Abstract
In the present study, we investigated the effect of dietary supplementation with the probiotic Bacillus amyloliquefaciens AV5 (OR647358) on the growth, serum and mucus immune responses, metabolomics, and lipid metabolism of Oreochromis niloticus. Fishes (27.2 ± 1.7 g and 9.0 ± 1.2 cm) were fed three distinct meals: a commercial diet (control-GC) and two treatment diets supplemented with probiotics at 106 (G1) and 108 cfu/g (G2), respectively, for 30 days. In the G2 group, the final weight, specific growth rate, weight gain rate, survival rate, and feed conversion ratio of the fish were significantly improved (p < 0.05). Lysozyme, myeloperoxidase, and alkaline phosphatase activities in the mucus of fish were significantly higher (p < 0.05) in the G1 and G2 groups. The serum total protein, superoxide dismutase, glutathione peroxidase, reactive oxygen species, and reactive nitrogen species levels were noticeably higher (p < 0.05) in fish fed G1 and G2. In addition, in the G1 and G2 groups, higher levels of enzymes involved in lipid metabolism, such as pyruvate kinase, 2-hydroxyethyl-ThPP, and dihydrolipoamide dehydrogenase, were increased. Distal gastrointestinal metabolites, such as glycerophospholipids and histidine, were observed. These findings strongly indicate that incorporating B. amyloliquefaciens AV5 at 108 cfu/g into commercial feeds positively influences fish growth, immunity, and lipid metabolism.
1. Introduction
In recent years, the increased production of Nile tilapia (Oreochromis niloticus) has been accompanied by an increase in infections and disease outbreaks, causing significant economic losses in the aquaculture industry [1]. Probiotics are a promising remedy for aquaculture, offering many advantages, such as enhancing fish growth, preserving the environment, and bolstering disease resilience, compared with alternative preventive approaches [2, 3]. Potential probiotic strains exhibit diverse characteristics that enable them to endure challenging conditions in the gastrointestinal tract. These traits include acid and bile salt tolerance, resilience against gastric juices, production of extracellular enzymes and antimicrobial substances capable of eliminating or inhibiting pathogen growth, adherence to intestinal mucus, and the capacity to reside in the gut [4]. Moreover, probiotic strains must fulfill biosafety criteria including nonhemolytic activity and antibiotic susceptibility [4]. Probiotic Bacillus species exhibit a remarkable array of mechanisms to inhibit fish pathogens, ranging from direct antibacterial actions such as bacteriocin production and lytic enzyme activity to indirect strategies such as immunostimulation and competitive exclusion [5]. Bacillus spp. are unique probiotics because they can produce spores. These spores produce nonpathogenic and nontoxic compounds, improve water quality, and make bacteria resistant to unfavorable conditions [4]. These characteristics distinguish them from other probiotics and provide advantages such as heat tolerance and extended shelf life [6]. Many studies have shown that certain types of Bacillus species are useful in aquaculture, particularly in Nile tilapia farming [7, 8, 9]. Catfish fed Bacillus amyloliquefaciens can boost their immune system [10]. Bacillus amyloliquefaciens YL-10 has the potential to be employed as a probiotic bacterium to prevent vibriosis infections in Haliotis discus hannai [11]. In a study by Liu et al. [12], adding B. subtilis E20 to the diet improved immune cells during phagocytosis and made Oplegnathus fasciatus less likely to cause infections. Metabolomics is a scientific discipline that helps uncover changes in metabolism by analyzing small molecules in biofluids, tissues, and organs, which play crucial roles in all biological processes [13, 14]. Nevertheless, the optimal levels at which B. amyloliquefaciens should be added to the diet of freshwater Nile tilapia to enhance immunological responses, metabolite levels, and lipid metabolism remain unclear [15, 16, 17]. This study investigated the impact of feed with varying levels of B. amyloliquefaciens AV5 on the growth, immune response, metabolomics, and lipid metabolism of Nile tilapia (O. niloticus).
2. Materials and Methods
2.1. Animal Ethics
The experiments were done according to the guidelines for the care and use of laboratory animals in China. The research received approval from the Ethics Committee for Animal Experiments at Guangdong Ocean University.
2.2. Experimental Fish and Rearing Condition
The healthy Nile tilapia fingerlings, O. niloticus (weight, 23.0 ± 1.5 g and length, 8.0 ± 1.2 cm), were sourced from Langye fish farm in Gaozhou, Guangdong province, China. These fingerlings exhibited no signs of bleeding, lethargy, ascites, or detachment of scales. They were transported and placed in plastic tanks at the Key Laboratory of Aquatic Animal Disease Control and Healthy Culture at Guangdong Ocean University. The fishes were given a 14-day period to acclimate before the commencement of the experimental study. The fishes were given commercial feed (Guangdong Yuehai Feed Group, Zhanjiang, China) twice daily, equivalent to 3% of their body weight. The fish's weight was measured weekly to evaluate growth and implement any required adjustments to the daily feeding ratio. Excrement and unconsumed food were periodically removed via siphoning. To prevent stress, the researchers consistently delivered aeration throughout the acclimation phase. Throughout this time frame, their health conditions were observed and assessed according to the study performed by Gobi et al. [18]. The water quality metrics were maintained at their basic levels throughout the probation and experimental trial periods, following the procedure undertaken by de Verdal et al. [19], with slight adjustments. Briefly, the conditions were as follows: temperature, 28.0 ± 1.5°C; dissolved oxygen, 6.8 ± 0.9 mg/L; pH level, 6.9 ± 1.2; and photoperiod, 13 hr of light followed by 11 hr of dark.
2.3. Strain Conditions
The strain B. amyloliquefaciens AV5 (OR647358) was obtained from the intestines of Nile tilapia in their natural habitat, and its molecular identity was determined using 16S rDNA gene sequencing (NCBI GenBank accession number OR647358). Previous studies have suggested that B. amyloliquefaciens has probiotic properties [20, 21]. The probiotic B. amyloliquefaciens AV5 used in this work was obtained from our laboratory's stock culture collection. It was preserved at −80°C in a 40% v/v solution of sterile glycerol in Luria–Bertani (LB) broth. To perform regular experiments, researchers obtained a preserved strain culture from the stocks kept at −80°C. This culture was then spread on nutrient agar and incubated overnight at 37°C in the presence of oxygen. We used a shaker incubator to cultivate a single isolated colony in 100 mL LB broth for 24 hr at 37°C. The cell density was determined by measuring the optical density at 600 nm (OD600) and then comparing it to the number of colony-forming units (cfu) obtained by serial dilution and spread plating on nutritional agar. The bacterial biomass was collected and centrifuged at 8,000 g for 20 min at 4°C. The liquid portion (supernatant) was extracted, and the bacteria pellets were diluted again in PBS at 106 and 108 cfu/mL.
2.4. Diets Preparation
Following the nutritional requirements of Nile tilapia, a basal diet from Guangdong Yuehai Feed Group (Zhanjiang, China) was used in the experiment. Table 1 displays the main ingredients and approximate composition of the basal diet according to the manufacturer's information. In the treatment trial, two different concentrations (106 and108 cfu/g) of probiotic B. amyloliquefaciens AV5 were added to the basal diet. The concentrations were chosen according to the studies conducted by Selim and Reda [22] and Thy et al. [23] with slight modifications. Briefly, probiotic suspensions were progressively sprayed (80 mL probiotic suspension/kg feed) on the commercial feed (Guangdong Yuehai Feed Group, Zhanjiang, China) before being blended gently using a mixer. The diets were dehydrated at 38°C in a drying cabinet and kept at 4°C until they were used again. The weekly assessment of the survivability of bacteria added to the diet was conducted using the technique developed by Abarike et al. [24], with minor adjustments. In a nutshell, 1.0 g of feed was thoroughly mixed with 9.0 mL of sterile saline solution (PBS), and further solutions were made in a series of dilutions up to 105. One hundred microliters of each was evenly inoculated into nutrient agar dishes in triplicate, incubated at 33°C for 24 hr, and the colonies were then determined. Mannitol egg yolk polymyxin (MYP) agar (Difco) was utilized to estimate the probiotic concentrations in the feed (cfu/g) [25]. Weekly feeds were meticulously prepared to maintain a consistent concentration of probiotics in the diets.
Table 1.
