Abstract
Models of healthy aging are typically based on the United States and Europe and may not apply to diverse and heterogeneous populations. In this study, our objectives were to conduct a meta-analysis to assess risk factors of cognition and functional ability across aging populations in Latin America and a scoping review focusing on methodological procedures. Our study design included randomized controlled trials and cohort, case–control and cross-sectional studies using multiple databases, including MEDLINE, the Virtual Health Library and Web of Science. From an initial pool of 455 studies, our meta-analysis included 38 final studies (28 assessing cognition and 10 assessing functional ability, n = 146,000 participants). Our results revealed significant but heterogeneous effects for cognition (odds ratio (OR) = 1.20, P = 0.03, confidence interval (CI) = (1.0127, 1.42); heterogeneity: I2 = 92.1%, CI = (89.8%, 94%)) and functional ability (OR = 1.20, P = 0.01, CI = (1.04, 1.39); I2 = 93.1%, CI = (89.3%, 95.5%)). Specific risk factors had limited effects, especially on functional ability, with moderate impacts for demographics and mental health and marginal effects for health status and social determinants of health. Methodological issues, such as outliers, inter-country differences and publication bias, influenced the results. Overall, we highlight the specific profile of risk factors associated with healthy aging in Latin America. The heterogeneity in results and methodological approaches in studying healthy aging call for greater harmonization and further regional research to understand healthy aging in Latin America.
Subject terms: Medical research, Databases, Ageing
Ibanez et al. performed a scoping review and meta-analysis of healthy aging studies across Latin America and report substantial heterogeneity in how risk factors affect cognitive and functional ability, underscoring the need for further regional research.
Main
The understanding of healthy aging and brain health has been informed primarily by studies from the United States and Europe1–5. These studies predominantly focus on cognitive abilities (that is, attention, problem-solving, learning and memory, among others) and functional abilities (that is, specific personal activities of daily living and higher-order instrumental skills), respectively6–8. Models are considered generalizable, as relatively small divergences in the aging process, irrespective of geographic or socioeconomic factors, have been assumed2,9,10. Previous reviews and meta-analyses in the United States and Europe reported consistent predictors of cognition and functional ability, including demographics (age and gender), cardiometabolic diseases, lifestyle (sleep problems, alcohol consumption and physical activity), mental health and social determinants of health (SDH) (education and socioeconomic status), particularly for high-income countries11–16.
However, there is a dearth of studies from other parts of the world, especially from regions with large social and health disparities4,9,10. The long-standing focus on high-income countries has inadvertently created a research evidence base that lacks representation from a broader spectrum of diverse populations and regions. Recently, prediction models of aging and brain–phenotype associations developed in high-income countries have shown poor reproducibility in more diverse populations1,2,10,17. These results indicate non-universal patterns markedly influenced by socioeconomic disparities and demographic variables, such as age and sex2,10,11,18. The differences across regions emphasize the need to move away from a one-size-fits-all approach to a more region-specific, tailored understanding of healthy aging and brain health.
In Latin American countries (LACs), the current prevalence of dementia stands at 8.5%, and it is expected to increase to 19.33% by 2050 (refs. 19,20). Such a projection significantly exceeds the prevalence estimates for Europe and the United States3, highlighting the need to contextualize the risk factors of the LACs21, a region of large inequalities and unique racial–ethnic admixtures9,10,20,19. Recent studies suggest that demographic factors impact healthy aging in a varied manner in LACs. This includes (1) a more significant impact from social and health disparities compared to age and sex1,2,5 and (2) less pronounced effects of the latter two compared to other regions1,5,22. We recently reported that cognition and functional abilities in healthy aging across LACs are influenced by heterogeneous factors that are different from other regions, mainly related to social and health disparities1. That study suggests that the disparities reflect non-universal effects of aging, deeply influenced by the numerous disparity-related cumulative exposures2,18 that escalate the risks associated with aging and dementia. However, except for the above-mentioned study, the cumulative evidence has many gaps and methodological flaws1,2,10,18,19. There is substantial heterogeneity regarding sample sizes, designs, populations, statistical approaches and outcomes. Most studies have not evaluated the interactions between different potential risk factors10. The focus on individual or a small number of countries within the region, combined with the lack of harmonization, can lead to a priori biases and non-representative regional findings20.
To address these gaps, we studied different factors influencing healthy aging, as reflected by cognition and functional ability across LACs, using meta-analytical and systematic review approaches (Fig. 1). We first assessed the overall effects of combined risk factors. We then performed separate meta-analyses of predictors, including demographics, health status, mental health symptoms, lifestyle factors and SDH. From an initial number of 455 studies, our meta-analyses included n = 146,005 participants and 38 final reports (28 for cognition and 10 for functional ability). We addressed the robustness of the results in terms of main effects, heterogeneity and multiple influences, considering different predictors, country differences, methods employed and publication bias. In addition, we performed a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) scoping review to address additional aspects related to the methodological procedures employed.
