Abstract
Objective
Treatment of anterior choroidal artery (AChA) aneurysms is frequently associated with ischemic complications. This study aimed to report the outcomes of treatment of unruptured AChA aneurysms in our hospital.
Methods
Between January 2015 and March 2022, 40 patients were treated for an unruptured AChA aneurysm in our hospital. Age, sex, aneurysm size, AChA branching type, treatment, occlusion rate, complications, modified Rankin Scale (mRS) score before surgery and after 90 days, and recurrence were investigated. The branching type was classified as internal carotid artery (ICA), neck, or dome type based on the location of the AChA origin.
Results
The mean age was 61.1 ± 1.9 years; 15 patients were men and 25 were women. The mean aneurysm diameter was 4.4 ± 0.3 mm. The branching type was ICA in four patients, neck in 35, and dome in one. Treatment was surgical clipping in 22 patients and endovascular coil embolization in 18 (14 with stent assistance). Motor-evoked potential (MEP) monitoring was used in all patients of the clipping group and 9 cases of the coiling group. Treatment complications occurred in eight patients (20%). mRS score worsened by more than one point 90 days after treatment in four patients (10%); however, the proportion of patients who experienced this did not significantly differ between the clipping and coiling groups. Although the odds of a thrombotic complication were higher with coiling than clipping, the difference was not significant (odds ratio: 10.2; P = 0.08). The rate of complete occlusion was lower in the coiling group (72.2% vs. 95.3%), but the difference was not significant. The median follow-up was 696 days (range: 99–2053). No aneurysm recurrence or rupture occurred.
Conclusion
AChA branching type is important for treatment decision-making in patients with AChA aneurysms. Rates of complications and occlusion do not significantly differ between clipping and coiling of AChA aneurysms. MEP monitoring may be useful in preventing thrombotic complications during coil embolization.
Keywords: anterior choroidal artery aneurysm, endovascular treatment, monitoring, clinical outcome
Introduction
Treatment of anterior choroidal artery (AChA) aneurysms is frequently associated with ischemic complications.1) Intraoperative electrophysiological monitoring of motor evoked potentials (MEPs)2) and use of indocyanine green video angiography during aneurysm surgery3) can reduce the incidence of these complications. In this study, we present the outcomes we have achieved with surgical clipping and coil embolization of unruptured AChA aneurysms since 2015. In addition, we discuss the optimal treatment strategy.
Materials and Methods
The study was approved by the ethics committee of Osaka National Hospital and performed in accordance with its guidelines (approval number: 21036). Informed consent was obtained using the opt-out method. Eligible patients could decline to participate via the ethics committee’s website.
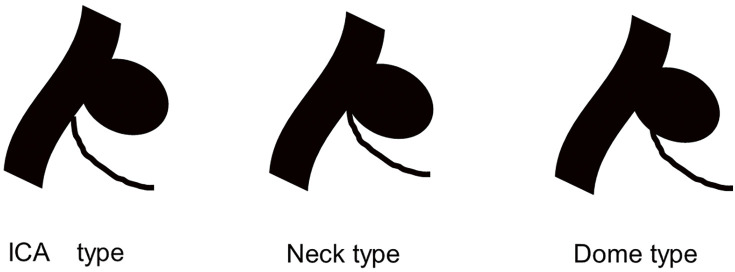
Among 627 consecutive patients with unruptured cerebral aneurysms treated in our hospital between January 2015 and March 2022, 40 harbored an AChA aneurysm (6.4%) and were included for analysis. The following data were recorded: age, sex, aneurysm size, AChA branching type, treatment, occlusion rate, surgical complications, modified Rankin Scale (mRS) score before treatment and after 90 days, and recurrence. The branching type was classified based on the location of AChA origin as internal carotid artery (ICA), neck, or dome (Fig. 1).4) In our hospital, clipping is generally the procedure of choice in patients with an ICA branching type because it is safe owing to the distance between the aneurysm neck and the AChA origin. We usually prefer coil embolization in those with neck or dome branching types, provided the AChA can be safely protected by a balloon or stent; however, clipping may be selected depending on laterality and the planned approach route.
Fig. 1. Illustrations of anterior choroidal artery branch types. With the internal carotid artery type, the anterior choroidal artery arises directly from the internal carotid artery. The neck type arises from the aneurysm neck, and the dome type arises from the dome. ICA, internal carotid artery.