Composition and proximate values of the basal diets (expressed as percentage dry weight) for Nile tilapia (O. niloticus) fingerling.
| Basal diet analysis | Raw materials and nutrient composition |
|---|---|
| Ingredients | Fish meal |
| Soybean meal | |
| Peanut meal | |
| Rapeseed meal | |
| Flour | |
| Calcium dihydrogen phosphate | |
| Zinc sulfate | |
| Ferrous sulfate | |
| Vitamin A | |
| Vitamin C | |
| Vitamin D3 | |
| Vitamin E | |
| Folic acid | |
|
| |
| Analyzed proximate composition | Crude protein ≥ 34.0% |
| Crude fat ≥ 2.50% | |
| Crude fiber ≤ 8.00% | |
| Crude ash ≤ 15.0% | |
| Water ≤ 11.0% | |
| Lysine ≥ 1.80% | |
| Calcium 0.5%–2.50% | |
| Total phosphorus 0.6%–2.0% | |
| NaCl ≤ 2.00% | |
2.5. Experimental Setup and Fish Management
Following a period of probation, 270 fish of uniform size (weighing 27.2 ± 1.7 g and 9.0 ± 1.2 cm in length), which were healthy and free from diseases, were divided into three groups: a control group (GC) with no probiotic inclusion and two groups with probiotic inclusion diets at the concentrations of 106 cfu/g (G1) and 108 cfu/g (G2), respectively. Nine plastic tanks were used, with each tank containing 30 fish, each group was kept with triplicates. The fish were fed three different diets: commercial feed GC (without probiotics), G1 (with probiotics at a concentration of 106 cfu/g), and G2 (with probiotics at a concentration of 108 cfu/g). The trial period lasted 30 days, the fishes were given a commercial diet or commercial diet-supplemented probiotics twice daily at 08:30 and 16:30, amounting to 5% of their body weight [22]. Measurements of fish weight on a weekly basis were conducted to assess their growth and to make any necessary adjustments to the daily feeding ratio. To ensure more precise feed conversion ratio (FCR) values, we measured the weight of the feed before each feeding session to ensure consistent feed intake. The diets were carefully prepared weekly to ensure optimal results. Throughout the trial duration, water was consistently replaced, and undigested feeds were frequently sucked out using the siphoning method.
2.6. Sample Collection
2.6.1. Growth Performance
The fishes were not fed for 24 hr before the end of the feeding trial. After completing the feeding experiment, each treatment group, consisting of 15 similarly sized fishes, was administered with the anesthetic MS-222 (100 mg/L) to make them unconscious before sample collection. The fish's growth performance, including final weight (Wt), weight gain rate (WGR), survival rate (SR), specific growth rate (SGR), and FCR, was calculated as follows: Weight gain rate (WGR, %) = (final fish weight (g)−initial fish weight (g))/initial fish weight (g) × 100, survival rate (SR, %) = 100 × final fish number/initial fish number, specific growth rate (SGR%) = 100 × ln(final fish weight (g))−ln(initial fish weight (g))/ time of the experiment covered, and feed conversion ratio (FCR) = total feed intake (g)/(final fish weight (g)-initial fish weight (g)), similar to the method described by Xue et al. [20].
2.6.2. Mucus and Serum Sampling
Skin mucus was collected using the methodology described by Gobi et al. [18]. Briefly, mucus was collected from the front to the back and mixed with tris-buffered saline (TBS), a solution containing 50 mM tris-HCl and 150 mM NaCl, at pH 8.0. The homogenate was centrifuged at 1,500 g for 10 min at 4°C. The resulting liquid above (supernatant) was preserved at −80°C. Following a 30-day feeding experiment, six fishes were randomly selected from each tank and subjected to 24 hr food deprivation. The fishes were immobilized using MS222 (Sigma-Aldrich, Louis, MO, USA) at 100 mg/L. Blood from each fish was extracted from the caudal vein using a 1 mL syringe and transferred into plastic Eppendorf tubes. The tubes were refrigerated at 4°C for 12 hr, after which the blood samples were centrifuged at 3,000 g for 10 min. This process resulted in serum extraction, and the collected serum was preserved at −80°C [18]. According to a study by Gobi et al. [18], immunological markers and antioxidant enzyme activities can be assessed using serum and mucus. Tissue samples were obtained from the distal intestines of six randomly selected fishes from each tank. The distal tissue was cleaned using saline solution (0.65%) on a sterile operating table. It was then transferred to microcentrifuge tubes and immediately submerged in liquid nitrogen (LN2).Finally, the tissue was stored at −80°C for further metabolomics investigation on the gastrointestinal tract [26].
2.7. Immunological Markers and Biochemical and Antioxidant Indices
2.7.1. Lysozyme (LYZ) Activity
We used the technique of Liu et al. [27] to evaluate LYZ activity in skin mucus and serum. In summary, we utilized commercially available assay kits from the Nanjing Jiancheng Institute in Nanjing, China, to quantify LYZ levels. LYZ activity was determined using a spectrophotometer (UV-6100; Shanghai Yuanxi Instrument Ltd., Shanghai, China) at a wavelength of 530 nm. Units per millilitre of serum or mucus per protein was used to determine the LYZ activity.
2.7.2. Reactive Oxygen Species (ROS) Activity
Intracellular respiratory burst activity, which involves the release of superoxide anions, was assessed by assessing the formation of ROS using the technique described by Rubio and Cerón [28]. Optical density was measured at 540 nm using a plate reader.
2.7.3. Reactive Nitrogen Species (RNS) Activity
The Griess reagent technique, which quantifies nitric oxide (NO) by converting it to nitrite, was used to assess NO in serum and skin mucus [29]. The concentration of nitrite in the culture medium was determined by utilizing a standard curve.
2.7.4. Superoxide Dismutase (SOD) Activity
The SOD activity was quantified using the technique described by Gobi et al. [18]. The variation in absorbance was quantified at 560 nm (∆A560). The SOD activity was quantified as U mg/protein.
2.7.5. Glutathione Peroxidase (GPx) Production
GPx activity was meticulously measured using the technique described by Dworzański et al. [30], which involves precisely measuring the oxidation of NADPH in the presence of H2O2 at a wavelength of 340 nm. GPx activity was quantified with the highest accuracy as nmol NADPH/min mg per protein.
2.7.6. Protein Analysis
The dye-binding method, a reliable and widely accepted technique, was employed to determine the protein content of serum and skin mucus, with bovine serum albumin (BSA, Sigma) as a reference, following the procedure described by Ghafoori et al. [31].
2.7.7. Alkaline Phosphatase (ALP) Production
ALP activity was measured using the technique described by Tang et al. [32]. A unit of activity was expressed as the quantity of enzyme needed to produce 1 mmol of the p-nitrophenol product in 1 min.
2.7.8. Myeloperoxidase (MPO) Analysis
The method described by Pulli et al. [33] was used to measure the activity of MPO in skin mucus and serum. A unit was defined as the quantity that resulted in a change in absorbance of one, and the production was represented as units per milligrams mucus or serum per protein.
2.8. Metabolomics
2.8.1. Intestine Preparation for Metabolite Examination
After the dissection of the samples into tiny fragments and the removal of fat-rich tissues, a solution of phosphate-buffered saline (PBS) was used to clean the gastrointestinal tract and remove any traces of blood. The intestinal fragments were first stored in LN2 and then moved to a freezer set at −80°C for future examination. After thawing, the tissues were mixed with pure water until they were completely homogenized (100 mg of tissue dissolved in 500 μL of ultrapurified H2O). The intestinal fragments were removed from the electropolish (EP) tube using 300 mL of CH₃OH. After a 10-min ultrasound, 20 L of normal internal material was extracted, followed by vigorous mixing for 30 s. The intestines were incubated at 20°C for 1 hr for further elimination of all proteins. After centrifugation (13,000 rpm, 15 min, 4°C), the liquid portion (supernatant) was transferred to a glass container for mass spectrometry (LC/MS) analysis. Additionally, 2 mL of fresh liquid chromatography was added to the sample [34]. For quality control, 20 µL taken ffrom each sample was used. Ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) analysis was performed using 180 µL of the supernatant [35].
2.8.2. Metabolites Extraction and (UHPLC)-LC-MS Analysis
LC-MS analysis was conducted using state ultra-high-performance liquid chromatography (UHPLC). We used a TripleTOF 5,600 mass spectrometer, a cutting-edge instrument, in conjunction with a UPLC BEH amide column, to ensure the highest level of accuracy. The mobile phase was then prepared. The mobile phase was composed of a solution containing 25 mM NH4OAC and 25 mM NH4OH in water (pH value of 9.75) along with acetonitrile. The gradient elution technique was used at a flow rate of 0.5 mL/min. The procedure consisted of the following steps: starting with 2% B for 1.5 min, 2%–100% B for 12 min, 100% B for 14.0 min; 100%–2% B for 14.1 min, and finally returning to 2% B for 17 min, where B is methanol (0.1% (v/v) formic acid water). The injection was administered in a volume of 3 µL. LC-MS analysis was performed using triple time-of-flight (TOF) mass spectrometry and information-dependent (IDA) MS/MS spectrometry. Imaging was initiated by activating the tandem hold using the mass spectrometry (MS/MS) spectrum after all scan surveying programs were executed with different preselected configurations. Throughout the process and evaluation, the MS data were not interrupted. Each loop was designed to fragment 12 precursor ions, with a collision energy of 30 V.
2.8.3. Metabolite Identification and Differential Analysis
Thévenot et al. [36] identified Q2, R2Y, and R2X as the measurement indices for orthogonal fractional least squares discriminant determination (OPLS-DA). The translation amounts of the X and Y matrices of the constructed model are denoted as R2X and R2Y, respectively, and the model's predictive ability is denoted as Q2. An accurate model was projected to have Q2 > 0.5, while an exceptional model was expected to have a Q2 value greater than 0.9. Ensuring alignment authentication is crucial for assessing dependability of the OPLS-DA model. Owing to its considerable importance, the model was maintained in its current state. When the Q2 and R2 values were equal to or lower than the variance metabolites, they were selected using the factors vital in the prediction (VIP) analysis. Furthermore, the VIP value of the OPLS-DA model, p value of the t-test, and method of combining the variance multiples were implemented to enhance the specific metabolites of biological replicates [37]. We used VIP > 1.5, p=0.01, and fold change (FC) > 3 as selection standards. The annotated metabolites utilized in this study were obtained from Kyoto Encyclopedia of Genes and Genomes (KEGG). This database plays a pivotal role in facilitating comprehensive analysis of metabolite content, expression data, and genes [36]. Metabolomics pathway analysis (MetPA) was employed in conjunction with the KEGG to process the metabolite pathways. Improvement in metabolic routes by differential metabolites was investigated using the KEGG database. Metabolic routes were considered enriched when x/n > y/N was reached. Significant enrichment of metabolic pathways was determined when the p value was <0.05. To prepare the data for heatmap clustering using differential metabolite intensity regions, a Z-value was used to standardize the results. Subsequently, it was visualized using a heatmap tool in R software. The analysis of chord plots was improved using the R package GO plot 1.0.2 [38].