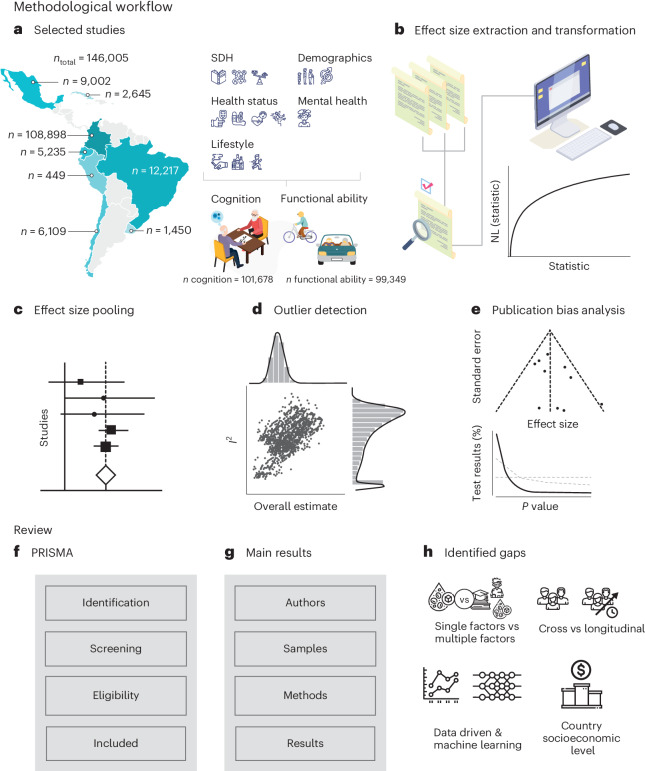
Fig. 1. Methodological workflow.
a–e, Meta-analysis. The left part of a (selected studies) presents the sample size (n) by each country. On the right side of a, we describe of the group of risk factors (demographic, SDH, health status, mental health and lifestyle) associated with the healthy aging outcomes (cognition and functional ability). b (effects size extraction and transformation) illustrates the procedure followed by three independent researchers to extract effect sizes of each risk factor for cognition and functional ability across studies. NL, natural logarithm. c, Effect size pooling: the panel displays the graphic representation of a forest plot, used to present pooled effect sizes of the studies assessed in this work (no real data are depicted in this panel). d (outlier detection) reveals the graphic representation of heterogeneity I2 across studies (y axis) versus the predicted overall estimate (no real data are depicted in this panel). e, Publication bias analysis: graphic representation of the funnel plot in the upper section and, in the lower section, a graph that depicts the strength of P value patterns across studies (no real data are depicted in this panel). The horizontal and diagonal dashed lines denote ‘Null of no effect’ and ‘Null of 33% power’, respectively. f–h, Scoping review. f, PRISMA: study stages following the PRISMA flowchart (no real data are depicted in this panel). g (main results) indicates the specific information extracted from the studies (no real data are depicted in this panel). h, Identified gaps. This panel provides the gaps identified in each study, including the inclusion of one versus many risk factors, the type of studies (cross-sectional, longitudinal or combined), the analytical approaches using data-driven insights and machine learning techniques and the consideration of countries with different socioeconomic backgrounds.
Results
Meta-analysis
A comprehensive exploration of literature published until 16 August 2023 was performed in multiple databases (including MEDLINE, Virtual Health Library and Web of Science; Fig. 2 and Supplementary File 1), leveraging a variety of keywords encompassing aging, brain health, lifestyle, demographics, mental health, SDH and other factors, specifically focusing on LACs. A team of three reviewers extracted detailed information from all the sourced articles, and the results were verified by an additional independent reviewer. Year of publication, effect size, type of effect size and control variables were obtained (data analysis section). Our research focused on five primary factors identified as critical determinants of healthy aging in previous studies1. These include insights from the Lancet Consortium for Dementia Prevention and Care3, encompassing demographics (age and sex), SDH (education, social isolation, socioeconomic status, ethnicity and race), health status (hypertension, diabetes and obesity), mental health status (depression, anxiety and stress-related disorders) and lifestyle factors (smoking, alcohol consumption and physical activity). Other factors relevant to aging were not included, given the lack of a minimum available number of reports. A complete list of studies included in all analyses for cognition1,23–49 and functional ability1,28,50–57 is included here and further described in Supplementary Table 1.
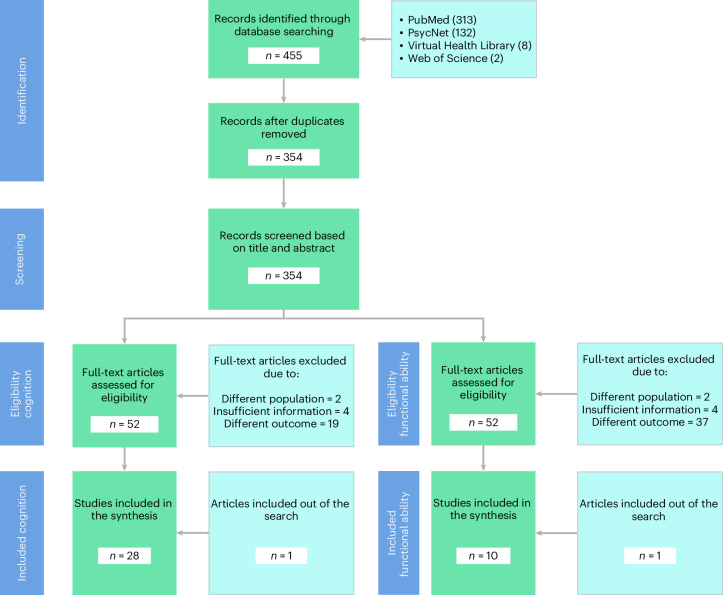
Fig. 2. PRISMA flowchart.
PRISMA methodology for searching and selecting studies. Using the search criteria, we identified 1,382 studies (PubMed 313, PsycNet 132, Virtual Health Library 8, Web of Science 2). After implementing a procedure to detect duplicates, we retained 354 studies for further analysis. Three independent evaluators assessed the relevance of the title and abstract of each study, ultimately selecting 52 studies. Four full-text articles were excluded due to insufficient or unsuitable information for our research interests, two because of a different population study and 19 for assessing different outcomes. In the end, 38 studies were selected for further analysis: 28 on risk factors for cognition and 10 on risk factors for functional ability.
