinic.
Much of the future work outlined above will involve the generation and utilization of better model systems, in vivo and in vitro. Syngeneic mice provide the benefit of an intact immune system [11, 13, 19, 106], which is clearly a better physiological mimic of physiology, with the trade-off that they preclude the use of human cells. In the context of loading, exercise models such as running [19] are more realistic in terms of clinical application, but controlled loading such as tibial compression [11••–14, 19••] provides for isolating the impacts of mechanical stimuli on bone tissue in the context of TIBD. In vitro, 3D models for osteocyte mechanobiology and TIBD are a necessity. You et al. has recently made strides in the development of a microfluidic chip that demonstrated mechanically loaded osteocytes in 3D reduced breast cancer extravasation through endothelialized channels [101•]. With other 3D osteocyte platforms coming online, combined with physiological in vivo models, the knowledge gained therein will lead to a better understanding of the intersection of loading, osteocytes, and TIBD, as well as better patient treatment options.
Funding
Cancer League of Colorado (ML, PO), National Science Foundation CMMI 2047187 (ML), Veteran’s Affairs Grant 1KBX00002929 (PO). CI supported by NIH/NCATS Colorado CTSA Grant Number TL1 TR002533; Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Conflict of Interest The authors declare no competing interests.
Code Availability Not applicable.
Data availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 662 2021. CA: A Cancer Journal for Clinicians. 2021;7(1);7–33. 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M, Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl. Cancer Inst. 2004;96(11):879–82. [DOI] [PubMed] [Google Scholar]
- 3.Grill V, Martin TJ. Hypercalcemia of malignancy. Rev. Endocr. Metab. Disord. 2000;1(4):253–63. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AM, Giangregorio L. Mechanical stimuli and bone health: what is the evidence? Curr. Opin. Rheumatol. 2012;24(5):561–6. [DOI] [PubMed] [Google Scholar]
- 5.Friedenreich CM, Stone CR, Cheung WY, Hayes SC. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020;4(1):pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurdana M Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol. Oncol. 2021;55(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev. Med. 2017;104:124–32. [DOI] [PubMed] [Google Scholar]
- 8.Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol. Nurs. Forum. 2004;31(5):977–83. [DOI] [PubMed] [Google Scholar]
- 9.Beaton R, Pagdin-Friesen W, Robertson C, Vigar C, Watson H, Harris SR. Effects of exercise intervention on persons with metastatic cancer: a systematic review. Physiother. Can. 2009;61(3):141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilk M, Kepski J, Kepska J, Casselli S, Szmit S. Exercise interventions in metastatic cancer disease: a literature review and a brief discussion on current and future perspectives. BMJ Support. Palliat. Care. 2020;10(4):404–10. [DOI] [PubMed] [Google Scholar]
- 11.••. Fan Y, Jalali A, Chen A, Zhao X, Liu S, Teli M, et al. Skeletal loading regulates breast cancer-associated osteolysis in a loading intensity-dependent fashion. Bone Res. 2020;8:9. First to show the damage-inducing loading reverses the protective effects against TIBD.
- 12.Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, Bonassar LJ, van der Meulen MCH, Fischbach C. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 2013;28(11):2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rummler M, Ziouti F, Bouchard AL, Brandl A, Duda GN, Bogen B, Beilhack A, Lynch ME, Jundt F, Willie BM. Mechanical loading prevents bone destruction and exerts anti-tumor effects in the MOPC315.BM.Luc model of myeloma bone disease. Acta Biomater. 2021;119:247–58. [DOI] [PubMed] [Google Scholar]
- 14.Ziouti F, Rummler M, Steyn B, Thiele T, Seliger A, Duda GN, et al. Prevention of bone destruction by mechanical loading is not enhanced by the Bruton’s tyrosine kinase inhibitor CC-292 in myeloma bone disease. Int. J. Mol. Sci. 2021;22(8):3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Liu H, Zhu L, Li X, Liu D, Song X, Yokota H, Zhang P. Ankle loading ameliorates bone loss from breast cancer-associated bone metastasis. FASEB J. 2019;33(10):10742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M, Liu H, Zhu L, Li X, Li J, Yang S, Liu D, Song X, Yokota H, Zhang P. Mechanical loading attenuates breast cancer-associated bone metastasis in obese mice by regulating the bone marrow microenvironment. J. Cell. Physiol. 2021;236:6391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagnotti GM, Chan ME, Adler BJ, Shroyer KR, Rubin J, Bain SD, Rubin CT. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone. 2016;90:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•. Liu S, Wu D, Sun X, Fan Y, Zha R, Jalali A, et al. Mechanical stimulations can inhibit local and remote tumor progression by downregulating WISP1. FASEB J. 2020;34(9):12847–59 Knee loading had distant, anti-tumorigenic effects on orthotopic mammary tumors.