2.9. Statistical Analysis
Our statistical analysis involved several tests. To examine the differences between groups, one-way analysis of variance (ANOVA) was conducted when the data variance was homogeneous. Differences between treatment groups were considered statistically significant at p < 0.05, using Tukey's honest significant difference (HSD) tests, and data were presented as mean ± SD. Receiver operating characteristic (ROC) curve analysis was conducted for metabolites to determine the area under curve (AUC), which compares the predictive ability of metabolites. Furthermore, we created graphical depictions of immune responses using GraphPad software LLC (version 9.0; San Diego, California).
3. Results
3.1. Growth Performance
As shown in Table 2, Wt, WGR, SR, and SGR of fish fed G2 were significantly higher than those fed G1 and the control diet (GC). There was no notable difference in the FCR for diets given to fish between G2 and G1. Fish that were fed the GC had a notably greater FCR (p < 0.05).
Table 2.
Growth indexes of Nile tilapia after 30 days of feeding with a commercial feed as a control diet (GC) and a diet supplemented with B. amyloliquefaciens AV5 at 106 cfu/g and 108 cfu/g as G1 and G2, respectively.
| Growth indexes | GC | G1 (106 cfu/g) | G2 (108 cfu/g) |
|---|---|---|---|
| Wi (g) | 23 ± 0.3 | 23 ± 0.4 | 23 ± 0.2 |
| Wt (g) | 42 ± 2.4a | 46 ± 1.5a | 55 ± 1.25b |
| WGR (%) | 82.2 ± 1.3a | 104.3 ± 1.4a | 139.1 ± 1.2b |
| SGR (%) | 1.42 ± 0.8a | 1.68 ± 0.4a | 2.07 ± 0.2b |
| FCR | 1.18 ± 0.1a | 1.02 ± 0.3a | 0.67 ± 0.2b |
| SR (%) | 98.19 ± 2.94 | 98.67 ± 1.92 | 99.98 ± 1.04 |
Note. Results are presented as the mean ± S.D (n = 3). Based on Tukey's test, values with distinct superscripts on the same line show a significant difference (p > 0.05). Thus, the following variables are defined: initial weight (Wi), final weight (Wt), weight gain rate (WGR), specific growth rate (SGR), feed conversion ratio (FCR), and survival rate (SR).
3.2. Biochemical Parameters
Figure 1(a) shows that after 30 days, the experimental diet groups had significantly higher protein concentrations in mucus and serum compared to the control group (p < 0.05). The G2 group showed the highest protein levels. Both G1 and G2 groups also had significantly higher ALP levels in the serum and mucus (p < 0.05) than the control group, with serum showing a more pronounced response. The G2 group had the highest ALP level, whereas the control group had the lowest level. Additionally, the G1 and G2 groups exhibited significantly higher MPO activity in skin mucus than the control group (p < 0.05). The G2 group showed the highest MPO activity in both the serum and mucus.
Figure 1.
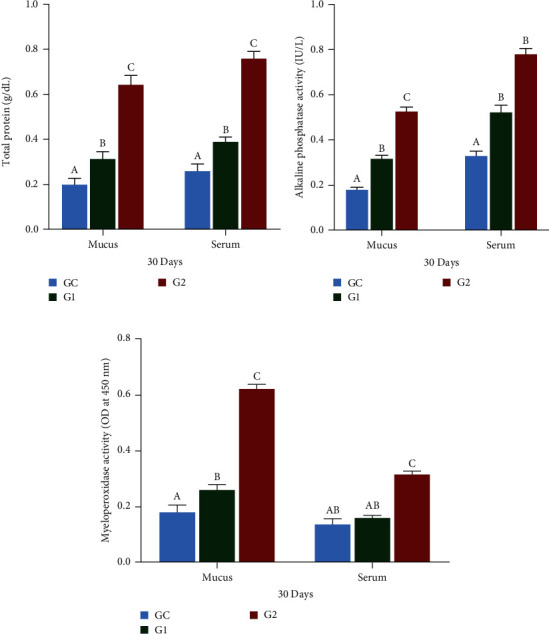
TP (a), ALP (b), and MPO (c) in mucus and serum of O. niloticus. Control: fish fed without probiotics (GC). G1 and G2: with commercial diet containing B. amyloliquefaciens AV5 at 106 and 108 cfu/g diet, respectively. TP denotes total protein, ALP denotes alkaline phosphatase, and MPO denotes myeloperoxidase activity. The values are shown as mean ± S.D of three replications. Based on Tukey's test, values with distinct letters on the same line show a significant difference (p < 0.05).
3.3. Immune Response Markers
After 30 days of treatment, LYZ levels in the serum and skin mucus of Nile tilapia significantly increased in the G1 and G2 groups compared to the control group (p < 0.05), as shown in Figure 2(a). The G2 group had the highest LYZ levels in both the serum and skin mucus. Skin mucus showed greater LYZ activity than serum.
Figure 2.
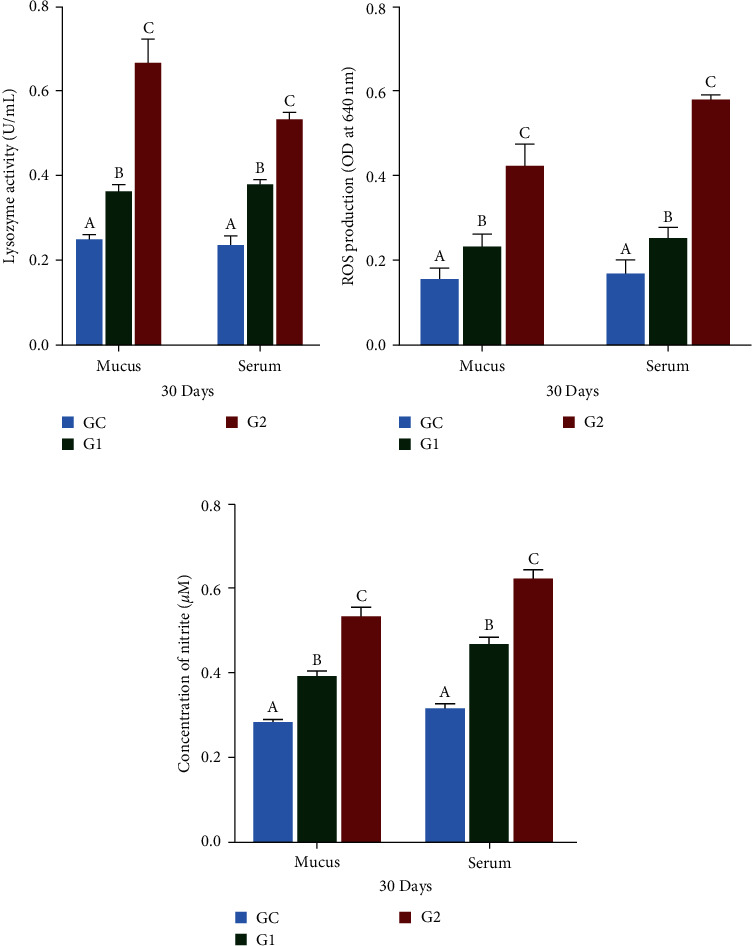
LYZ (a), ROS (b), and RNS (c) in mucus and serum of O. niloticus. Control: fish fed without probiotics (GC). G1 and G2: with commercial diet containing B. amyloliquefaciens AV5 at 106 and 108 cfu/g diet, respectively. LYZ denotes lysozyme activity, ROS denotes reactive oxygen, and RNS denotes reactive nitrogen activity. The values are shown as mean ± S.D of three replications. Based on Tukey's test, values with distinct letters on the same line show a significant difference (p < 0.05).
3.4. Antioxidant Indices
After 30 days of feeding, the generation of ROS in the serum and mucus substantially increased (p < 0.05) in the G1 and G2 groups relative to the control group (GC), as shown in Figure 2(b). ROS levels were higher in the serum than in the skin mucus, with the G2 group showing the highest levels and the GC the lowest levels. Similarly, the synthesis of RNS was significantly increased (p < 0.05) in the G1 and G2 groups relative to the GC group (Figure 2(c)). RNS production was also higher in serum than in skin mucus, with the G2 group exhibiting the highest levels and the GC group the lowest. SOD and GPx in the serum and mucus of Nile tilapia were significantly increased (p < 0.05) in the G1and G2 groups compared to those in the GC group, as shown in Figures 3(a) and 3(b). The G2 group exhibited the highest levels of SOD and GPx activities, followed by G1 and GC.