Egger’s test58 and enhanced funnel plots59 were used to gauge publication bias, complemented by a p-curve test assessing the empirical evidence strength and potential biases60. The I2 index61 and Graphic Display of Heterogeneity (GOSH62) plots provided an assessment of heterogeneity, supported by subgroup analyses across different countries (Methods). Given the existence of high heterogeneity, outlier data points were identified and excluded in further analyses to maintain data integrity63. Leveraging random effects models, factors were analyzed with the Paule–Mandel estimator64 using Knapp–Hatung adjustments65 to minimize false discovery rates. The age ranges in most studies were above 59 years. However, some studies included individuals aged 35 years and older. Table 1 provides a detailed description of the age ranges used across these studies, along with details of studies assessing cognition and functional ability in each country and the meta-analysis. Table 2 presents the major results of the meta-analyses of cognition, and Table 3 presents the results of the meta-analyses of functional ability.
Table 1.
Details of studies assessing cognition and functional ability in each country and meta-analysis
| Cognition | Functional ability | Age ranges | ||||
|---|---|---|---|---|---|---|
| Country | n = Full | n = W/o outliers | n = Full | n = W/o outliers | Full | |
| All predictors | Brazil | 10,080 | 8,492 | 2,215 | 2,215 | 35–>90 |
| Chile | 4,339 | 4,339 | 470 | 470 | 60–89 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | 31,680 | 31,680 | 31,680 | >64 | |
| Colombia | 43,933 | 416 | 64,614 | 41,271 | 60–96 | |
| Cuba | 2,645 | 2,645 | – | – | 60–>75 | |
| Mexico | 9,387 | 9,118 | – | – | 50–>65 | |
| Peru | – | – | 449 | 449 | >59 | |
| Demographics | Brazil | 4,486 | 3,563 | 1,907 | 1,626 | 35–>80 |
| Chile | 2,955 | 2,955 | 470 | 470 | 60–89 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | 31,680 | 31,680 | – | >64 | |
| Colombia | 23,759 | 416 | 64,614 | 64,614 | 60–96 | |
| Cuba | 2,645 | 2,645 | – | – | 60–>75 | |
| Mexico | 1,109 | 1,109 | – | – | >49 | |
| Peru | – | – | 449 | 449 | >59 | |
| Health | Brazil | 6,081 | 6,081 | 1,779 | 1,413 | 35–>80 |
| Chile | 1,571 | 1,571 | 470 | 470 | 60 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | 31,680 | 31,680 | 31,680 | >64 | |
| Colombia | 23,343 | – | 64,614 | 64,614 | >64 | |
| Cuba | 2,645 | 1,846 | – | – | 65–>75 | |
| Mexico | 2,416 | 1,826 | – | – | >64 | |
| Peru | – | – | 449 | 449 | >59 | |
| Mental health | Brazil | 1,875 | 1,875 | – | – | >59 |
| Chile | 1,571 | 1,571 | – | – | >59 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | – | 31,680 | 31,680 | >64 | |
| Colombia | 416 | 416 | 47,037 | 47,037 | 60–>80 | |
| Cuba | 1,846 | 1,846 | – | – | 65–>75 | |
| Mexico | 1,291 | 1,291 | – | – | >54 | |
| Peru | – | – | – | – | >59 | |
| Lifestyle | Brazil | 4,964 | 3,376 | 1,413 | 1,413 | 35–>80 |
| Chile | 2,955 | 1,384 | – | – | >59 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | 31,680 | 31,680 | 31,680 | >64 | |
| Colombia | 43,517 | 23,343 | 47,037 | 47,037 | >64 | |
| Cuba | 1,846 | 1,846 | – | – | 65–>75 | |
| Mexico | 4,495 | 4,495 | – | – | >50 | |
| Peru | – | – | – | – | >59 | |
| SDH | Brazil | 5,368 | 3,780 | 2,050 | 2,050 | 35–>90 |
| Chile | 1,384 | 1,384 | – | – | >59 | |
| (Colombia, Ecuador, Chile, Uruguay) | 31,680 | 31,680 | 31,680 | 31,680 | >64 | |
| Colombia | 23,759 | 416 | 64,614 | 41,271 | 60–96 | |
| Cuba | 2,645 | 2,645 | – | – | 65–>75 | |
| Mexico | 3,414 | 3,414 | – | – | >50 | |
| Peru | – | – | 449 | 449 | >59 | |
Table 2.
Meta-analyses of cognition
| k | OR | 95% CI | P | t | 95% PI | I2 | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| All factors | Random effects model | 28 | 1.2006 | (1.0127, 1.4234) | 0.0363 | 2.2 | (0.5912, 2.4382) | 92.1% | (89.8%, 94.0%) |
| Outliers removed | 24 | 1.2651 | (1.1443, 1.3987) | <0.0001 | 4.85 | (1.0272, 1.5582) | 16.9% | (0.0%, 49.4%) | |
| Demographics | Random effects model | 15 | 1.5098 | (1.1905, 1.9147) | 0.0023 | 3.72 | (0.7144, 3.1909) | 89.2% | (83.9%, 92.8%) |
| Outliers removed | 13 | 1.7386 | (1.3997, 2.1595) | <0.0001 | 5.56 | (1.0247, 2.9496) | 48.2% | (1.7%, 72.7%) | |
| Health | Random effects model | 16 | 1.2856 | (1.0136, 1.6305) | 0.0397 | 2.25 | (0.5822, 2.8385) | 80.1% | (68.4%, 87.4%) |
| Outliers removed | 13 | 1.2259 | (1.0601, 1.4175) | <0.0001 | 3.05 | (0.8819, 1.7040) | 70.4% | (47.9%, 83.2%) | |
| Mental health | Random effects model | 10 | 1.6803 | (1.1848, 2.3830) | 0.0084 | 3.36 | (0.6409, 4.4051) | 84.9% | (73.9%, 91.2%) |
| Outliers removed | 9 | 1.8175 | (1.2699, 2.6012) | 0.0049 | 3.84 | (0.7416, 4.4543) | 61.2% | (19.6%, 81.3%) | |
| Lifestyle | Random effects model | 13 | 1.04 | (0.7765, 1.3930) | 0.7747 | 0.29 | (0.3836, 2.8197) | 93.4% | (90.4%, 95.4%) |
| Outliers removed | 10 | 0.9623 | (0.7490, 1.2363) | 0.7364 | −0.35 | (0.4841, 1.9129) | 83.4% | (70.9%, 90.5%) | |
| SDH | Random effects model | 15 | 0.9994 | (0.6110, 1.6348) | 0.998 | 0 | (0.1656, 6.0331) | 98.2% | (97.8%, 98.6%) |
| Outliers removed | 13 | 1.0658 | (0.7997, 1.4205) | 0.6373 | 0.48 | (0.4734, 2.3996) | 77.2% | (61.2%, 86.6%) |
PI, prediction interval.