- 19.••. Wang S, Pei S, Wasi M, Parajuli A, Yee A, You L, et al. Moderate tibial loading and treadmill running, but not overloading, protect adult murine bone from destruction by metastasized breast cancer. Bone. 2021:116100. First to demonstrate that in vivo moderate aerobic exercise was protective against breast cancer bone metastatic bone loss.
- 20.Bonewald LF. The Amazing Osteocyte. J. Bone Miner. Res. 2011;26:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson EG, Delgado-Calle J. The emerging role of osteocytes in cancer in bone. JBMR Plus. 2019;3(3):e10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006;8:455–98. [DOI] [PubMed] [Google Scholar]
- 23.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif. Tissue Int. 2014;94(1):5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17(10):1231–4. [DOI] [PubMed] [Google Scholar]
- 25.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17(10):1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. [DOI] [PubMed] [Google Scholar]
- 27.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y, Uehara S, Udagawa N, Takahashi N. Regulation of bone metabolism by Wnt signals. J. Biochem. 2016;159(4):387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016;12(4):203–21. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–66. [DOI] [PubMed] [Google Scholar]
- 31.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotkin LI. Apoptotic osteocytes and the control of targeted bone resorption. Curr Osteoporos Rep. 2014;12(1):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baschuk N, Rautela J, Parker BS. Bone specific immunity and its impact on metastasis. Bonekey Rep. 2015;4:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CWO, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006;169(3):987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kappaB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102(6):2259–67. [DOI] [PubMed] [Google Scholar]
- 36.Ponzetti M, Rucci N. Updates on Osteoimmunology: What’s new on the cross-talk between bone and immune system. Front Endocrinol (Lausanne). 2019;10:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H, Sada A, Ikeda K, Matsui T, Katayama Y. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18(5):749–58. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Systemic tumor necrosis factor α mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor α–transgenic mice. Arthritis Rheum. 2004;50(1):265–76. [DOI] [PubMed] [Google Scholar]
- 40.Weigelt B, Peterse JL. van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5(8):591–602. [DOI] [PubMed] [Google Scholar]
- 41.Le Pape F, Vargas G, Clezardin P. The role of osteoclasts in breast cancer bone metastasis. J Bone Oncol. 2016;5(3):93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clezardin P Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011;13(2):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guise TA. The vicious cycle of bone metastases. J. Musculoskelet. Neuronal Interact. 2002;2(6):570–2. [PubMed] [Google Scholar]
- 44.Ihle CL, Owens P. Integrating the immune microenvironment of prostate cancer induced bone disease. Mol. Carcinog. 2020;59(7):822–9. [DOI] [PubMed] [Google Scholar]
- 45.Gobel A, Dell’Endice S, Jaschke N, Pahlig S, Shahid A, Hofbauer LC, et al. The role of inflammation in breast and prostate cancer metastasis to bone. Int. J. Mol. Sci. 2021;22(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hesse E, Schröder S, Brandt D, Pamperin J, Saito H, Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5(9):e125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: An overview. Oncol. Rev. 2017;11(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int. J. Cancer. 2011;131(6):1466–71. [DOI] [PubMed] [Google Scholar]
- 49.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26(6):1391–401. [DOI] [PubMed] [Google Scholar]
- 50.Zhou JZ, Riquelme MA, Gu S, Kar R, Gao X, Sun L, Jiang JX. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35(43):5597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekita A, Matsugaki A, Ishimoto T, Nakano T. Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J. Struct. Biol. 2017;197(3):260–70. [DOI] [PubMed] [Google Scholar]
- 52.Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Puschel K, Djuric M, et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013;7(9):7542–51. [DOI] [PubMed] [Google Scholar]
- 53.Lee J-H, Kim H-N, Kim K-O, Jin WJ, Lee S, Kim H-H, Ha H, Lee ZH. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012;72(13):3175–86. [DOI] [PubMed] [Google Scholar]
- 54.Xiang L, Gilkes DM. The contribution of the immune system in bone metastasis pathogenesis. Int. J. Mol. Sci. 2019;20(4):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tucci M, Stucci S, Strippoli S, Dammacco F, Silvestris F. Dendritic cells and malignant plasma cells: an alliance in multiple myeloma tumor progression? Oncologist. 2011;16(7):1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 2012;189(9):4258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate–mediated mmp-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67(23):11438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsamraae M, Cook LM. Emerging roles for myeloid immune cells in bone metastasis. Cancer Metastasis Rev. 2021;40(2):413–425, 10.1007/s10555-021-09965-3. [DOI] [PubMed] [Google Scholar]