Figure 3.
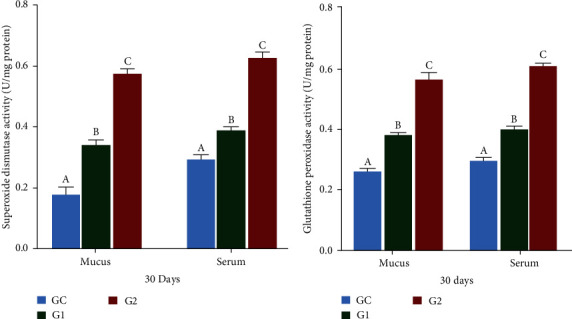
SOD (a) and GPx (b) in mucus and serum of O. niloticus. Control: fish fed without probiotics (GC). G1 and G2: with commercial diet containing B. amyloliquefaciens AV5 at 106 and 108 cfu/g diet, respectively. SOD denotes superoxide dismutase activity and GPx denotes glutathione peroxidase. The values are shown as mean ± S.D of three replications. Based on Tukey's test, values with distinct letters on the same line show a significant difference (p < 0.05).
3.5. Metabolomics
3.5.1. Principal Component Analysis (PCA) and OPLS-DA Response
PCA of the intestinal metabolites in the G2 and GC control groups, as shown in Figure 4(a), effectively differentiated the two groups with no overlap. The permutation test showed high predictability and repeatability, with R2 and Q2 values of 0.942 and 0.1094, respectively. The OPLS-DA score diagram also indicated a distinct separation between the GC and G2 groups, with a legitimate permutation test yielding R2Y and Q2 values of 0.995 and 0.78, respectively, in positive mode (Figure 4(b)). All the sample groups fell within the 95% Hotelling's T2 ellipse, with the OPLS-DA model showing Q2 = 0.78, R2Y = 0.995, and R2X = 0.722.
Figure 4.
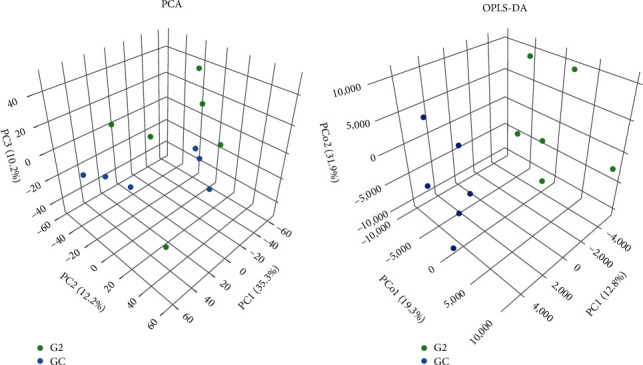
(a) Principal component analysis of LC-MS metabolite profiles (R2 = 0.942 and Q2 = 0.1094) and (b) a pairwise evaluation utilizing an OPLS-DA score graph (Q2 = 0.78, R2Y = 0.995, and R2X = 0.722) in the Nile tilapia GC and G2 groups. GC, control diet group with no probiotics and G2, with 108 cfu/g of B. amyloliquefaciens AV5.
3.5.2. Glycolysis/Gluconeogenesis and Lipid Metabolism
Figure 5 illustrates the relationship between glycolysis/gluconeogenesis and the related pathways. The metabolic response was influenced by the addition of B. amyloliquefaciens AV5 to the diet of O. niloticus according to the KEGG. The inclusion of B. amyloliquefaciens AV5 activated many differentially expressed genes (DEGs) related to glycolysis. In addition, lipid digestion and absorption were significantly modulated in O. niloticus. Figure 6(a) illustrates the complex interrelationships between lipid metabolism and the corresponding physiological mechanisms. After supplementing O. niloticus meal with B. amyloliquefaciens AV5, an increase in the activities of pyruvate kinase, 2-hydroxyethyl-ThPP, and dihydrolipoamide dehydrogenase was observed. Our findings indicate a strong correlation between lipid metabolic routes, as demonstrated by the involvement of multiple enzymes (glycerol-3-phosphate acyltransferase, 1-acylglycerol-3-phosphate acyltransferase, and diacylglycerol acyltransferase) in both the processes.
Figure 5.
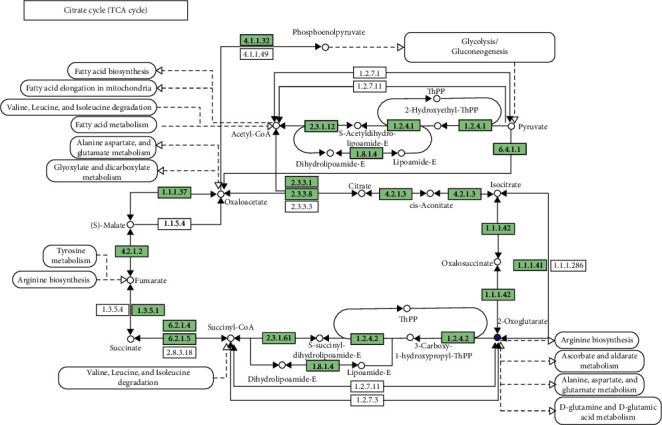
The differentially expressed genes in the gluconeogenesis/glycolysis routes are annotated in the KEGG database in Nile tilapia. GC, control diet group with no probiotics and G2, with 108 cfu/g of B. amyloliquefaciens AV5.
Figure 6.
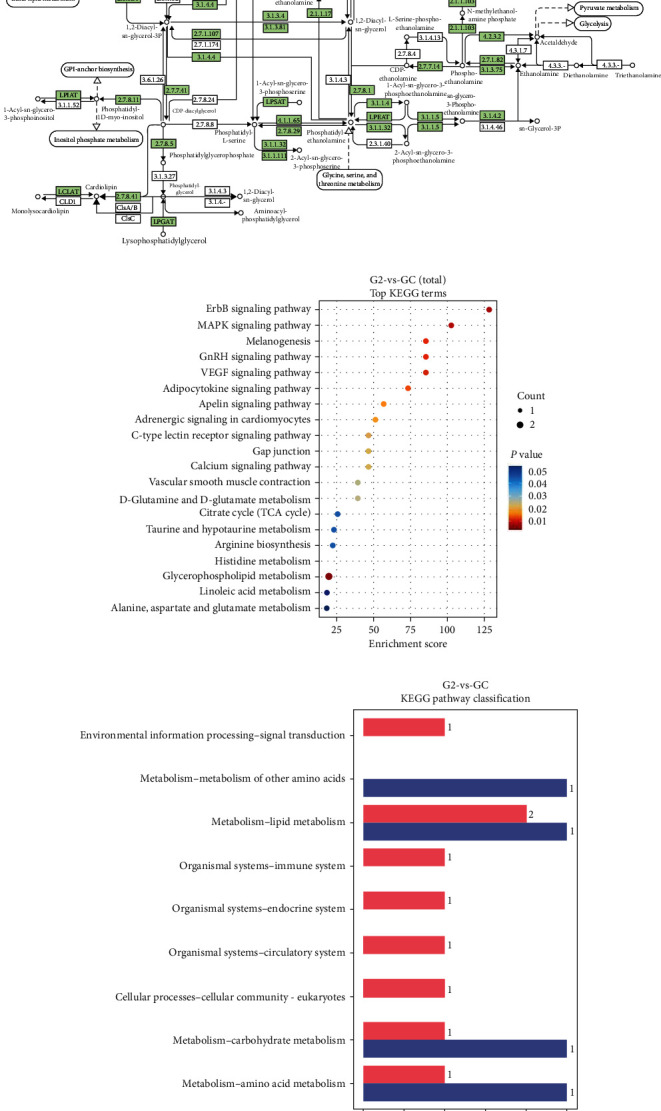
The Kyoto Encyclopedia of Genes and Genomes (KEGG) showing (a) lipid metabolism/glycerophospholipids route that differentially expressed genes, (b) enrichment of differential metabolites routes, and (c) classification of all the assembled unigenes in Nile tilapia commercial fed diet included with B. amyloliquefaciens AV5. GC, control group with no probiotics and G2, with 108 cfu/g of B. amyloliquefaciens AV5.
3.5.3. Differential Metabolites Response
The evaluation of various pathways for enrichment, as depicted in Figure 6(b), identified differential metabolites in 20 pathways. Histidine and glycerophospholipid metabolic pathways showed significant abundance, indicating modulation of crucial biological and cellular processes by the experimental diets (p < 0.05). In Figure 6(c), O. niloticus fed with G1 and G2 diets exhibited elevated levels of differential metabolites compared to those fed with GC diets, with the G2 group showing higher upregulation than the GC control. Figure 7 presents a heatmap illustrating the metabolite reactions of O. niloticus. A notable metabolic shift was observed in the experimental groups, with higher levels of 3-hydroxyoctadecanoylcarnitine and 6-methylheptadecanoylcarnitine in the G2 group compared to the GC group.
Figure 7.