Table 3.
Meta-analyses of functional ability
| k | OR | 95% CI | P | t | 95% PI | I2 | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| All predictors | Random effects model | 10 | 1.2088 | (1.0470,1.3956) | 0.0153 | 2.99 | (0.8248, 1.7717) | 93.1% | (89.3%, 95.5%) |
| Outliers removed | 9 | 1.2795 | (1.242, 1.4562) | 0.0023 | 4.39 | (0.9472, 1.7283) | 75.9% | (53.8%, 87.5%) | |
| Demographics | Random effects model | 9 | 1.1232 | (0.7593, 1.6613) | 0.5132 | 0.68 | (0.3381, 3.7311) | 95.6% | (93.4%, 97.1%) |
| Outliers removed | 7 | 1.137 | (0.8681, 1.4891) | 0.2887 | 1.16 | (0.5685, 2.2740) | 94.8% | (91.6%, 96.8%) | |
| Health | Random effects model | 8 | 1.4634 | (0.8708, 2.4595) | 0.1264 | 1.73 | (0.3152, 6.7956) | 79.6% | (60.2%, 89.5%) |
| Outliers removed | 7 | 1.1921 | (1.1260, 1.2622) | 0.0003 | 7.53 | (1.0688, 1.3298) | 58.5% | (4.3%, 82%) | |
| Mental health | Random effects model | 2 | 1.083 | (0.9878, 1.1874) | 0.0577 | 11.01 | – | – | – |