Aneurysm occlusion was assessed using angiography immediately after coil embolization and using three-dimensional computed tomography angiography 1 week after clipping. Severe neurological deterioration was defined as a >1-point increase between the pretreatment and 90-day follow-up mRS scores. MEP monitoring (MEE-1216; Nihon Kohden, Tokyo, Japan) was performed in both surgical clipping and endovascular coil embolization procedures. Indocyanine green video angiography (Diagnogreen; Daiichi Sankyo Company, Tokyo, Japan) and microvascular Doppler ultrasonography (DVM-4500; Hadeco, Kanagawa, Japan) were used in surgical clipping procedures. For coil embolization, we mainly used an 8 Fr FUBUKI (Asahi Intecc, Aichi, Japan) guiding catheter, AXS Vecta 71 (Stryker, Kalamazoo, MI, USA) or Cerulean DD6 Plus (Medikit, Tokyo, Japan) distal access catheter, Excelsior SL-10 (Stryker) microcatheter, and Synchro Select (Stryker) micro guidewire. Dual antiplatelet therapy (mainly aspirin and clopidogrel) was introduced 2 weeks prior to coil embolization. Immediately before the procedure, light transmission aggregometry was performed to assess platelet aggregation capacity. Poor responders underwent medication changes accordingly. After coil embolization, patients received a 2-day continuous infusion of argatroban in addition to dual antiplatelet therapy.
Continuous variables were compared using the two-sided Student’s t-test. Categorical variables were compared using the chi-squared test or Fisher’s exact test as appropriate. P <0.05 was considered significant. Statistical analyses were conducted using JMP Pro 17 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and treatment outcomes
In all, 15 patients were men and 25 were women. The mean age was 61.1 ± 1.9 years (range: 32–78). The mean aneurysm diameter was 4.4 ± 0.3 mm (range: 1.3–12.6). AChA branching type was ICA in four cases, neck in 35, and dome in one. Treatment was clipping in 22 patients and coiling in 18 (stent assistance was used in 14 of these). MEP monitoring was used in all clipping group patients and nine coiling group patients. The median follow-up was 696 days (range: 99–2053). Patient characteristics of the entire cohort are shown in Table 1.
Table 1. Patient characteristics.
| N = 40 | |
|---|---|
| Age: mean ± SD (years) | 61.9 ± 1.9 |
| Sex | |
| Male | 15 |
| Female | 25 |
| Size of aneurysms (range) (mm) | 4.4 ± 0.3 |
| AChA branching type | |
| ICA type | 4 |
| Neck type | 35 |
| Dome type | 1 |
| Operation | |
| Clip | 22 |
| Coil | 18 |
| (Stent-assisted) | (14) |
AChA, anterior choroidal artery; ICA, internal carotid artery; SD, standard deviation
Mean aneurysm diameter and dome–neck ratio did not significantly differ between the clipping and coiling groups, nor did the rate of complete aneurysm occlusion (95.4% and 72.2%, respectively; P = 0.07). Two surgical complications (both oculomotor nerve palsies) occurred in the clipping group. In the coiling group, six complications occurred: thrombosis in three patients, puncture site hematoma in one, vessel perforation in one, and inadequate stent deployment in one. The incidence of complications did not significantly differ between the groups. Severe neurological deterioration after treatment occurred in three clipping group patients (13.6%) and one coiling group patient (5.6%); the difference was not significant. Although the odds of a thrombotic complication were higher with coiling than clipping, the difference was not significant (odds ratio: 10.2; P = 0.08). None of the thrombotic complications were associated with severe neurological deterioration. No aneurysm recurrence or rupture occurred during follow-up. Outcomes and complications according to the group are shown in Table 2.
Table 2. Outcomes and complications by operation.
| Clip | Coil | P | |
|---|---|---|---|
| No. of patient | 22 | 18 | |
| Size of aneurysms | 4.0 | 4.8 | 0.23 |
| D/N ratio | 1.4 | 1.5 | 0.31 |
| Operative complications | Thrombosis 0 | Thrombosis 3 | 0.08 (OR: 10.2) |
| Oculomotor nerve palsy 2 | Puncture hematoma 1 Vessel perforation 1 Poor stent deployment 1 |
0.64 | |
| AN occlusion CO | 21 | 13 | 0.07 (OR: 8.1) |
| Worsening of mRS ≥1 at 90 days after operation | Oculomotor nerve palsy 2 CSDH 1 |
Pain of puncture site 1 | 0.61 |
AN, aneurysm; CO, complete occlusion; CSDH, chronic subdural hematoma; mRS, modified Rankin Scale; OR, odds ratio
Treatment, complications, and outcomes according to AChA branching type
Treatment performed, the incidence of complications, and the complete occlusion rate did not significantly differ between the ICA, neck, and dome branch types. However, all eight complications occurred in the neck branch group; four resulted in severe neurological deterioration (Table 3).