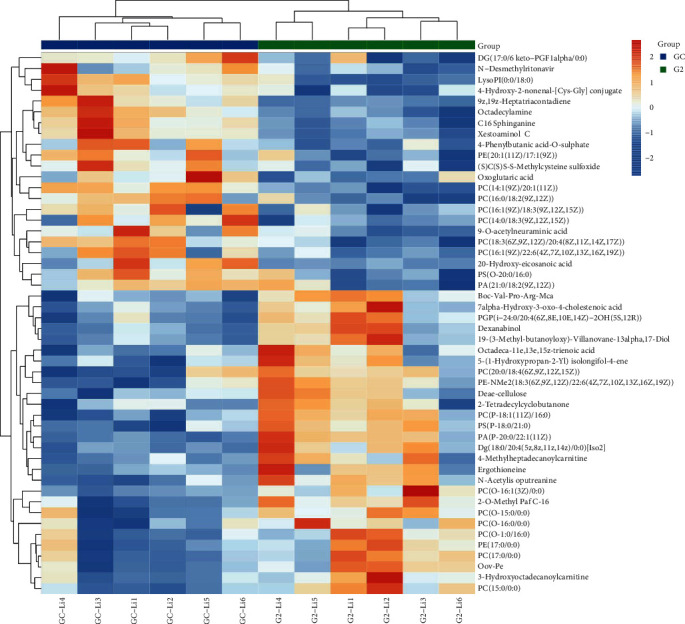
The heatmap illustrates the unsupervised hierarchical clustering in Nile tilapia, displaying the changes in metabolite levels relative to the median metabolite level. GC, control diet group with no probiotics and G2, 108 cfu/g of B. amyloliquefaciens AV5. Note: The reader is directed to the Web version of this study for a description of the color references in this figure's legend.
4. Discussion
A probiotic-supplemented diet is an environmentally friendly preventive measure for fish health and growth in aquaculture [4]. Probiotics enhance lipid metabolism and immunity [39], and Bacillus strains are increasingly being used because of their significant probiotic benefits [2]. Dietary components affect the microbiota and its metabolites, and probiotics alter the composition of the gut microbiome to positively influence host health [2]. Metabolomics is a powerful method for demonstrating metabolic changes induced by probiotic ingestion in host tissues, organs, and biofluids. In contrast, lipidomics examines lipids in various cells and biofluids [40]. Limited research has explored the effects of Bacillus probiotics added to the diets of O. niloticus on lipid metabolism and metabolites [41]. The present investigation found that supplementing O. niloticus diets with B. amyloliquefaciens AV5 significantly increased Wt, WGR, SR, and SGR. The probiotic group (G2) showed the most favorable outcomes (Table 2). The greater Wt and WGR in the B. amyloliquefaciens treatments may be due to nutrient consumption, as indicated by the significantly reduced FCR in these groups. Comparable research has shown that Bacillus cereus greatly enhances the growth performance of crustaceans [41]. Numerous Bacillus species can enhance host growth, feed efficiency, and weight gain [18]. Supplementing Nile tilapia diets with Bacillus licheniformis improves growth and feed utilization [24]. B. amyloliquefaciens also boosts growth in O. niloticus and yellow catfish [20, 42]. The probiotic B. licheniformis enhances growth in Mozambique tilapia [18]. Probiotics promote fish weight gain by improving intestinal morphology, appetite, vitamin synthesis, and digestion [20]. Probiotics can also enhance the immune system by adhering to and colonizing fish intestines [43, 44]. Further studies are required to determine the effect of probiotics, including B. amyloliquefaciens, on the digestive enzyme activity of fish. Probiotic bacteria interact with the immune system through microbe-associated molecular patterns (MAMPs) such as polysaccharides (CPs), peptidoglycan (PGN), lipoteichoic acids, and lipoprotein [43]. Pattern recognition receptors (PRRs), such as nucleotide oligomerization domain (NOD)-like receptors (NLRs), toll-like receptors (TLRs), and C-type lectin receptors (CLRs), enable immune cells to bind MAMPs [43, 45]. This finding lends credence to the idea that supplementing fish feed with a live culture of B. amyloliquefaciens maintains high probiotic levels and boosts immune response. Probiotics improve immunological responses by interacting with the immune cells. Labeo rohita, Cyprinus carpio, and O. mossambicus showed higher mucus and serum protein levels when fed B. subtilis KADR1, Lactococcus lactis, and B. licheniformis Dahb1, respectively [18, 46, 47]. Similarly, O. niloticus fed with B. amyloliquefaciens AV5 exhibited elevated mucin and serum protein levels. Key defense proteins, such as agglutinins, lectins, LYZ, and immunoglobulins, are linked to serum and mucus proteins [18]. These findings support the development of probiotic-based fish feed to enhance health and immune responses in aquaculture and indicate that B. amyloliquefaciens AV5 can increase serum and mucus protein levels in fishes. In Persian sturgeon, a diet with B. licheniformis increased serum and mucus ALP activity [48]. O. niloticus showed elevated ALP activity in both serum and mucus when fed Lactobacillus plantarum [49]. Enterococcus faecium in the diet of Rutilus rutilus caspicus also increased ALP activity in its mucus [50]. Similarly, our study found that O. niloticus had higher ALP levels in mucus and serum when supplemented with B. amyloliquefaciens AV5. ALP, a lysosomal enzyme, activates macrophages and serves as an antibacterial agent [18]. Neutrophils release MPO from azurophilic granules during respiratory bursts, producing hypochlorous acid to eliminate pathogenic bacteria [51]. Degranulation activates the halide synthesis pathway and releases MPO and various antimicrobial enzymes. MPO activity increased in the serum of O. mossambicus fed B. licheniformis, L. rohita fed B. amyloliquefaciens, and Catla catla fed B. amyloliquefaciens [10, 18, 21]. The mucus of O. mossambicus also showed increased MPO levels with B. licheniformis supplementation [18]. Our study found elevated MPO activity in both the serum and mucus of O. niloticus when fed B. amyloliquefaciens AV5 for 30 days. LYZ is a bactericidal peptide crucial for fish innate immunity. It stimulates phagocytes and the complement system, breaks down bacterial cell walls, and prevents biofilm formation [18, 43, 52]. LYZ levels increased in O. niloticus fed B. safensis NPUST1 at 106 and 107 cfu/g and B. subtilis WB60 at 107 cfu/g [53, 54]. Our research found that Nile tilapia fed B. amyloliquefaciens AV5 had higher LYZ activity in the serum and mucus. Red sea bream showed increased serum LYZ activity with B. subtilis, and O. mossambicus had elevated mucus and serum LYZ levels with B. licheniformis Dahb1 [18, 55]. C. catla showed increased mucus LYZ with B. amyloliquefaciens FPTB16 at 108 and 109 cfu/g [10, 18], and O. niloticus had higher mucus LYZ with B. licheniformis [24]. Phagocytes eliminate bacterial invaders through respiratory bursts that generate ROS and inhibit pathogenic infections in fish [18]. Elevated ROS levels were observed in yellow perch fed a mixture of Bacillus species for 6 weeks and in Pengze crucian carp supplemented with B. cereus [56, 57]. Increased ROS levels were also seen in the serum and mucus of Mozambique tilapia fed B. licheniformis Dahb1at 107 cfu/mL and C. catla fed B. amyloliquefaciens FPTB16 at 108 and 109 cfu/g [10, 18]. Our research showed elevated ROS levels in the serum and mucus of Nile tilapia fed B. amyloliquefaciens AV5. In addition to ROS activity, NO is an effector molecule involved in the immune response and is connected to activated granulocytes in fish [10]. Infections can be restricted by activating macrophages, which generate RNS [10]. NO is a key regulator of immune processes with direct antimicrobial effects [43]. Elevated serum NO levels have been observed in grass carp fed B. subtilis [58]. Higher NO levels were also noted in C. catla fed B. amyloliquefaciens FPTB16 at 108 and 109 cfu/g [10] and in O. mossambicus-fed B. licheniformis at 107 cfu/mL [18]. Our research found that after 30 days of feeding O. niloticus diets containing B. amyloliquefaciens AV5, NO generation increased in both serum and skin mucus, following a pattern similar to that of oxygen radical production. During phagocytosis, vertebrates produce ROS, such as hydroxyl radicals (-OH), hydrogen peroxide (H2O2), and superoxide anions (O2−), which are highly effective germicides. Antioxidant enzymes protect cells from damage caused by ROS [59]. The most prevalent antioxidant enzymes in fishes are GPx, SOD, and catalase (CAT) [60]. O. mossambicus supplemented with B. licheniformis for 4 weeks showed increased GPx and SOD levels in serum and mucus [18]. After 8 weeks, C. catla fed B. amyloliquefaciens (109 and 108 cfu/g) had elevated GPx and SOD levels in serum and skin mucus [10]. Ctenopharyngodon idella supplemented with B. licheniformis also showed increased GPx and SOD expression in mucus [61]. Grass carp treated with B. subtilis for 42 days had higher serum SOD and GPx levels [62]. Similarly, O. niloticus fed B. amyloliquefaciens AV5 (106 and 108 cfu/g) for 30 days exhibited increased GPx and SOD activities in serum and mucus. Metabolomics is now used in aquaculture research to tackle the long-standing challenges related to health, nutrition, enhancement, and genetics that have plagued the aquaculture industry [40]. Metabolomic methods have been proposed for various purposes, including gene expression product analysis, biomarker identification, metabolite characterization, and variation tracking [63]. Metabolomics is one of these new omics-based methods that can quantify and analyze biomolecules and metabolites that represent an animal's reaction to both internal and external influences [40]. Metabolomics is a highly effective method for investigating metabolic changes that occur in host biofluids, tissues, and organs after the intake of probiotics [64]. In our study, we employed PCA metabolite analysis to assess the effects of supplementing Nile tilapia diets with B. amyloliquefaciens AV5. The results revealed distinct nonoverlapping between the control group (GC) and the G2 group (Figure 4(a)), consistent with previous findings by Yin et al. [40], indicating distinct clustering of metabolic products. OPLS-DA confirmed that intestines from both the treatment and control groups fell within the 95% ellipse of Hotelling's T2 test, suggesting suitability for evaluating distal gut metabolites postsupplementation with B. amyloliquefaciens AV5. In addition, OPLS-DA analysis revealed notable differences in metabolites between the treatment and control groups (Figure 4(b)). Glycolysis and gluconeogenesis are vital metabolic routes for ATP generation in cells [65].They involve multiple enzymes and substrates to combine molecules and form complex compounds [65] that are essential for energy acquisition, protein synthesis, and reproduction [40]. Glycolysis is particularly important for glucose balance, converting glucose into pyruvate to produce ATP for cellular energy [66].