| – | – | – | – | – | – | – | – | – | |
| Lifestyle | Random effects model | 4 | 1.3086 | (0.6875, 2.4906) | 0.2756 | 1.33 | (0.2154, 7.9499) | 99.4% | (99.2%, 99.6%) |
| – | – | – | – | – | – | – | – | – | |
| SDH | Random effects model | 8 | 1.2273 | (0.9382, 1.6055) | 0.1144 | 1.8 | (0.6113, 2.4641) | 99.0% | (98.7%, 99.2%) |
| Outliers removed | 7 | 1.3401 | (1.0338, 1.7371) | 0.0328 | 2.76 | (0.7314, 2.4555) | 99.1% | (98.8%, 99.3%) |
PI, prediction interval.
Cognition
Our research identified 38 articles, and 28 were included in the analysis of cognition. Sample size of each meta-analysis is provided in Table 1. Supplementary Table 1 summarizes the articles and the main results.
All factors
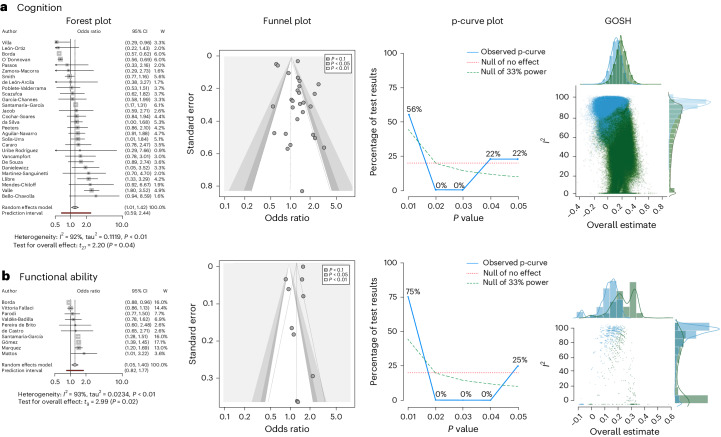
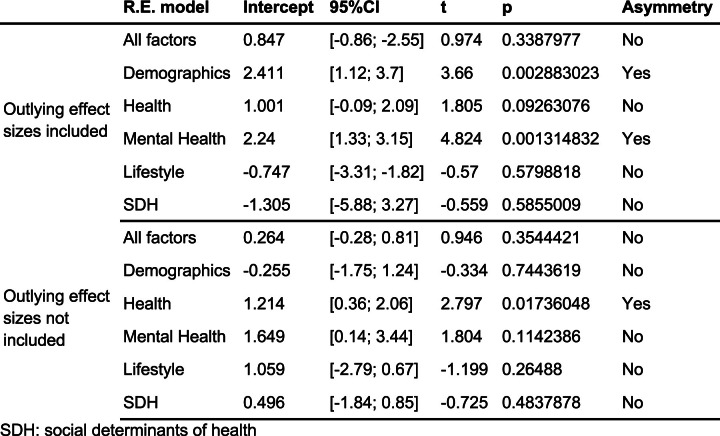
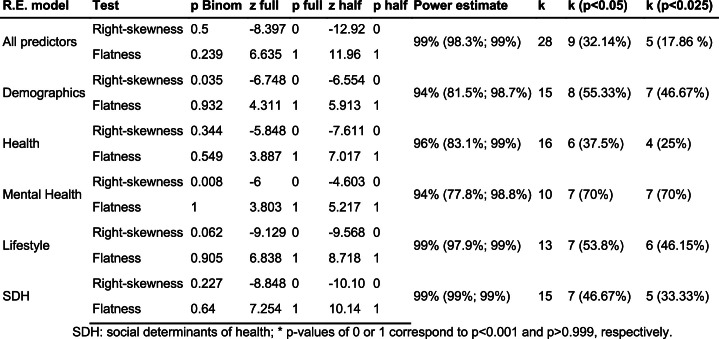
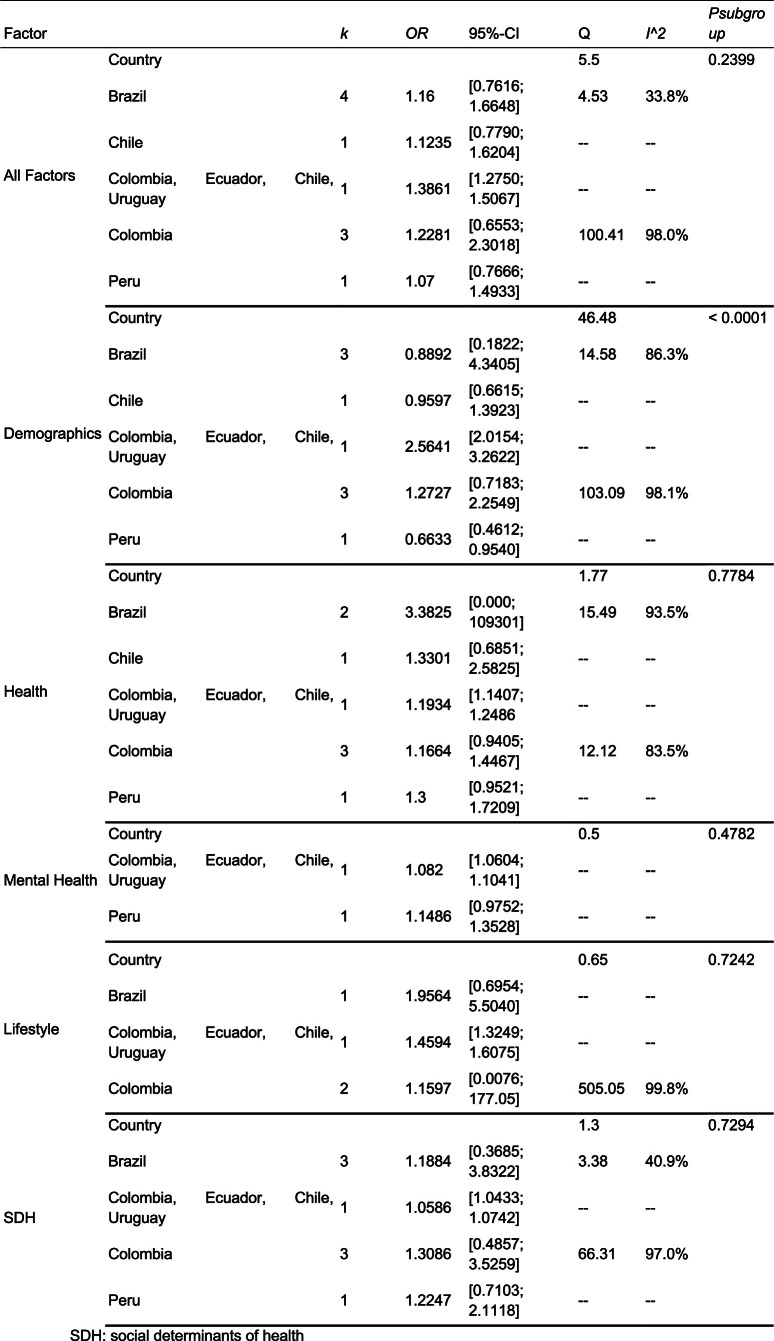
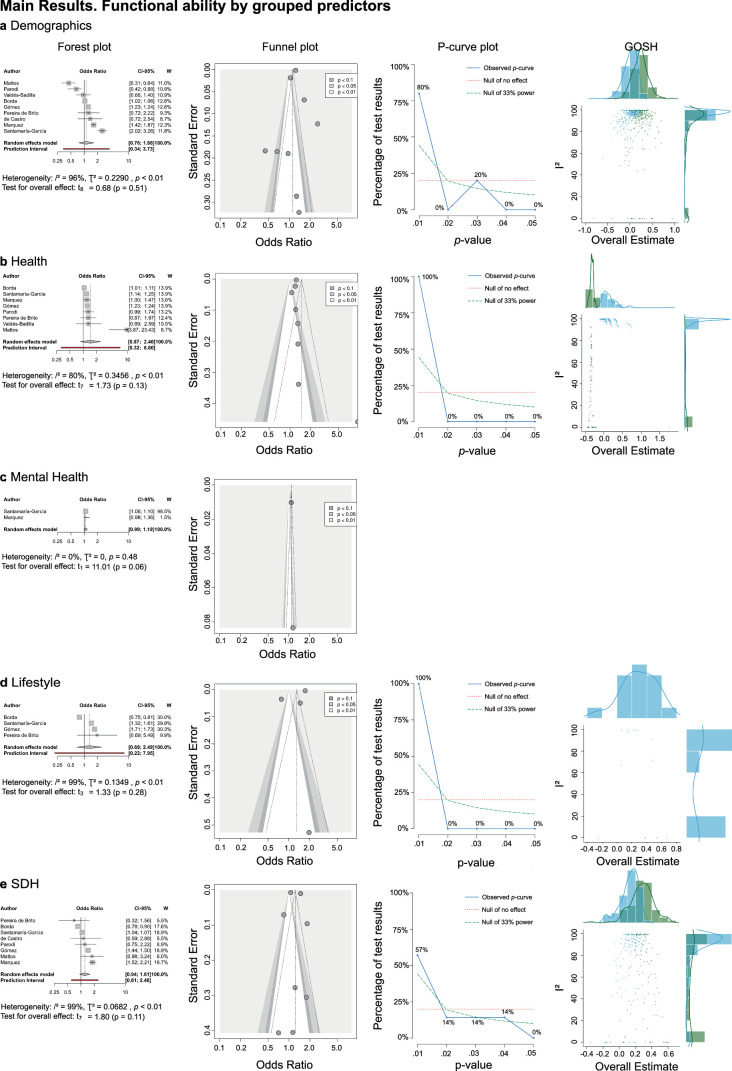
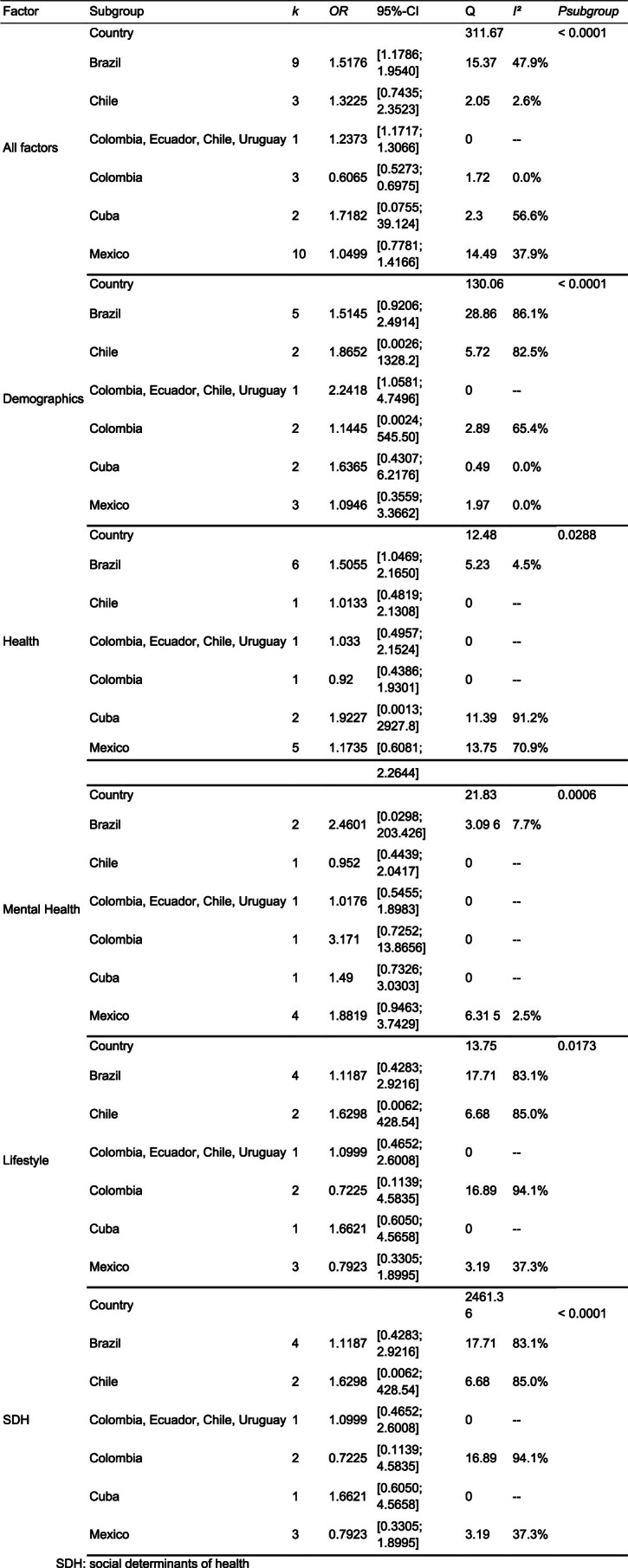
The meta-analysis combining all factors (k = 28) reached statistical significance (odds ratio (OR) = 1.2006, P = 0.0363, confidence interval (CI) = (1.0127, 1.4234)), although pronounced heterogeneity was detected (I2 = 92.1%, CI = (89.8%, 94%)). After excluding outliers, the heterogeneity markedly decreased (I2 = 16.9%, CI = (0.0%, 49.4%); Supplementary Figs. 1–3). Concurrently, the pooled effect size exhibited enhanced statistical significance with more precise CIs (OR = 1.2651, P < 0.0001, CI = (1.1443, 1.3987); Table 1 and Fig. 3a). Subgroup analysis showed significant differences between countries (P < 0.0001), and effect sizes ranged from 0.6065 to 1.7182 (Extended Data Table 1). Egger’s test (P = 0.3388; Extended Data Table 2) did not indicate asymmetry (Fig. 3a). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −8.397, P < 0.001, z half = −12.92, P < 0.001, power estimate = 99%, CI = (98.3%, 99%); Fig. 3a and Extended Data Table 3).
