Abstract
Background
The aim was to assess therapeutic outcomes and tolerance in patients with metastatic castration resistant prostate cancer (mCRPC) treated with androgen receptor targeted agents (ARTA) treatment at one oncological center in the Czech Republic.
Materials and methods
Retrospective analysis of 64 patients with mCRPC treated with abiraterone (50 patients) and enzalutamide (14 patients) in the first line of this disease was conducted. Kaplan-Meier analysis was used to calculate progression free survival (PFS) and overall survival (OS). We performed a multivariate analysis of risk factors for treatment outcomes (PFS, OS) by Cox regression analysis.
Results
The median follow-up was 28.4 months. The median PFS was 15.4 months [95% confidence interval (CI): 12.3–18.5], median OS was 38.2 months (95% CI: 19.9–56.5). Regression analysis demonstrated a favorable prognostic effect on PFS in patients with reduction of PSA ≥ 50 %, in patients with early reduction of prostate-specific antigen (PSA) ≥ 50% within 3 months, in patients younger than 74 years and in overall performance status (PS) 0. Regression analysis demonstrated a favorable prognostic effect on OS in patients with reduction of PSA ≥ 50 %, in patients with early reduction of PSA ≥ 50 % within 3 months and in patients with overall PS 0. Adverse effects grade 3–4 were reported in 17 (27.9%) patients in abirateron arm and in 1 (7.1%) patient in enzalutamide arm.
Conclusion
The analysis of patients with mCRPC treated with ARTA in the first line showed that ARTA represents an effective and safe therapy and contributes to longer survival.
Keywords: castrate resistant prostate cancer, metastases, targeted therapy, androgen receptor, abiraterone, enzalutamide
Introduction
Prostate cancer is the most common cancer in men [1]. Locally limited disease usually receives local treatment such as surgery or radiotherapy. Currently, the hypofractionated schedule is preferred to conventional fractionation schedule of radiation treatment [2]. The standard of advanced prostate cancer therapy is androgen deprivation therapy (ADT). However, after varying lengths of time, many patients develop resistence to ADT. Such patients are categorized as castration-resistant prostate cancer (CRPC). In 1996, a clinical study was published that established the effect of mitoxantrone on the symptomatology of metastatic CRPC (mCRPC), however, no extension of overall survival (OS) has been demonstrated [3]. Eight years later, in 2004, the results of two clinical studies, TAX 327 and SWOG 9916, demonstrated a significant prolongation of OS in patients with mCRPC treated by docetaxel [4, 5]. Further changes in therapy standards came after 2010, when cabazitaxel and radium 223 were introduced into therapeutic practice [6, 7].
Other published works, however, confirmed that despite castrate levels of testosterone, the activation of the androgen receptor (AR) significantly affects the progression of the disease [8–10]. Several studies proved a persisting concentration of dihydrotestosterone (DHT) in prostate cancer cells, even though ADT was given [11–13]. Some studies even demonstrated a higher concentration of DHT in tumor tissue than DHT concentration detected in healthy men [14]. It led to further broadening of therapy options aiming to affect the AR activation pathway. We are talking about the androgen receptor targeted agents (ARTA) treatment. Two substances are currently used in patients with mCRPC — enzalutamide and abiraterone.
Abiraterone is an active metabolite that origins from abiraterone acetate by conversion in the liver. Abiraterone belongs to the group of selective inhibitors of 17α-hydroxylase/C17,20-lyase (CYP17) enzyme. This enzyme is necessary for the synthesis of testosterone in the testicular tissue and adrenal glands, but also in the prostate cancer cells. Inhibiting CYP17 leads to reduction of corticoid production. On the basis of feedback reaction, there is an overproduction of adrenocorticotropic hormone (ACTH) with a subsequent increase of mineralocorticoid concentration. This results in hypokalemia, arterial hypertension, fluid retention and edema. Administration of prednisone aiming to suppress the overproduction of ACTH is, therefore, a necessary part of the abiraterone treatment. Phase 3 of the COU-AA-302 clinical study assessed abiraterone in 1088 chemotherapy-naive patients with mCRPC. The patients were treated by a combination of abiraterone in a daily dose of 1000 mg (4 × 250 mg in one daily dose) and prednisone in a daily dose of 10 mg (5 mg in 2 daily doses) or placebo with prednisone. The randomization was 1:1. Patients with performance status (PS) 0–1 according to Eastern Cooperative Oncology Group (ECOG were included into the trial. According to BPI–SF (Brief Pain Inventory-Short Form) asymptomatic (score 0–1) or mildly symptomatic (score 2–3) patients were included. Patients with visceral metastases were not included in the clinical trial. The primary objective was to determine radiographic progression-free survival (rPFS) and OS. The primary analysis was published in 2013 and confirmed a statistically significant extension of median rPFS [16.5 vs. 8.3 months, hazadr ratio (HR): 0.53; 95% confidence interval (CI): 0.45–0.62, p < 0.0001] and also median OS (median not reached vs. 27.2 months, HR: 0.75; 95% CI: 0.61–0.93, p = 0.01). Compared to placebo-treated patients, the patients treated with abiraterone showed efficacy in the secondary objectives of the clinical trial: time to prostate specific antigen (PSA) progression, time to administration of opiates due to cancer pain, time to initiation of cytotoxic therapy and time to worsening of the overall condition of a patient according to Eastern Cooperative Oncology Group (ECOG) by at least 1 point [15]. The final analysis was published in 2015 at median follow-up of 49.2 months. A significant increase of OS was proved in patients treated with abiraterone (34.7 vs. 30.3 months, HR: 0.81, 95% CI: 0.70–0.93, p = 0.003) [16].