Conversely, gluconeogenesis converts noncarbohydrate compounds, such as fatty acids and amino acids, into glucose [65]. Pyruvate kinase converts pyruvate into phosphoenolpyruvate (PEP), which is crucial for ATP synthesis [67]. PEP is vital for glycolysis, which is the process of converting glucose into ATP [67]. The coenzyme 2-hydroxyethyl-ThPP aids in converting pyruvate to acetyl-CoA, which is essential for the citric acid cycle and ATP production [68]. It also facilitates electron transport, thereby promoting ATP generation [69]. The dihydrolipoamide dehydrogenase enzyme is essential for both the pyruvate dehydrogenase complex and S-succinyl dihydrolipoamide and acetyl dihydrolipoamide-E, facilitating electron transport in pyruvate to acetyl-CoA conversion for increased ATP production [69]. S-acetyl dihydrolipoamide-E transfers electrons from pyruvate to NAD+, whereas S-succinyl dihydrolipoamide transfers electrons from NADH to CoQ, which is crucial for ATP production in the citric acid cycle [70, 71]. Nile tilapia fed diets containing G1 and G2 showed elevated levels of pyruvate kinase, 2-hydroxyethyl-ThPP, and dihydrolipoamide dehydrogenase, which enhanced the glycolytic pathway activity (Figure 5). This suggests that highly unsaturated phospholipids may protect liver cell membranes, improve mitochondrial function, promote lipid metabolism, and reduce extra fat levels in the fish [70]. To the best of our knowledge, research on metabolomics in fish using UPLC-MS is limited, with few results and no comprehensive literature review to elucidate our findings. Therefore, further investigation in this area is recommended. Glycerophospholipids are essential lipids involved in various biological processes [72]. Enzymes such as diacylglycerol acyltransferase, glycerol-3-phosphate acyltransferase, and acyl-CoA-1-acylglycerol-3-phosphate acyltransferase are key in their biosynthesis, regulating their synthesis, remodeling, and degradation to maintain membrane structure [72]. The study found that glycerol-3-phosphate acyltransferase, 1-acylglycerol-3-phosphate acyltransferase, and diacylglycerol acyltransferase are crucial for lipid metabolism and that experimental diets improved these metabolic pathways compared to the control group (Figure 6(a)). Further research is needed to examine glycerophospholipids in fish fed diets supplemented with B. amyloliquefaciens or other probiotics. Histidine and glycerophospholipid metabolisms are vital for numerous biological activities [73]. Histidine, an essential amino acid, is crucial for protein synthesis, histamine production, pH control, and creation of histidine-containing dipeptides and neurotransmitters [73]. Glycerophospholipid metabolism maintains proper membrane structure and function because glycerophospholipids are critical components of cellular membranes [73]. Both histidine and glycerophospholipid metabolism are essential for cellular integrity and proper functioning of various organs and systems. Additionally, histidine and glycerophospholipid metabolism has been linked to various disorders and diseases [72]. Understanding the enzymes involved in glycerophospholipid metabolism is crucial for gaining insights into the mechanisms that regulate membrane structure and function. In this study, histidine and glycerophospholipid metabolism were enriched in the experimental diets compared to the control group (Figure 6(b)). However, few reports exist on histidine and glycerophospholipid metabolism in fish following probiotic supplementation. Researchers have aimed to discern metabolic pathways from distinct metabolites to establish their relationships. This study focused on quantifying the metabolic response of Nile tilapia to commercial diets containing B. amyloliquefaciens AV5. The metabolic pathway study revealed that the treatment diets (G1 and G2) had a greater impact on lipid, carbohydrate, and amino acid metabolism than the control diet (GC) in Nile tilapia (Figure 6(c)). However, its effect on glucose metabolism remains unclear. Protein metabolism has been found to enhance amino acid degradation. The functions of 3-hydroxyoctadecanoylcarnitine and 6-methylheptadecanoylcarnitine are still unknown because of the limited research on these compounds in fish. Given the general role of carnitines in cellular metabolism, these compounds may be involved in lipid metabolism or energy production [74]. Optimal carnitine activity is necessary for substrate utilization in oxidative processes [74]. In this study, 3-hydroxyoctadecanoylcarnitine and 6-methylheptadecanoylcarnitine levels were higher in fish fed commercial diets supplemented with B. amyloliquefaciens AV5 than in those fed commercial diets alone (GC; Figure 7). The addition of B. amyloliquefaciens AV5 to fish diets may lead to increased levels of carnitine-associated metabolites. The results of this investigation suggest that supplementing commercial fish diets with B. amyloliquefaciens AV5 could potentially improve their health and lipid metabolism by modulating carnitine-related metabolites. However, further research is needed to determine the precise role of carnitines in fish physiology and metabolism.
5. Conclusions
In this study, the effects of adding B. amyloliquefaciens AV5 to commercial fish diets on immunological response (serum and mucus), metabolomics, and lipid metabolism after 30 days of feeding were assessed. The growth, immune parameters, and activities of antioxidant enzymes in the serum and mucus of O. niloticus were enhanced by adding 106 and 108 cfu/g of B. amyloliquefaciens AV5. Moreover, the regulatory effect on glycolysis/gluconeogenesis pathways and lipid metabolism was determined by modifying the morphology of histidine and glycerophospholipids. Probiotic administration at a dosage of 108 cfu/g had the best effects in this study and should be recommended for application in aquaculture.
Acknowledgments
The authors express their gratitude to all the laboratory members for their valuable suggestions on this work. This study was funded by National Natural Science Foundation of China (grant numbers U20A2065, 32073006, and 32002426), Natural Science Foundation of Guangxi Province (grant number 2020GXNSFAA297243), and Natural Science Foundation of Guangdong Province (grant number 2021B0202040002).
Data Availability
Data will be provided upon request.
Ethical Approval
The experiments were done by the guidelines for the care and use of laboratory animals in China. The research received approval from the Ethics Committee for Animal Experiments at Guangdong Ocean University.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Vicent Michael Shija contributed to writing the original draft, review, and editing, software, methodology, formal analysis, data curation, and conceptualization. Glory Emanuel Zakaria, Kwaku Amoah, Junwei Huang, and Zhong Yong contributed to writing the review and editing. Li Yi contributed to writing the review and editing, visualization, and software. Fortunatus Masanja contributed to visualization, software, and investigation. Jia Cai contributed to writingthe original draft, review, and editing, supervision, project administration, and conceptualization.