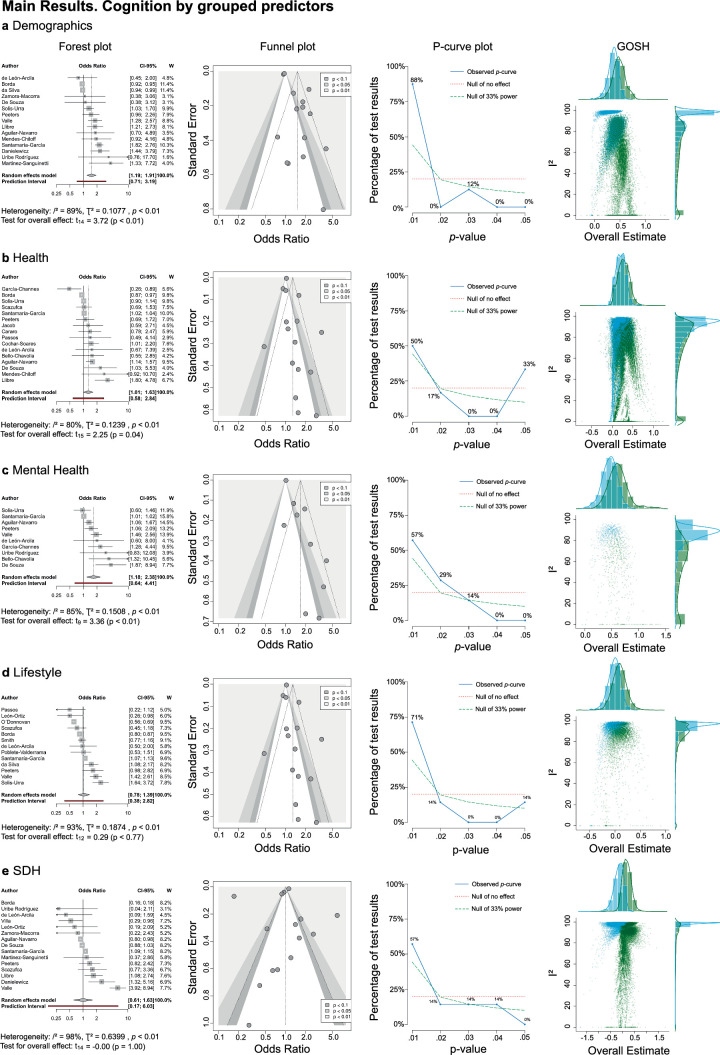
Fig. 3. Meta-analysis across all risk factors for cognition and functional ability.
a, Cognition. Forest plot shows k studies in random effects model (first author, OR, CI and weights). The random effects model results (cognition: k = 28, n = 102,064, OR = 1.2006, P = 0.0363, CI = (1.0127, 1.4234); functional ability: k = 10, n = 99,428, OR = 1.2088, P = 0.0153, CI = (1.0470, 1.3956)) are reported with Knapp–Hartung correction for false discovery rate, the prediction interval and heterogeneity values (I2 and tau2). W, weights. b, Functional ability. Forest plot shows k studies in random effects model (first author, OR, CI and weights). The random effects model results (functional ability: k = 10, n = 99,428, OR = 1.2088, P = 0.0153, CI = (1.0470, 1.3956)) are reported with Knapp–Hartung correction for false discovery rate, the prediction interval and heterogeneity values (I2 and tau2). For a and b, contour-enhanced funnel plot shows effect sizes, standard errors and significance; p-curve analysis shows the accumulation of P values over the significant studies (observed p-curve), the no-effect curve and 33% power curve; and the GOSH shows distribution for all 2k−1 possible study combinations (1 million randomly selected models when 2k−1 > 106) in blue and leaving out the most negatively influential study in green.
Extended Data Table 1.
Meta-analyses of cognition by country
Extended Data Table 2.
Publication bias test (Egger’s test) of cognition
Extended Data Table 3.
Analyses of p-curve of cognition
Demographic factors
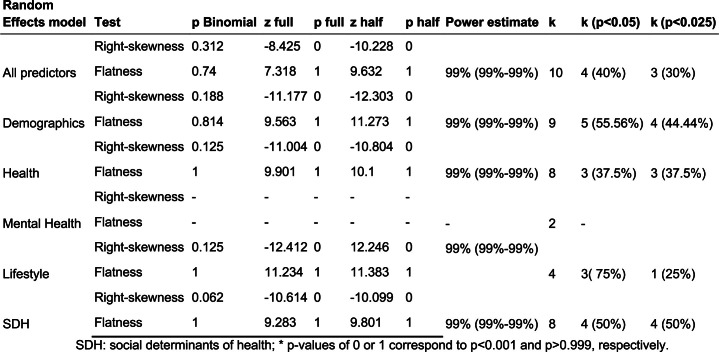
Demographics factors (age and sex, k = 15) reached statistical significance (OR = 1.5098, P = 0.0023, CI = (1.1905, 1.9147)), with high heterogeneity (I2 = 89.2%, CI = (83.9%, 92.8%)). The pooled effect size without outliers reached statistical significance (OR = 1.2651, P < 0.0001, CI = (1.1443, 1.3987)), with moderate heterogeneity (I2 = 48.2%, CI = (1.7%, 72.7%); Table 1 and Extended Data Fig. 1a). Subgroup analysis showed significant differences between countries (P < 0.0001), and effect sizes ranged from 1.0946 to 2.2418 (Extended Data Table 1). Egger’s test (P = 0.0288) indicated asymmetry (Extended Data Table 2 and Extended Data Fig. 1b), and the p-curve analysis showed significant results for both half and full p-curve tests, suggesting the presence of a true non-zero effect (z full = −6.748, P < 0.001, z half = −6.554, P < 0.001, power estimate = 94%, CI = (81.5%, 98.4%); Extended Data Table 3 and Extended Data Fig. 1a).
Extended Data Fig. 1. Metanalysis across different risk factors for Cognition.