- 59.Costanzo-Garvey DL, Keeley T, Case AJ, Watson GF, Alsamraae M, Yu Y, Su K, Heim CE, Kielian T, Morrissey C, Frieling JS, Cook LM. Neutrophils are mediators of metastatic prostate cancer progression in bone. Cancer Immunol. Immunother. 2020;69(6):1113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, et al. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front. Oncol. 2020;10(2232). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones JD, Sinder BP, Paige D, Soki FN, Koh AJ, Thiele S, et al. Trabectedin reduces skeletal prostate cancer tumor size in association with effects on m2 macrophages and efferocytosis. Neoplasia. 2019;21(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roca H, McCauley LK. Efferocytosis and prostate cancer skeletal metastasis: Implications for intervention. Oncoscience. 2018;5(5–6):174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 1987;219(1):1–9. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J. Bone Miner. Res. 2001;16(1):148–56. [DOI] [PubMed] [Google Scholar]
- 65.Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351–7. [DOI] [PubMed] [Google Scholar]
- 66.Vainionpaa A, Korpelainen R, Leppaluoto J, Jamsa T. Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos. Int. 2005;16(2):191–7. [DOI] [PubMed] [Google Scholar]
- 67.von Stengel S, Kemmler W, Kalender WA, Engelke K, Lauber D. Differential effects of strength versus power training on bone mineral density in postmenopausal women: a 2-year longitudinal study. Br. J. Sports Med. 2007;41(10):649–55 discussion 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stengel SV, Kemmler W, Pintag R, Beeskow C, Weineck J, Lauber D, Kalender WA, Engelke K. Power training is more effective than strength training for maintaining bone mineral density in postmenopausal women. J. Appl. Physiol. 2005;99(1):181–8. [DOI] [PubMed] [Google Scholar]
- 69.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–83. [DOI] [PubMed] [Google Scholar]
- 70.Galea GL, Paradise CR, Meakin LB, Camilleri ET, Taipaleenmaki H, Stein GS, et al. Mechanical strain-mediated reduction in RANKL expression is associated with RUNX2 and BRD2. Gene X. 2020;5:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luff SA, Papoutsakis ET. Megakaryocytic maturation in response to shear flow is mediated by the activator protein 1 (AP-1) transcription factor via mitogen-activated protein kinase (MAPK) mechanotransduction. J. Biol. Chem. 2016;291(15):7831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soves CP, Miller JD, Begun DL, Taichman RS, Hankenson KD, Goldstein SA. Megakaryocytes are mechanically responsive and influence osteoblast proliferation and differentiation. Bone. 2014;66:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metzger CE, Narayanan SA. The role of osteocytes in inflammatory bone loss. Front Endocrinol (Lausanne). 2019;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. [DOI] [PubMed] [Google Scholar]
- 75.Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, et al. Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum. 2009;60(11):3336–45. [DOI] [PubMed] [Google Scholar]
- 76.Liao C, Cheng T, Wang S, Zhang C, Jin L, Yang Y. Shear stress inhibits IL-17A-mediated induction of osteoclastogenesis via osteocyte pathways. Bone. 2017;101:10–20. [DOI] [PubMed] [Google Scholar]
- 77.Metzger CE, Anand Narayanan S, Phan PH, Bloomfield SA. Hindlimb unloading causes regional loading-dependent changes in osteocyte inflammatory cytokines that are modulated by exogenous irisin treatment. NPJ Microgravity. 2020;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matthews CE, Moore SC, Arem H, Cook MB, Trabert B, Hakansson N, et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J. Clin. Oncol. 2020;38(7):686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–39. [DOI] [PubMed] [Google Scholar]
- 80.Williams PT. Significantly greater reduction in breast cancer mortality from post-diagnosis running than walking. Int. J. Cancer. 2014;135(5):1195–202. [DOI] [PubMed] [Google Scholar]
- 81.Uth J, Fristrup B, Sorensen V, Helge EW, Christensen MK, Kjaergaard JB, et al. One year of Football Fitness improves L1-L4 BMD, postural balance, and muscle strength in women treated for breast cancer. Scand. J. Med. Sci. Sports. 2021;31:1545–57. [DOI] [PubMed] [Google Scholar]
- 82.Vehmanen L, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Huovinen R, Ruohola J, et al. Five-year follow-up results of aerobic and impact training on bone mineral density in early breast cancer patients. Osteoporos. Int. 2021;32(3):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta-analysis. Osteoporos. Int. 2018;29(2):287–303. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol. Nurs. Forum. 2007;34(3):627–33. [DOI] [PubMed] [Google Scholar]
- 85.Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res. Treat. 2011;127(2):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campbell KL, Weller S, Cormie P, Lane KN, Rauw JM, Goulart J. Enhancing safety of exercise for individuals with bone metastases: screening recommendations developed through Delphi consensus process. J. Clin. Oncol. 2020;38(15_suppl):e24042–e. [Google Scholar]