Table 3. Treatment strategies and outcomes by AChA branching type.
| ICA | Neck | Dome | P | |
|---|---|---|---|---|
| No. of patients | 4 | 35 | 1 | |
| Clip | 4 | 18 | 0 | 0.11 |
| Coil | 0 | 17 | 1 | |
| Operative complications | 0 | 8 | 0 | 0.65 |
| AN occlusion CO | 4 | 30 | 0 | 0.24 |
| Worsening of mRS ≥1 at 90 days after operation | 0 | 4 | 0 | 1.0 |
AChA, anterior choroidal artery; AN, aneurysm; CO, complete occlusion; mRS, modified Rankin Scale
Subanalysis of the neck type group
Among the 17 patients in the neck type group who underwent coil embolization, six experienced a complication. The complication was thrombotic in three patients (transient AChA ischemia during the procedure, intraprocedural obstruction of the AChA, and AChA infarction on postprocedural MRI, respectively); all three had undergone coiling with stent assistance, and one exhibited reduced MEP amplitudes during the procedure. In the patient with reduced MEP amplitudes, both blood flow and MEP amplitudes improved after administration of heparin through a microcatheter, urokinase through a distal access catheter, and ozagrel through an intravenous line. None of the complications caused a worsening of the mRS score. Outcomes and complications in the neck type group are shown in Table 4.
Table 4. Treatment strategies and outcomes of neck type.
| Neck type clip | Neck type coil | P | |
|---|---|---|---|
| No. of patients | 18 | 17 | |
| Operative complications | Thrombosis 0 (0%) | Thrombosis 3 (17.6%) | 0.10 (OR: 8.9) |
| Oculomotor nerve palsy 2 | Puncture hematoma 1 Vessel perforation 1 Poor stent deployment 1 |
0.65 | |
| AN occlusion CO | 17 (94.4%) | 13 (76.5%) | 0.17 |
| Worsening of mRS ≥1 at 90 days after operation | Oculomotor nerve palsy 2 CSDH 1 |
Pain of puncture site 1 | 0.60 |
AN, aneurysm; CO, complete occlusion; CSDH, chronic subdural hematoma; mRS, modified Rankin Scale; OR, odds ratio
Discussion
In a study comparing surgical clipping and coil embolization of AChA aneurysms, Meguro et al.5) reported no significant difference in complications or postoperative mRS worsening; however, ruptured aneurysms were included in their analysis. Another AChA aneurysm study in which >90% were treated using coil embolization reported a 1% incidence of procedure-related complications and no re-rupture.6) Other studies reporting good endovascular outcomes7–11) suggest that coil embolization may be the best choice when treating these aneurysms; however, clipping is still widely used. We also found no significant difference in the incidence of complications or occlusion rate between the two modalities. Although the odds of thrombotic complications were considerably higher with coiling, the difference was not significant. Furthermore, complications did not have a direct impact on neurological outcomes. MEP monitoring can help avoid serious thrombotic complications when performing coiling or stent placement to treat an AChA aneurysm. If a decline in MEP amplitude is detected before coil deployment or stent implantation, they should be retrieved immediately. If deployment or implantation has already been completed, additional antithrombotic agents such as ozagrel or heparin should be considered.
Our comparison of complete occlusion rates showed no significant difference between clipping and coiling; however, our sample size was small and possibly underpowered. The lower rate of complete occlusion in the coiling group may be because AChA aneurysms are typically not filled tightly with coils to avoid complications. In a study of AChA clipping outcomes before and after the introduction of intraoperative MEP monitoring and indocyanine green video angiography, Aoki et al.1) reported significantly better outcomes. Combined monitoring is now an essential part of clipping procedures for AChA aneurysms.12) In one of our patients, a complication was probably averted when the disappearance of MEPs alerted us to a problem during endovascular treatment. Kawamoto et al. reported that AChA territory ischemia can occur even when the AChA demonstrates angiographic filling; they hypothesized that the cause may be the occlusion of small vessels that are hidden by the main trunk.4) Therefore, MEP monitoring should be useful in the endovascular treatment of AChA aneurysms. However, MEP monitoring cannot detect infarcts in branches that perfuse non-pyramidal tracts.13,14)
In our experience, thrombotic AChA territory complications can still occur when MEP monitoring is used, which has also been previously reported.15) Nonetheless, MEP monitoring remains useful as response changes can indicate severe motor paralysis. Regarding the association between AChA branching type and endovascular complications, Kang et al. reported significantly more symptomatic complications in patients with a neck type.7) Similarly, in our study, all complications occurred in patients with a neck type.