Enzalutamide belongs to a group of nonsteroidal antiandrogens. Contrary to the previous antiandrogens (e.g. bicalutamide), enzalutamide affects the AR on three levels. Phase 3 clinical trial PREVAIL, published in 2014, assessed patients with mCRPC treated with enzalutamide without previous chemotherapy. A total of 1717 patients were studied and randomized in 1:1 allocation ratio for enzalutamide (160 mg/day) or placebo. The primary objective was to determine OS and rPFS. The clinical study demonstrated a significant prolongation of OS (HR: 0.71; 95% CI: 0.60–0.84, p < 0.001). Similarly, the one-year survival rate was significantly higher in patients treated with enzalutamide (65% vs. 14%, HR: 0.19; 95% CI: 0.15–0.23, p < 0.001) [17]. Another analysis of this trial was published in 2017 confirming a significant increase of median OS in patients treated with enzalutamide (35.3 vs. 31.3 months, HR: 0.77; 95% CI: 0.67–0.88, p = 0.0002) [18].
Based on the results mentioned in clinical trials, abiraterone and enzalutamide were introduced into clinical practice in the Czech Republic. The aim was to assess therapeutic outcomes and tolerance in patients treated with ARTA treatment at one oncological center in the Czech Republic.
Materials and methods
The presented work is a retrospective analysis of 64 patients with mCRPC treated with abiraterone (50 patients) and enzalutamide (14 patients) in the first line of this disease. The median follow-up was 28.4 months.
Abiraterone was administered to patients in one daily oral dose of 1000 mg (2 tablets of 500 mg). and prednisone in total daily dose 10 mg.
Enzalutamide was administered to patients in one daily oral dose of 160 mg (4 tablets of 40 mg). We did not indicate prednisone in patients treated with enzalutamide.
Patients were treated at outpatient care regularly every 28 days. The clinical examination consisted of assessment of clinical condition and tolerance to therapy. Patients were indicated for regular blood tests (blood count, biochemistry panel, PSA and testosterone levels). Patients who did not undergo orchiectomy continued the pharmacological ADT. Patients with bone metastases were indicated for denosumab or bisphosphonates administration. The abiraterone or enzalutamide treatment was administered until disease progression or unacceptable toxicity according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.
Progression was defined as elevation of PSA combined with deterioration of the clinical condition of the patient or progression in imaging [computed tomography (CT) examination according to Response Evaluation Criteria In Solid Tumours (RECIST) 1.1. and bone scintigraphy using technetium 99m according to Prostate Cancer Clinical Trials Working Group (PCWG2) criteria] [19, 20]. Progression-free survival (PFS) was defined as the period from the date of the initiation of ARTA treatment until the day of disease progression or the date of the last follow-up visit in patients without progression (censored data). OS was defined as time from date of the initiation of ARTA treatment until date of all-cause death or the date of the last follow-up visit in living patients. (censored data). PFS and OS estimation were calculated according to the Kaplan-Meier analysis. Using the Cox regression analysis, we evaluated the effect of selected factors on overall survival. HR was calculated with 95% CI. Values HR smaller than 1 represent a risk reduction (favorable prognostic effect), whereas values above 1 represent a negative prognostic factor. All tests were assessed in a standard way at a significance level of α = 0.05. SPSS statistical software was used for calculation.