References
- 1.Kuebutornye F. K. A., Abarike E. D., Lu Y., et al. Mechanisms and the role of probiotic Bacillus in mitigating fish pathogens in aquaculture. Fish Physiology and Biochemistry . 2020;46:819–841. doi: 10.1007/s10695-019-00754-y. [DOI] [PubMed] [Google Scholar]
- 2.Kuebutornyea F. K. A., Wang Z., Lu Y., et al. Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish & Shellfish Immunology . 2020;97:83–85. doi: 10.1016/j.fsi.2019.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Liu H., Wang S., Cai Y., et al. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses, and tilapia, Oreochromis niloticus disease resistance. Fish & Shellfish Immunology . 2016;60(2017):326–333. doi: 10.1016/j.fsi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ringø E. Probiotics in shellfish aquaculture. Aquaculture and Fisheries . 2020;5(1):1–27. doi: 10.1016/j.aaf.2019.12.001. [DOI] [Google Scholar]
- 5.Sharifuzzaman S. M., Austin B. Diagnosis and Control of Diseases of Fish and Shellfish . John Wiley & Sons, Ltd.; 2017. Probiotics for disease control in aquaculture; pp. 189–222. [DOI] [Google Scholar]
- 6.Kavitha M., Raja M., Perumal P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822) Aquaculture Reports . 2018;11:59–69. doi: 10.1016/j.aqrep.2018.07.001. [DOI] [Google Scholar]
- 7.Adorian T. J., Jamali H., Farsani H. G., et al. Effects of probiotic bacteria Bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian sea bass, Lates calcarifer (Bloch) Probiotics and Antimicrobial Proteins . 2019;11:248–255. doi: 10.1007/s12602-018-9393-z. [DOI] [PubMed] [Google Scholar]
- 8.Kuebutornye F. K. A., Abarike E. D., Lu Y. A review on the application of Bacillus as probiotics in aquaculture. Fish & Shellfish Immunology . 2019;87:820–828. doi: 10.1016/j.fsi.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Sookchaiyaporn N., Srisapoome P., Unajak S., Areechon N. Efficacy of Bacillus spp. isolated from Nile tilapia Oreochromis niloticus Linn. on its growth and immunity, and control of pathogenic bacteria. Fisheries Science . 2020;86:353–365. doi: 10.1007/s12562-019-01394-0. [DOI] [Google Scholar]
- 10.Das A., Nakhro K., Chowdhury S., Kamilya D. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla) Fish & Shellfish Immunology . 2013;35(5):1547–1553. doi: 10.1016/j.fsi.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Xiaolonga G., Caihuan K., Mo Z., Xian L., Fucun W., Ying L. Effects of the probiotic Bacillus amyloliquefaciens on the growth, immunity, and disease resistance of Haliotis discus hannai. Fish & Shellfish Immunology . 2019;94:617–627. doi: 10.1016/j.fsi.2019.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Liu C.-H., Chiu C.-H., Wang S.-W., Cheng W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish & Shellfish Immunology . 2012;33(4):699–706. doi: 10.1016/j.fsi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Mozzia F., Ortiza M. E., Bleckwedela J., De Vuystb L., Pescumaa M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Research International . 2013;54(1):1152–1161. doi: 10.1016/j.foodres.2012.11.010. [DOI] [Google Scholar]
- 14.Cevallos-Cevallos J. M., Reyes-De-Corcuera J. I., Etxeberria E., Danyluka M. D., Rodrick G. E. Metabolomic analysis in food science: a review. Trends in Food Science & Technology . 2009;20(11-12):557–566. doi: 10.1016/j.tifs.2009.07.002. [DOI] [Google Scholar]
- 15.Pantami H. A., Bustamam M. S. A., Min C. C., et al. Integration of spleen 1H NMR-based metabolomics revealed the immunomodulatory effect. Bima Journal of Science and Technology . 2023;7(1):165–189. [Google Scholar]
- 16.Bustamam M. S. A., Azam A. A., Pantami H. A., Shaari K., Min C. C. The immunostimulant effects of Isochrysis galbana supplemented diet on the spleen of red hybrid tilapia (Oreochromis spp.) evaluated by nuclear magnetic resonance metabolomics. Aquaculture Nutrition . 2022;2022:22. doi: 10.1155/2022/1154558.1154558 [DOI] [Google Scholar]
- 17.Atef S., Ahmed O. M., Abo-Al-Ela H. G., Said M. M. Dietary Bacillus species modulate lipid metabolism-related parameters, growth, water quality, and bacterial load in Nile tilapia (Oreochromis niloticus) Animal Feed Science and Technology . 2024;310 doi: 10.1016/j.anifeedsci.2024.115943.115943 [DOI] [Google Scholar]
- 18.Gobi N., Vaseeharan B., Chen J.-C., et al. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish & Shellfish Immunology . 2018;74:501–508. doi: 10.1016/j.fsi.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 19.de Verdal H., Vandeputte M., Mekkawy W., Chatain B., Benzie J. A. H. Quantifying the genetic parameters of feed efficiency in juvenile Nile tilapia Oreochromis niloticus. BMC Genetics . 2018;19 doi: 10.1186/s12863-018-0691-y.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue M., Wu Y., Hong Y., et al. Effects of dietary Bacillus amyloliquefaciens on the growth, immune responses, intestinal microbiota composition and disease resistance of yellow cat fish, Pelteobagrus fulvidraco. Frontiers in Cellular and Infection Microbiology . 2022;12 doi: 10.3389/fcimb.2022.1047351.1047351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan M. I. R., Kamilya D., Choudhury T. G., Rathore G. Dietary administration of a host-gut derived probiotic Bacillus amyloliquefaciens COFCAU_P1 modulates immune-biochemical response, immune-related gene expression, and resistance of Labeo rohita to Aeromonas hydrophila infection. Aquaculture . 2022;546 doi: 10.1016/j.aquaculture.2021.737390.737390 [DOI] [Google Scholar]
- 22.Selim K. M., Reda R. M. Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefaciens. Fish & Shellfish Immunology . 2015;44(2):496–503. doi: 10.1016/j.fsi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Thy H. T. T., Tri N. N., Quy O. M., Kannika K., Unajak S., Areechon N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus) Fish & Shellfish Immunology . 2017;60:391–399. doi: 10.1016/j.fsi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Abarike E. D., Cai J., Lu Y., et al. Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response, and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunology . 2018;82:229–238. doi: 10.1016/j.fsi.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Zokaeifar H., Balcázar J. L., Kamarudin M. S., Sijam K., Arshad A., Saad C. R. Selection and identification of non-pathogenic bacteria isolated from fermented pickles with antagonistic properties against two shrimp pathogens. Journal of Antibiotics . 2012;65:289–294. doi: 10.1038/ja.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R.-X., Amenyogbe E., Lu Y., Jin J.-H., Xie R.-T., Huang J.-S. Effects of low-temperature stress on intestinal structure, enzyme activities and metabolomic analysis of juvenile golden pompano (Trachinotus ovatus) Frontiers in Marine Science . 2023;10 doi: 10.3389/fmars.2023.1114120.1114120 [DOI] [Google Scholar]
- 27.Liu J., UllahKhan F., Jin S., et al. Indexing serum and mucous biochemical parameters of endangered Chinese sturgeon Acipenser sinensis with implications for health assessment. Journal of Fish Biology . 2024;104(4):1180–1192. doi: 10.1111/jfb.15662. [DOI] [PubMed] [Google Scholar]
- 28.Rubio C. P., Cerón J. J. Spectrophotometric assays for evaluation of reactive oxygen species (ROS) in serum: general concepts and applications in dogs and humans. BMC Veterinary Research . 2021;17 doi: 10.1186/s12917-021-02924-8.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry . 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 30.Dworzański J., Strycharz-Dudziak M., Kliszczewska E., et al. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLOS ONE . 2020;15(3) doi: 10.1371/journal.pone.0230374.e0230374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghafoori Z., Heidari B., Farzadfar F., Aghamaali M. Variations of serum and mucus lysozyme activity and total protein content in the male and female Caspian kutum (Rutilus frisii kutum, Kamensky 1901) during reproductive period. Fish & Shellfish Immunology . 2014;37(1):139–146. doi: 10.1016/j.fsi.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Tang Z., Chen H., He H., Ma C. Assays for alkaline phosphatase activity: progress and prospects. TrAC Trends in Analytical Chemistry . 2019;113:32–43. doi: 10.1016/j.trac.2019.01.019. [DOI] [Google Scholar]
- 33.Pulli B., Ali M., Forghani R., et al. Measuring myeloperoxidase activity in biological samples, global metabolic profiling of animal and human tissues via UPLC-MS. PLOS ONE . 2013;8(7) doi: 10.1371/journal.pone.0067976.e67976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Want E. J., Masson P., Michopoulos F., et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nature Protocols . 2013;8:17–32. doi: 10.1038/nprot.2012.135. [DOI] [PubMed] [Google Scholar]
- 35.Amoah K., Dong X.-H., Tan B.-P., et al. Ultra-performance liquid chromatography-mass spectrometry-based untargeted metabolomics reveals the key potential biomarkers for castor meal-induced enteritis in juvenile hybrid grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus ♂) Frontiers in Nutrition . 2022;9 doi: 10.3389/fnut.2022.847425.847425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thévenot E. A., Roux A., Xu Y., Ezan E., Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. Journal of Proteome Research . 2015;14(8):3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J. Y.-Chang, Li Y.-F., Zhou L., Zhang D.-P. Comparative metabolomics unveils molecular changes and metabolic networks of syringing against hepatitis B mice by untargeted mass spectrometry. RSC Advances . 2019;10(1):461–473. doi: 10.1039/C9RA06332C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji W., An K., Wang C., Wang S. Bioinformatics analysis of diagnostic biomarkers for Alzheimer’s disease in peripheral blood based on sex differences and support vector machine algorithm. Hereditas . 2022;159 doi: 10.1186/s41065-022-00252-x.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringø E., Harikrishnan R., Soltani M., Ghosh K. The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals . 2022;12(21) doi: 10.3390/ani12213016.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin J., Hong X., Ma L., Liu R., Bu Y. Non-targeted metabolomic profiling of atrazine in Caenorhabditis elegans using UHPLC-QE Orbitrap/MS. Ecotoxicology and Environmental Safety . 2020;206 doi: 10.1016/j.ecoenv.2020.111170.111170 [DOI] [PubMed] [Google Scholar]