This study has several limitations. It was retrospective and conducted in a single center. In addition, as noted above, the sample size was small and may have been underpowered. Moreover, only one patient with a dome branching type was included because treating patients with this type is associated with a high risk of poor outcomes. Selection bias was present because we tended to select clipping for patients with ICA branching type.
Conclusion
In this small retrospective study of AChA aneurysm treatment, rates of complications and aneurysm occlusion did not significantly differ between clipping and coiling. MEP monitoring may be useful in preventing thrombotic complications during coil embolization. Several factors may influence treatment selection, such as the presence and location of other aneurysms, patient comorbidities, aneurysm size, endovascular access considerations, and patient preference. AChA branching type is also an important consideration. Treatment strategy should be based on a comprehensive assessment of these factors.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing the draft version of this manuscript.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1).Aoki T, Noguchi K, Komaki S, et al. Clinical outcome of surgical clipping for anterior choroidal artery aneurysm. Surg Cereb Stroke 2015; 43: 442–447. (in Japanese) [Google Scholar]
- 2).Suzuki K, Kodama N, Sasaki T, et al. Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg 2003; 98: 507–514. [DOI] [PubMed] [Google Scholar]
- 3).Raabe A, Nakaji P, Beck J, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green video angiography during aneurysm surgery. J Neurosurg 2005; 103: 982–989. [DOI] [PubMed] [Google Scholar]
- 4).Kawamoto S, Fukaya S, Abe Y, et al. Anterior choroidal artery aneurysms: clinical characteristics and surgical outcome. Surg Cereb Stroke 2017; 45: 177–182. (in Japanese) [Google Scholar]
- 5).Meguro T, Kuwahara K, Tomita Y, et al. Ischemic complications of anterior choroidal artery aneurysm treatment. No Shinkei Geka 2014; 42: 917–923. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 6).Roh HK, Jeong EO, Kim KH, et al. Treatment results of anterior choroidal artery aneurysms treated mostly with coil embolization: a single-center experience. J Cerebrovasc Endovasc Neurosurg 2022; 24: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Kang HS, Kwon BJ, Kwon OK, et al. Endovascular coil embolization of anterior choroidal artery aneurysms. J Neurosurg 2009; 111: 963–969. [DOI] [PubMed] [Google Scholar]
- 8).Kim BM, Kim DI, Chung EC, et al. Endovascular coil embolization for anterior choroidal artery aneurysms. Neuroradiology 2008; 50: 251–257. [DOI] [PubMed] [Google Scholar]
- 9).Wang Y, Yu J. Endovascular treatment and angiographic characteristics of aneurysms at the origin of the anterior choroidal artery. Front Neurol 2022; 13: 832604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Al Fauzi A, Rahmatullah MI, Suroto NS, et al. Comparison of outcomes between clipping and endovascular coiling in anterior choroidal artery aneurysm: a systematic review. Neurosurg Rev 2023; 46: 276. [DOI] [PubMed] [Google Scholar]
- 11).Ideguchi M, Hadeishi H. Ischemic complications after surgical clipping of anterior choroidal artery aneurysms. Surg Cereb Stroke 2019; 47: 337–342. (in Japanese) [Google Scholar]
- 12).Ota T, Mizutani T. Surgical techniques for clipping the internal carotid artery-anterior choroidal artery aneurysm. Surg Cereb Stroke 2013; 41: 176–182. (in Japanese) [Google Scholar]
- 13).Byoun HS, Oh CW, Kwon OK, et al. Intraoperative neuromonitoring during microsurgical clipping for unruptured anterior choroidal artery aneurysm. Clin Neurol Neurosurg 2019; 186: 105503. [DOI] [PubMed] [Google Scholar]
- 14).Sato T, Itakura T, Suzuki K, et al. Motor evoked potential monitoring and novel laser light imaging system to simultaneously visualize light and near-infrared fluorescence images in aneurysm surgery. Surg Cereb Stroke 2020; 48: 168–172. (in Japanese) [Google Scholar]
- 15).Irie T, Yoshitani K, Ohnishi Y, et al. The efficacy of motor-evoked potentials on cerebral aneurysm surgery and new-onset postoperative motor deficits. J Neurosurg Anesthesiol 2010; 22: 247–251. [DOI] [PubMed] [Google Scholar]