Results
Patients characteristic
The average age of patients was 73.3 (61 to 92) years, 29 (45.3%) of the patients were ≥ 75 years old. All the cases, histologically, were acinar adenocarcinoma. Histologically, GS 6–7 was found in 33 (51.6%) patients; GS 8–10 was found in 31 patients (48.4%). Primarily metastatic disease was diagnosed in 26 (40.6%) patients. In 38 (59.4%) patients, the disease was diagnosed in a localized clinical stage. The most common metastatic site was the bone (53 patients, 82.8%). Twenty-eight (43.8%) patients had metastases in the lymph nodes and in 2 patients (3.1%) we detected visceral metastases (liver and lungs). Primary local treatment (radiotherapy or radical prostatectomy) was indicated in a total of 37 (57.8%) patients with a primarily localized disease. Eleven patients (17.2%) underwent radical prostatectomy, of whom 10 patients also underwent a subsequent adjuvant or salvage radiotherapy. A total of 26 (40.6%) patients were treated by primary radiotherapy. Primary ADT (orchiectomy, administration of LHRH analogs or LHRH antagonists) was indicated in all patients before the initiation of ARTA. The median time of administration of primary ADT was 23.0 (6 to 120) months. Median PSA before the start of ARTA was 20.7 (0.3–1588) ng/ml. PS was assessed according to the ECOG classification. PS 0 was found in 31 (48.4%) and PS 1 in 33 (51.6%) patients. The cohort of patients is shown in Table 1.
Table 1.
Patients characteristics
| All patients (64) N (%) | Abiraterone group (50) N (%) | Enzalutamide group (14) N (%) | |
|---|---|---|---|
|
| |||
| Age | |||
| Median (range), years | 71 (61–92) | 73(61–87) | 69(62–92) |
| ≥ 75 years | 29 (45.3) | 24 (48.0) | 5 (35.7) |
|
| |||
| Disease at the time of diagnosis | |||
| Metastatic | 26 (40.6) | 18 (36.0) | 8 (57.1) |
| Localized | 38 (59.4) | 32 (64.0) | 6 (42.9) |
|
| |||
| Gleason score (GS) | |||
| GS 6–7 | 33 (51.6) | 27 (54.0) | 6 (42.9) |
| GS 8–10 | 31 (48.4) | 23 (46.0) | 8 (57.1) |
|
| |||
| Primary local treatment | |||
| Radical prostatektomy | 11 (17.2) | 9 (18.0) | 2 (14.3) |
| Radical radiotherapy | 36 (56.3) | 30 (60.0) | 6 (42.9) |
|
| |||
| Metastases localization | |||
| Bone | 53 (82.8) | 39 (78.0) | 14 (100.0) |
| Lymphatic | 28 (43.8) | 24 (48.0) | 4 (28.6) |
| Visceral | 2 (3.1) | 1 (2.0) | 1 (7.1) |
|
| |||
| Duration of primary ADT | |||
| Median (range), months | 23.0 (6–120) | 20.5 (6–120) | 26.5(8–120) |
|
| |||
| Performance status (PS ECOG) | |||