- 41.Muthukrishnan S., Hoong M. C., Chen W. W., Natrah I. Efficacy of Bacillus cereus strain BP-MBRG/1b and prebiotic fructooligosaccharides dietary supplementation on growth performance and disease resistance of Macrobrachium rosenbergii (De Mann) towards Aeromonas hydrophila AH-1N. Aquaculture Research . 2021;52:1657–1665. doi: 10.1111/are.15018. [DOI] [Google Scholar]
- 42.Kuebutornye F. K. A., Tang J., Cai J., et al. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status, and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture . 2020;527 doi: 10.1016/j.aquaculture.2020.735440.735440 [DOI] [Google Scholar]
- 43.Shija V. M., Amoah K., Cai J. Effect of Bacillus probiotics on the immunological responses of Nile Tilapia (Oreochromis niloticus): a review. Fishes . 2023;8(7) doi: 10.3390/fishes8070366.366 [DOI] [Google Scholar]
- 44.Di Z., Wu S., Feng W., Jakovlić I., Tran N. T., Xiong F. Adhesion and colonization properties of potentially probiotic Bacillus paralicheniformis strain FA6 isolated from grass carp intestine. Fisheries Science . 2020;86:153–161. doi: 10.1007/s12562-019-01385-1. [DOI] [Google Scholar]
- 45.Kwon Y., Park C., Lee J., et al. Regulation of bone cell differentiation and activation by microbe-associated molecular patterns. International Journal of Molecular Sciences . 2021;22(11) doi: 10.3390/ijms22115805.5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramesh D., Souissi S. Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquaculture Research . 2018;49:367–377. doi: 10.1111/are.13467. [DOI] [Google Scholar]
- 47.Feng J., Chang X., Zhang Y., Yan X., Zhang J., Nie G. Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response, and disease resistance against Aeromonas hydrophila. Fish & Shellfish Immunology . 2019;93:73–81. doi: 10.1016/j.fsi.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Darafsh F., Soltani M., Abdolhay H. A., Mehrejan M. S. Efficacy of dietary supplementation of Bacillus licheniformis and Bacillus subtilis probiotics and Saccharomyces cerevisiae (yeast) on the hematological, immune response, and biochemical features of Persian sturgeon (Acipenser persicus) fingerlings. Iranian Journal of Fisheries Sciences . 2020;19(4):2024–2038. [Google Scholar]
- 49.Mohammadi G., Rafiee G., Abdelrahman H. A. Effects of dietary Lactobacillus plantarum (KC426951) in biofloc and stagnant-renewal culture systems on growth performance, mucosal parameters, and serum innate responses of Nile tilapia Oreochromis niloticus. Fish Physiology and Biochemistry . 2020;46(3):1167–1181. doi: 10.1007/s10695-020-00777-w. [DOI] [PubMed] [Google Scholar]
- 50.Tarkhani R., Imani A., Hoseinifar S. H., et al. Comparative study of host-associated and commercial probiotic effects on serum and mucosal immune parameters, intestinal microbiota, digestive enzymes activity, and growth performance of roach (Rutilus rutilus caspicus) fingerlings. Fish & Shellfish Immunology . 2020;98:661–669. doi: 10.1016/j.fsi.2019.10.063. [DOI] [PubMed] [Google Scholar]
- 51.Mokhtar D. M., Zaccone G., Alesci A., Kuciel M., Hussein M. T., Sayed R. K. A. Main components of fish immunity: an overview of the fish immune system. Fishes . 2023;82(2) doi: 10.3390/fishes8020093.93 [DOI] [Google Scholar]
- 52.Ellis A. E. Innate host defense mechanisms of fish against viruses and bacteria. Developmental & Comparative Immunology . 2001;25(8-9):827–839. doi: 10.1016/S0145-305X(01)00038-6. [DOI] [PubMed] [Google Scholar]
- 53.Wu P.-S., Liu C.-H., Hu S.-Y. Probiotic Bacillus safensis NPUST1 administration improves growth performance, gut microbiota, and innate immunity against Streptococcus iniae in Nile tilapia (Oreochromis niloticus) Microorganisms . 2021;9(12) doi: 10.3390/microorganisms9122494.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Won S., Hamidoghli A., Choi W., et al. Effects of Bacillus subtilis WB60 and Lactococcus lactis on growth, immune responses, histology and gene expression in Nile tilapia, Oreochromis niloticus. Microorganisms . 2019;8(1) doi: 10.3390/microorganisms8010067.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaineldin A. I., Hegazi S., Koshio S., et al. Bacillus subtilis as a probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish & Shellfish Immunology . 2018;79:303–312. doi: 10.1016/j.fsi.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Eissa N., Wang H.-P., Yao H., Abou-ElGheit E. S. Mixed Bacillus species enhance the innate immune response and stress tolerance in yellow perch subjected to hypoxia and air-exposure stress. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-25269-z.6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang G., Cao H., Jiang W., et al. Dietary supplementation of Bacillus cereus as probiotics in Pengze crucian carp (Carassius auratus var. Pengze): Effects on growth performance, fillet quality, serum biochemical parameters and intestinal histology. Aquaculture Research . 50:2207–2217. doi: 10.1111/are.14102. [DOI] [Google Scholar]
- 58.Lin Y.-S., Saputra F., Chen Y.-C., Hu S.-Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio) Fish & Shellfish Immunology . 2018;86:410–419. doi: 10.1016/j.fsi.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Wang Y., Xiaohalati X., et al. A bioinspired manganese-organic framework ameliorates ischemic stroke through its intrinsic nanozyme activity and upregulating endogenous antioxidant enzymes. Advanced Science . 2023;10(20) doi: 10.1002/advs.202206854.e2206854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogueji E., Nwani C., Mbah C., Iheanacho S., Nweke F. Oxidative stress, biochemical, lipid peroxidation, and antioxidant responses in Clarias gariepinus exposed to acute concentrations of ivermectin. Environmental Science and Pollution Research . 2020;27:16806–16815. doi: 10.1007/s11356-019-07035-4. [DOI] [PubMed] [Google Scholar]
- 61.Qin L., Xiang J., Xiong F., et al. Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier, and disease resistance of grass carp (Ctenopharyngodon idella) Fish & Shellfish Immunology . 2019;97:344–350. doi: 10.1016/j.fsi.2019.12.040. [DOI] [PubMed] [Google Scholar]
- 62.Tang Y., Han L., Chen X., Xie M., Kong W., Wu Z. Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under Aeromonas hydrophila challenge. Probiotics and Antimicrobial Proteins . 2019;11:545–558. doi: 10.1007/s12602-018-9409-8. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Liang D., Yang Q., et al. The effect of partial replacement of fish meal by soy protein concentrates on growth performance, immune responses, gut morphology, and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) Fish & Shellfish Immunology . 2019;98:619–631. doi: 10.1016/j.fsi.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 64.Chung H.-J., Sim J.-H., Min T.-S., Choi H.-K. Metabolomics and lipidomics approaches in the science of probiotics. Journal of Medicinal Food . 2018;21(11):1086–1095. doi: 10.1089/jmf.2017.4175. [DOI] [PubMed] [Google Scholar]
- 65.Mekuchi M., Sakata K., Yamaguchi T., Koiso M., Kikuchi J. Trans-omics approaches used to characterize fish nutritional biorhythms in Leopard coral grouper (Plectropomus leopardus) Scientific Reports . 2017;7(1) doi: 10.1038/s41598-017-09531-4.9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao J., Zeng F., Liao S., Cao L., Zhou Y. Effects of glycolysis on the polarization and function of tumor-associated macrophages (Review) International Journal of Oncology . 2023;62(6) doi: 10.3892/ijo.2023.5518.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue W. W., Yan S. W., Zhang R., et al. Characterization of a novel pyruvate kinase from Trichinella spiralis and its participation in sugar metabolism, larval molting and development. PLOS Neglected Tropical Diseases . 2022;16(10) doi: 10.1371/journal.pntd.0010881.e0010881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krivoruchko A., Zhang Y., Siewers V., Chen Y., Nielsen J. Microbial acetyl-CoA metabolism and metabolic engineering. Metabolic Engineering . 2015;28:28–42. doi: 10.1016/j.ymben.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Stobbe M. D., Houten S. M., van Kampen A. H. C., Wanders R. J. A., Moerland P. D. Improving the description of metabolic networks: the TCA cycle as an example. The FASEB Journal . 2012;26(9):3625–3636. doi: 10.1096/fj.11-203091. [DOI] [PubMed] [Google Scholar]
- 70.Feng Y., Zhang L., Fu J., et al. Characterization of glycolytic pathway genes using RNA-Seq in developing kernels of Eucommia ulmoides. Journal of Agricultural and Food Chemistry . 2016;64(18):3712–3731. doi: 10.1021/acs.jafc.5b05918. [DOI] [PubMed] [Google Scholar]
- 71.Bénit P., Goncalves J., El Khoury R., et al. Succinate dehydrogenase, succinate, and superoxides: a genetic, epigenetic, metabolic, environmental explosive crossroad. Biomedicines . 2022;10(8) doi: 10.3390/biomedicines10081788.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutlu A. S., Duffy J., Wang M. C. Lipid metabolism and lipid signals in aging and longevity. Developmental Cell . 2021;56(10):1394–1407. doi: 10.1016/j.devcel.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Z., Ge F., Che Y., Wu S., Dong X., Song D. Metabolomics coupled with pathway analysis provides insights into sarco-osteoporosis metabolic alterations and estrogen therapeutic effects in mice. Biomolecules . 2022;12(1) doi: 10.3390/biom12010041.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghonimy A., Zhang D. M., Farouk M. H., Wang Q. The impact of carnitine on dietary fiber and gut bacteria metabolism and their mutual interaction in monogastrics. International Journal of Molecular Sciences . 2018;19(4) doi: 10.3390/ijms19041008.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon request.