- 87.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 2016;76(14):4032–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aveseh M, Nikooie R, Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J. Physiol. 2015;593(12):2635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abdalla DR, Murta EF, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7, 12-dimethylbenzanthracene. Eur. J. Cancer Prev. 2013;22(3):251–8. [DOI] [PubMed] [Google Scholar]
- 90.Abdalla DR, Gomes BBM, Murta EFC, Michelin MA. Bone marrow-derived dendritic cells under influence of experimental breast cancer and physical activity. Oncol. Lett. 2017;13(3):1406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wennerberg E, Lhuillier C, Rybstein MD, Dannenberg K, Rudqvist NP, Koelwyn GJ, Jones LW, Demaria S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget. 2020;11(4):452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36(6):1030–8. [DOI] [PubMed] [Google Scholar]
- 93.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared to young-adult mice following 1 week of axial tibial compression. J. Bone Miner. Res. 2010;25(9):2006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017;67(5):378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pagnotti GM, Adler BJ, Green DE, Chan ME, Frechette DM, Shroyer KR, Beamer WG, Rubin J, Rubin CT. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51(3):570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–4. [DOI] [PubMed] [Google Scholar]
- 97.Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann. Biomed. Eng. 2011;39(5):1379–89. [DOI] [PubMed] [Google Scholar]
- 98.Brown MJ, Morris MA, Akam EC. An exploration of the role of exercise in modulating breast cancer progression in vitro: a systematic review and meta-analysis. Am. J. Phys. Cell Phys. 2021;320(3):C253–C63. [DOI] [PubMed] [Google Scholar]
- 99.Baldelli G, De Santi M, Gervasi M, Annibalini G, Sisti D, Hojman P, et al. The effects of human sera conditioned by high-intensity exercise sessions and training on the tumorigenic potential of cancer cells. Clin. Transl. Oncol. 2021;23(1):22–34. [DOI] [PubMed] [Google Scholar]
- 100.Ma YV, Lam C, Dalmia S, Gao P, Young J, Middleton K, et al. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J. Cell. Biochem. 2018;119(7):5665–75. [DOI] [PubMed] [Google Scholar]
- 101.•. Mei X, Middleton K, Shim D, Wan Q, Xu L, Ma YV, et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr Biol (Camb). 2019;11(4):119–29 New 3D microfluidic platform for investigating osteocytes, loading, and breast cancer.
- 102.Ma YV, Xu L, Mei X, Middleton K, You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell. Biochem. 2019;120(5):7590–601. [DOI] [PubMed] [Google Scholar]
- 103.Heveran CM, Schurman CA, Acevedo C, Livingston EW, Howe D, Schaible EG, Hunt HB, Rauff A, Donnelly E, Carpenter RD, Levi M, Lau AG, Bateman TA, Alliston T, King KB, Ferguson VL. Chronic kidney disease and aging differentially diminish bone material and microarchitecture in C57Bl/6 mice. Bone. 2019;127:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J. Bone Miner. Metab. 2004;22(6):524–9. [DOI] [PubMed] [Google Scholar]
- 105.Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, Yoneda T, Mohammad KS, Plotkin LI, Roodman GD, Bellido T. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76(5):1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ziouti F, Soares AP, Moreno-Jimenez I, Rack A, Bogen B, Cipitria A, et al. An early myeloma bone disease model in skeletally mature mice as a platform for biomaterial characterization of the extracellular matrix. J Oncol. 2020;2020:3985315–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.•. Pin F, Prideaux M, Huot JR, Essex AL, Plotkin LI, Bonetto A, et al. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021; Non-bone metastatic cancers induce osteocyte direct remodeling.
- 108.•. Wang W, Sarazin BA, Kornilowicz G, Lynch ME. Mechanically-loaded breast cancer cells modify osteocyte mechanosensitivity by secreting factors that increase osteocyte dendrite formation and downstream resorption. Front. Endocrinol. 2018;9(352) Breast cancer signals impair osteocyte mechanoresponse.
- 109.Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene. 2019;38(23):4540–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang X, He Y, Tian S, Zhu F, Huang B, Zhang J, Chen Z, Wang H. Fluid shear stress increases osteocyte and inhibits osteoclasts via downregulating receptor-activator of nuclear factor kappaB (RANK)/osteoprotegerin expression in myeloma microenvironment. Med. Sci. Monit. 2019;25:5961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 2011;26(11):2634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu LH, Shao H, Ma YV, You L. OCY454 Osteocytes as an in Vitro Cell Model for Bone Remodeling Under Mechanical Loading. J. Orthop. Res. 2019;37:1681–9. [DOI] [PubMed] [Google Scholar]
- 113.McBride SH, Silva MJ. Adaptive and Injury Response of Bone to Mechanical Loading. Bonekey Osteovision. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