| PS 0 | 31 (48.4) | 24 (48.0) | 7 (50.0) |
| PS 1 | 33 (51.6) | 26 (52.0) | 7 (50.0) |
|
| |||
| Pretreatment PSA | |||
| Median (range) [ng/ml] | 20.7 (0.3–1588) | 20.1 (0.3–1588) | 21.6 (0.9–1153) |
ADT — androgen deprivation therapy; ECOG — Eastern Cooperative Oncology Group; PSA — prostate-specific antigen
Results
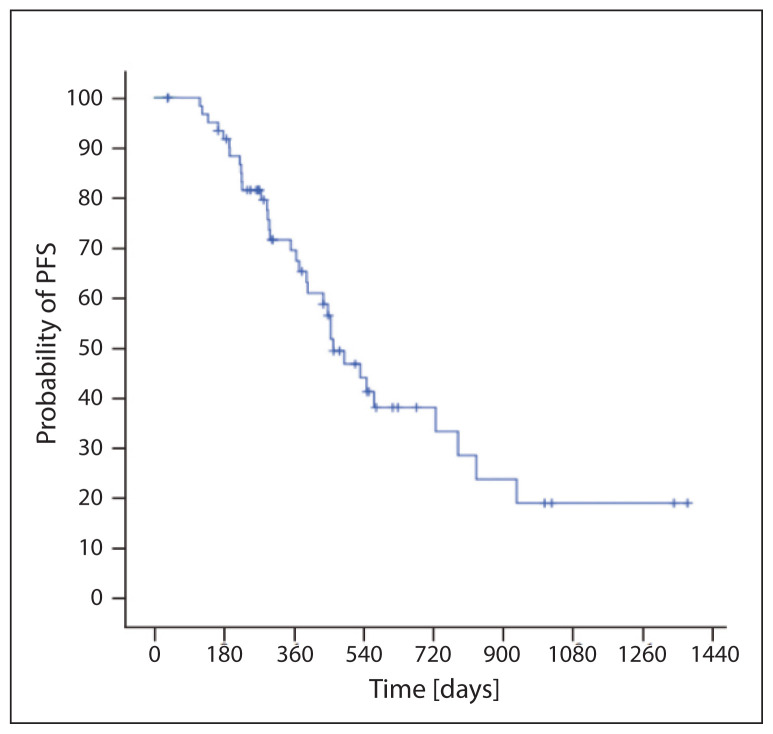
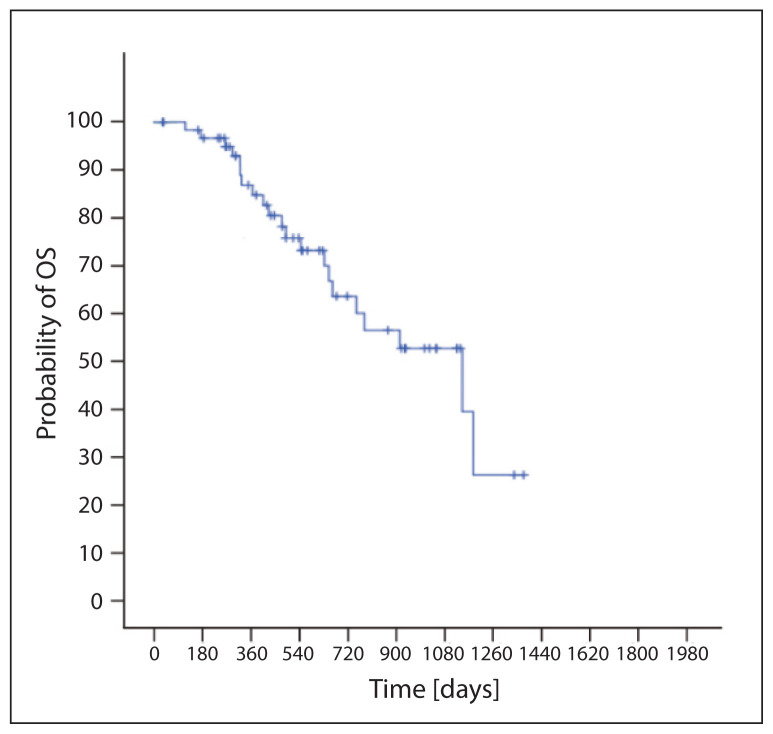
Median of nadir PSA during ARTA was 2.6 (0–848) ng/ml. Reduction in PSA ≥ 50% was observed in 53 (82.8%) patients; an early 3-month reduction was shown in 44 (68.8%) patients. Progression was found in 34 patients (53%); median PFS was 15.4 months (95% CI 12.3–18.5) (Fig. 1). A total of 21 patients died (33%); the median OS was 38.2 months (95% CI 19.9–56.5) (Fig. 2). We proved, in regression analysis, a statistically significant favorable prognostic effect on PFS in patients with reduction of PSA ≥ 50%, in patients with early reduction of PSA ≥ 50% within 3 months from the initiation of ARTA treatment, in patients younger than 74 years and in overall PS 0. We did not establish the effect of GS values or length of previous ADT on PFS. Similarly, patients with primarily a metastatic disease did not have worse PFS (Tab. 2). In regression analysis we proved a statistically significant favorable prognostic effect on OS in patients with reduction of PSA ≥ 50%, in patients with early reduction of PSA ≥ 50% within 3 months from the initiation of ARTA treatment and in patients with overall PS 0. We did not establish the effect of GS values, age or length of previous ADT on PFS. Similarly, patients with primarily a metastatic disease did not have a worse OS (Tab. 3).
Figure 1.
Progression free survival (PFS)
Figure 2.
Overall survival (OS)
Table 2.
Factor impact on progression free survival
| Factors | HR | 95% CI | p-value |
|---|---|---|---|
|
| |||
| PSA decrease ≥ 50 % | 0.416 | 0.187–0.927 | 0.032 |
| Early PSA decrease ≥ 50 % (≤ 3 vs. > 3 months) | 0.402 | 0.157–0.865 | 0.012 |
| Primary ADT duration (> 12 vs. ≤ 12 months) | 0.622 | 0.232–1.607 | 0.630 |
| Age (< 74 vs. ≥ 74 years) | 0.409 | 0.201–0.835 | 0.014 |
| Gleason score (6–7 vs. 8–10) | 0.946 | 0.479–1.865 | 0.872 |
| PS (0 vs. 1) | 0.149 | 0.061–0.366 | 0.001 |
| Primary localized vs. metastatic disease | 0.588 | 0.280–1.234 | 0.160 |
PSA — prostate-specific antigen; ADT — androgen deprivation therapy; PS — performance status; HR — hazard ratio; CI — confidence interval
Table 3.
Factor impact on overal survival
| Factors | HR | 95% CI | p-value |
|---|---|---|---|
|
| |||
| PSA decrease ≥ 50 % | 0.388 | 0.122–0.930 | 0.038 |
| Early PSA decrease ≥ 50 % (≤ 3 vs. > 3 months) | 0.330 | 0.120–0.910 | 0.032 |
| Primary ADT duration (> 12 vs. ≤ 12 months) | 0.735 | 0.281–1.588 | 0.845 |
| Age (< 74 vs. ≥ 74 years) | 0.431 | 0.168–1.101 | 0.079 |
| Gleason score (6–7 vs. 8–10) | 0.932 | 0.391–2.227 | 0.876 |
| PS (0 vs. 1) | 0.115 | 0.026–0.498 | 0.004 |
| Primary localized vs. metastatic disease | 0.691 | 0.264–1.807 | 0.451 |
PSA — prostate-specific antigen; ADT — androgen deprivation therapy; PS — performance status; HR — hazard ratio; CI — confidence interval
Some of the patients were subsequently treated by another line of therapy after progression on ARTA treatment. Symptomatic therapy was indicated for the remaining patients. The reason was deterioration of the overall condition in the case of disease progression or declination of further therapy by the patient. We indicated follow-up treatment in a total of 19 patients (55.9%). The most common type of follow-up treatment was application of chemotherapy — docetaxel. The overview of follow-up treatments is shown in Table 4.
Table 4.
Subsequent therapy after first-line androgen receptor targeted agents (ARTA)
| Subsequent treatment | Patients with ARTA discontinuation (N, %) | ||
|---|---|---|---|
|
| |||
| All patients (n = 34) | Patients with abiraterone progression (n = 31) | Patients with enzalutamide progression (n = 3) | |
|
| |||
| All (N, %) | 19 (55.9) | 17 (54.8) | 2 (66.7) |
| Chemotherapy | 9 (26.5) | 9 (29.0) | 0 |
| Second-line ARTA | 5 (14.7) | 3 (9.7) | 2 (66.7) |
| Radium 223 | 5 (14.7) | 5 (16.1) | 0 |
Tolerance
Abiraterone treatment was tolerated well. Adverse effects of abiraterone were reported in a total of 53 (86.9%) patients. Grade 3–4 toxicity was described in 17 (27.9%) patients. The abiraterone treatment was terminated due to toxicity in one patient who suffered a grade 4 skin reaction. The most common adverse effect was fatigue, diarrhea and elevation of ALT or AST. In 23 (37.7%) patients, we reduced the simultaneous administration of prednisone to a dose of 5 mg/day. The most common cause was worsened compensation of diabetes (10 patients), increased food intake with weight gain (8 patients) and occurrence of small subcutaneous hematomas (8 patients). After reducing the dose of prednisone, we did not observe any worsening of abiraterone toxicity. Similarly, enzalutamide therapy was well tolerated, too. Adverse effects of enzalutamide were reported in a total of 10 (78.5%) patients. Grade 3–4 toxicity was found in 1 (7.1%) patient. The most common symptoms included fatigue, diarrhea and nausea.
Discussion
In our patients treated with ARTA before the docetaxel chemotherapy, we have proven median of PFS 15.4 months and median of OS 38.2 months. Registration study COU-AA-302 assessing abiraterone showed median rPFS 16.5 months and median OS 34.7 months [16]. The clinical study PREVAIL assessing enzalutamide showed median rPFS 20.8 months and median OS 35.3 months [18]. The median PFS could have been lower in our group because a portion of the patients who had elevation of PSA and concurrent clinical deterioration of their condition were assessed for progression earlier than in the cases indicated by radiographic examination. On the other hand, the length of OS in our group was slightly longer than in registration studies COU-AA-302 and PREVAIL. The reason could have been a larger portion of patients subsequently treated with another antineoplastic treatment. It was 55% of the patients in our group versus 44% in the COU-AA-302 study and 43.8% in the PREVAIL trial. In our group, we had a larger percentage of patients older than 75 years compared to registration studies. The cause could have also been age preference of ARTA therapy in older patients before administering more toxic chemotherapy. In 2021, a retrospective work was published evaluating therapy results of ARTA in patients with mCRPC. The work demonstrated lower median OS for abiraterone (24 months) and enzalutamide (29 months) compared to our group and registration studies. The cause could have been a higher proportion of patients with worse prognostic factors such as higher GS, as more than third of the patients had PS 2–3 [21]. The results of a prospective clinical trial evaluating abiraterone in 454 patients treated in clinical practice demonstrated the median PFS 17.3 (95% CI: 14.1–19.4) months and median OS 37.3 (95% CI: 36.5 — not reached) months. There was a higher percentage of patients above 75 years old in the study and higher proportion of patients with visceral disease than in our group [22].
The determination for an optimum treatment sequence for mCRPC has been widely discussed, both in conferences and in professional literature. Currently, there is no available unambiguous molecular predictive factor for selection of an optimum treatment for mCRPC that we could use in a common clinical practice. The most studied predictive factor for the administration of ARTA in patients with mCRPC is the splice variant of AR called AR-V7. The AR-V7 variant is most commonly determined from the circulating tumor cells [23]. A clinical study published in 2014 showed that the detection of AR-V7 in circulating tumor cells of mCRPC patients is associated with resistance to enzalutamide or abiraterone. A total of 31 patients treated with enzalutamide and 31 patients treated with abiraterone were evaluated. Patients with AR-V7 showed a significantly shorter median OS in both groups, shorter survival without PSA progression or radiographic progression, compared to patients without AR-V7 detection [24]. A larger study evaluating 202 patients treated with ARTA similarly showed shorter PFS and OS in patients with the presence of AR-V7[25]. Another clinical trial, on the other hand, did not confirm these findings [26]. It appears that the incidence of AR-V7 increases after a ARTA treatment, reaching the incidence of 19–34%, compared to 3% before initiating mCRPC therapy [23]. Testing for this biomarker is not standard procedure and administration of ARTA in clinical practice is not currently dependent on its analysis. Currently, we have in Czech Republic five lines available for patients with mCRPC It is a proven fact that patients who undergo more lines of therapy of mCRPC have longer overall survival rates [27]. In our clinical practice choosing the type of treatment depends on the clinical characteristics of patients which include: performance status, comorbidities, presence of symptoms, localization of metastases (bone vs. visceral). Furthermore, it is important to take into consideration potential toxicity that differs in the individual types of planned therapy.
In our group, within the regression analysis we showed a favorable prognostic effect of PSA ≥ 50% reduction on the extension of PFS and OS. Particularly, those patients who experienced a rapid decline of PSA ≥ 50% within three months from starting ARTA benefited significantly. The favorable effect of PSA reduction has been repeatedly documented in clinical practices both in chemotherapy and ARTA [28–31]. With regard to the simplicity of testing, evaluation of PSA dynamics may serve for the prediction of treatment results even in clinical practice. We also proved a significant effect on prolongation of OS and PFS in patients with PS 0. This fact can be associated with a low volume of the disease which does not manifest when in performance status PS 0, however, in PS 1 patients, the restrictions may be caused by clinical manifestation of more advanced disease. Furthermore, the PS 0 patient group, too, has fewer comorbidities which can also affect therapy outcomes, especially in long-term treatment. Possible proof of overall better condition may also be our observation, where patients < 75 years showed a favorable prognostic effect on the extension of PFS. We did not see any effect of GS on therapy results in our patients. GS values proved a significant prognostic effect on OS and formation of metastases in patients after local treatment [32–34]. Predictive significance of GS on ARTA effect is not clear. Fizazi in his analysis of clinical trials COU-AA-301 and COU-AA-302 did not prove an impact of GS value on the effect of abiraterone [35]. However, another smaller analysis proved that high GS is an independent factor for worse therapy results of abiraterone [36]. At the EUA conference, more practical results were brought in 2016 by a published post hoc analysis of registration study COU-AA-302. The analysis demonstrated that the greatest benefit was noticed in patients who were asymptomatic, had entry concentration of PSA ≤ 80 ng/ml and whose Gleason score was ≤ 7 [37]. In clinical practice, GS 8–10 is currently not a contraindication for administering ARTA to our patients. A more important reality, however, is timely initiation of therapy, if greater disease progression and onset of symptoms or significant PSA elevation have not occurred yet. Within the regression analysis, we did not demonstrate any significant effect of previous ADT on PFS or OS in our group. Some data suggest that a shorter time of primary ADT may be a negative predictive factor to subsequent ARTA in patients with mCRPC [38]. A work was published in 2021 that evaluated the prognostic effect of duration of primary ADT on subsequent treatment with abiraterone or enzalutamide in patients with mCRPC. The duration of ADT was assessed in 3 groups (< 12 months, 12–36 months, > 36 months). Patients with longer duration of ADT showed a significant effect on extension of median OS (17.3; 19.9 and 31.6 months, p = 0.001). The significance of longer duration of ADT was seen both in chemotherapy-naive patients and in patients after chemotherapy [39].
The presented analysis shows a higher number of patients treated with abiraterone than enzalutamide. This is related to the fact that abiraterone was approved as a covered treatment at an earlier date than was enzalutamide. We do not have an efficacy comparison of these medicines on the basis of data from prospective studies. A meta-analysis of 24 cohorts reciprocally compared enzalutamide with abiraterone. The study compared ARTA before and after giving docetaxel. Before administering docetaxel to patients treated with enzalutamide the median OS was 31.1 months (95% CI: 29.3–32.9). In patients treated with abiraterone the median OS was 25.2 months (95% CI: 23.7–26.6). The difference was 5.9 months in favor of enzalutamide (HR: 0.81, p < 0.001). An interesting thing was a higher ratio of patients with GS 8–10 treated with enzalutamide (52% vs. 42%), which, however, did not correlate with median pre-treatment PSA concentration, which was higher in patients treated with abiraterone (32.7 vs. 184 ng/ml). The authors also made an adjustment to GS, where the difference in median OS was even more distinct in favor of enzalutamide. Similarly, median PFS was also significantly longer in patients treated with enzalutamide (15.8 vs. 7.4 months, HR: 0.47, p < 0.0001) [40].
In our clinical practice, we indicate the type of ARTA based on comorbidities of the patients. In patients with cardiovascular disease or diabetes we prefer to give enzalutamide, while in patients with a history of brain disease we prefer abiraterone. However, it is always important to consider possible drug interactions of the planned ARTA therapy.
Conclusion
The our analysis of patients with mCRPC treated with abiraterone or enzalutamide in the first line showed that ARTA represents an effective and safe therapy and contributes to longer survival of our mCRPC patients.
Acknowledgements
Scientific Board, Regional Hospital Liberec
Footnotes
Author contributions: S.A. — manuscript writing; JB — data interpretation; VS — data collection; JD — data interpretation; HK — statistical analysis; IR — manuscript writing, literature search, data collection, data interpretation.
Conflict of interest: The authors state that there are no conflicts of interest regarding the publication of this article.
Ethical permission: Ethical approval was not necessary for the preparation of this article.
Funding: None declared.
References
- 1.Soni A, Jadhav GK, Manocha S, et al. Comparative evaluation of hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate and high risk prostate cancer. Rep Pract Oncol Radiother. 2022;27(6):1001–1009. doi: 10.5603/RPOR.a2022.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osoba D, Tannock IF, Ernst DS, et al. Use of palliative end points to evaluate the effects of mitoxantrone and low-dose prednisone in patients with hormonally resistant prostate cancer. J Clin Oncol. 1994;12(4):689–694. doi: 10.1200/JCO.1994.12.4.689. [DOI] [PubMed] [Google Scholar]
- 3.Pond GR, Sonpavde G, de Wit R, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Oudard S, Ozguroglu M, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 6.Benabdallah N, Lu P, Abou DS, et al. ALSYMPCA Investigators. Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(9):1384–1393. doi: 10.1007/s00259-013-2427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor AO. Progression of metastatic castrate-resistant prostate cancer: impact of therapeutic intervention in the post-docetaxel space. J Hematol Oncol. 2011;4:18. doi: 10.1186/1756-8722-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 10.Geller J, Albert J, Loza D, et al. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res. 1979;33(1B):103–111. [PubMed] [Google Scholar]
- 11.Mohler JL, Gregory CW, Ford OH, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumagai J, Hofland J, Erkens-Schulze S, et al. Intratumoral conversion of adrenal androgen precursors drives androgen receptor-activated cell growth in prostate cancer more potently than de novo steroidogenesis. Prostate. 2013;73(15):1636–1650. doi: 10.1002/pros.22655. [DOI] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, de Bo, et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096. Erratum in: N Engl J Med 2013; 368(6)584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan CJ, Smith MR, Fizazi K, et al. COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 16.Beer TM, Armstrong AJ, Rathkopf DE, et al. PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol. 2017;71(2):151–154. doi: 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Collette L. Re: Design and endpoints of clinical trials in patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Eur Urol. 2008;54(2):462. doi: 10.1016/j.eururo.2008.04.086. [DOI] [PubMed] [Google Scholar]
- 20.Demirci A, Bilir C, Gülbağcı B, et al. Comparison of real-life data of abiraterone acetate and enzalutamide in metastatic castration-resistant prostate cancer. Sci Rep. 2021;11(1):14131. doi: 10.1038/s41598-021-93659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Procopio G, Chiuri VE, Giordano M, et al. Real-world experience of abiraterone acetate plus prednisone in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: long-term results of the prospective ABItude study. ESMO Open. 2022;7(2):100431. doi: 10.1016/j.esmoop.2022.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Büchler T, Bobek V, Kološtová K. Testování varianty androgenového receptori AR-V7 pro výběr pacientů s kastračně refrakterním metastazujícím karcinomem prostaty k léčbě novými hormonálními léky. Klin Onkol. 2018;3(1):9–14. doi: 10.14735/amko20189. [DOI] [PubMed] [Google Scholar]
- 23.Antonarakis E, Lu C, Wang H, et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/nejmoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo J, Luber B, Wang H, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second- Line Abiraterone and Enzalutamide. J Clin Oncol. 2017;35(19):2149–2156. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernemann C, Schnoeller TJ, Luedeke M, et al. Expression of AR-V7 in Circulating Tumour Cells Does Not Preclude Response to Next Generation Androgen Deprivation Therapy in Patients with Castration Resistant Prostate Cancer. Eur Urol. 2017;71(1):1–3. doi: 10.1016/j.eururo.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Angelergues A, Efstathiou E, Gyftaki R, et al. Results of the FLAC European Database of Metastatic Castration-Resistant Prostate Cancer Patients Treated With Docetaxel, Cabazitaxel, and Androgen Receptor-Targeted Agents. Clin Genitourin Cancer. 2018;16(4):e777–e784. doi: 10.1016/j.clgc.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AJ, Tannock IF, de Wit R, et al. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46(3):517–525. doi: 10.1016/j.ejca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Halabi S, Armstrong AJ, Sartor O, et al. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J Clin Oncol. 2013;31(31):3944–3950. doi: 10.1200/JCO.2013.50.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XuS, Ryan CJ, Stuyckens K, et al. Correlation between Prostate-Specific Antigen Kinetics and Overall Survival in Abiraterone Acetate-Treated Castration-Resistant Prostate Cancer Patients. Clin Cancer Res. 2015;21(14):3170–3177. doi: 10.1158/1078-0432.CCR-14-1549. [DOI] [PubMed] [Google Scholar]
- 30.Conteduca V, Crabb SJ, Scarpi E, et al. Association Between Early PSA Increase and Clinical Outcome in Patients Treated with Enzalutamide for Metastatic Castration Resistant Prostate Cancer. Mol Diagn Ther. 2016;20(3):255–263. doi: 10.1007/s40291-016-0196-1. [DOI] [PubMed] [Google Scholar]
- 31.Epstein JI, Allsbrook WC, Amin MB, et al. ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 32.Kryvenko ON, Epstein JI, Epstein JI, et al. Update on the Gleason grading system for prostate cancer: results of an international consensus conference of urologic pathologists. Adv Anat Pathol. 2006;13(1):57–59. doi: 10.1097/01.pap.0000202017.78917.18. [DOI] [PubMed] [Google Scholar]
- 33.Buhmeida A, Pyrhönen S, Laato M, et al. Prognostic factors in prostate cancer. Diagn Pathol. 2006;1:4. doi: 10.1186/1746-1596-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fizazi K, Flaig TW, Stöckle M, et al. Does Gleason score at initial diagnosis predict efficacy of abiraterone acetate therapy in patients with metastatic castration-resistant prostate cancer? An analysis of abiraterone acetate phase III trials. Ann Oncol. 2016;27(4):699–705. doi: 10.1093/annonc/mdv545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azria D, Massard C, Tosi D, et al. An ambispective observational study in the safety and efficacy of abiraterone acetate in the French temporary authorizations for use (ATU): Predictive parameters of response. J Clin Oncol. 2012;30(5_suppl):149–149. doi: 10.1200/jco.2012.30.5_suppl.149. [DOI] [Google Scholar]
- 36.Miller K, Carles J, Gschwend JE, et al. The Phase 3 COUAA-302 Study of Abiraterone Acetate Plus Prednisone in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Stratified Analysis Based on Pain, Prostate-specific Antigen, and Gleason Score. Eur Urol. 2018;74(1):17–23. doi: 10.1016/j.eururo.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Loriot Y, Massard C, Albiges L, et al. Personalizing treatment in patients with castrate-resistant prostate cancer: A study of predictive factors for secondary endocrine therapies activity. J Clin Oncol. 2012;30(5_suppl):213–213. doi: 10.1200/jco.2012.30.5_suppl.213. [DOI] [Google Scholar]
- 38.Di Stefano RF, Tucci M, Turco F, et al. Prognostic role of early PSA drop in castration resistant prostate cancer patients treated with abiraterone acetate or enzalutamide. Minerva Urol Nefrol. 2020;72(6):737–745. doi: 10.23736/S0393-2249.20.03708-X. [DOI] [PubMed] [Google Scholar]
- 39.Fang M, Nakazawa M, Antonarakis ES, et al. Efficacy of Abiraterone and Enzalutamide in Pre- and Postdocetaxel Castration-Resistant Prostate Cancer: A Trial-Level Meta-Analysis. Prostate Cancer. 2017;2017:8560827. doi: 10.1155/2017/8560827. [DOI] [PMC free article] [PubMed] [Google Scholar]