Each panel illustrates the major effects for each factor analyzed: panel a focuses on demographics; panel b on health; panel c on mental health; panel d on lifestyle; and panel e on Social Determinants of Health (SDH). The forest plot shows -k studies using the random effects model (first author, odds ratio, confidence interval, and weights). The random effects model results (Demographics: k = 15, n = 66634, OR = 1.5098, p-value = 0.0023, CI = [1.1905; 1.9147]; Health: k = 16, n = 67736, OR = 1.2856, p-value = 0.0397, CI = [1.0136; 1.6305]; Mental health symptoms: k = 16, n = 38679, OR = 1.6803, p-value = 0.0084, CI = [1.1848; 2.3830]; Lifestyle: k = 13, n = 89457, OR = 1.04, p-value = 0.7747, CI = [0.7765; 1.3930]; SDH: k = 15, n = 68250, OR = 0.9994, p-value = 0.998, CI = [0.6110; 1.6348]) are reported with Knapp-Hartung correction for false discovery rate, the prediction interval, and heterogeneity values (I², tau²). Contour-enhanced funnel plot showing effect sizes, standard errors, and significance. P-curve analysis, showing the accumulation of p-values over the significant studies (observed p-curve), the no-effect curve and 33% power curve. GOSH (Graphic Display of Heterogeneity) shows the distribution for all 2k-1 possible study combinations (1 million randomly selected models when 2k-1 > 106) in blue and leaving out the most negatively influential study in green.
Health status
The health factors meta-analysis (cardiometabolic risks and other diseases, k = 16) was significant (OR = 1.2856, P = 0.0397, 95% CI = (1.0136, 1.6305)) and revealed high heterogeneity (I2 = 80.1%, CI = (68.4%, 87.4%)). After excluding outliers, heterogeneity slightly decreased (I2 = 70.4%, CI = (47.94%, 83.2%); Supplementary Figs. 1–3). Concurrently, the pooled effect size exhibited enhanced statistical significance with narrower CIs (OR = 1.2259, P < 0.0001, 95% CI = (1.0601, 1.4175); Table 1 and Extended Data Fig. 1b). Subgroup analysis showed significant differences between countries (P = 0.0288), and effect sizes ranged from 0.9227 to 1.9227 (Extended Data Table 1). Egger’s test (P = 0.0926) did not indicate asymmetry (Extended Data Table 2 and Extended Data Fig. 1c). p-curve analysis showed significant results for both half and full p-curve tests, evidencing the presence of a true non-zero effect (z full = −5.848, P < 0.001, z half = −7.611, P < 0.001, power estimate = 96%, CI = (83.1%, 99%); Extended Data Table 3 and Extended Data Fig. 1b).
Mental health symptoms
Meta-analysis of mental health symptoms (k = 16) had significant effects (OR = 1.6803, P = 0.0084, CI = (1.1848, 2.3830)) and exhibited high heterogeneity (I2 = 84.9%, CI = (73.9%, 91.2%)). The pooled effect size without outliers was significant (OR = 1.8175, P = 0.0049, CI = (1.2699, 2.6012); Table 1, Extended Data Fig. 1c and Supplementary Figs. 1–3) and showed decreased heterogeneity (I2 = 61.2%, CI = (19.6%, 81.3%)). Subgroup analysis yielded significant differences between countries (P = 0.0006), and effect sizes ranged from 0.952 to 1.3.171 (Extended Data Table 1). Egger’s test (P = 0.0013) indicated asymmetry (Extended Data Table 2 and Extended Data Fig. 1d). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −6, P < 0.001, z half = −4.603, P < 0.001, power estimate = 94%, CI = (77.8%, 98.8%); Extended Data Table 3 and Extended Data Fig. 1c).
Lifestyle
The lifestyle factors metanalysis (physical activity, nutrition, alcohol consumption and smoking, k = 13) was not significant (OR = 1.04, P = 0.7747, 95% CI = (0.7765, 1.3930)) and showed high heterogeneity (I2 = 93.4%, CI = (90.4%, 95.4%)). After excluding outliers, the random effects model remained non-significant (P > 0.05), and heterogeneity slightly decreased (Extended Data Table 3, Extended Data Fig. 1d and Supplementary Figs. 1–3). Subgroup analysis showed significant differences between countries (P = 0.0173), and effect sizes ranged from 0.7225 to 1.6621 (Extended Data Table 1). Egger’s test (P = 0.5799) did not indicate asymmetry (Extended Data Table 2 and Extended Data Fig. 1c). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −9.129, P < 0.001, z half = −9.568, P < 0.001, power estimate = 99%, CI = (97.9%, 99%); Extended Data Table 3 and Extended Data Fig. 1d).
SDH
The SDH factors meta-analysis (educational attainment and socioeconomic status, k = 15) did not reach significance (OR = 0.9994, P = 0.998, 95% CI = (0.6110, 1.6348)) and showed high heterogeneity (I2 = 98.2%, CI = (97.8%, 98.6%)). After excluding outliers, the random effects model remained not statistically significant (P > 0.05), and heterogeneity (I2 = 77.2%, CI = (61.2%, 86.6%)) slightly decreased (Extended Data Table 3, Extended Data Fig. 1e and Supplementary Figs. 1–3). Subgroup analysis by country showed significant differences (P < 0.0001), and effect sizes ranged from 0.7225 to 1.6621 (Extended Data Table 1). Egger’s test (P = 0.5855) did not indicate asymmetry (Extended Data Table 2 and Extended Data Fig. 1c). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −8.848, P < 0.001, z half = −10.10, P < 0.001, power estimate = 99%, CI = (99%, 99%); Extended Data Table 3 and Extended Data Fig. 1e).
Functional ability
Ten studies were included as they assessed specifically risk factors of functional ability (Supplementary Table 1 summarizes the articles and the main results). Sample size included in each meta-analysis is provided in Table 1.
All factors
The meta-analysis including all factors (k = 10) achieved significant effects (OR = 1.2088, P = 0.0153, CI = (1.0470, 1.3956)). However, there was pronounced heterogeneity (I2 = 93.1%, CI = (89.3%, 95.5%)). After excluding outliers, the heterogeneity remained high (I2 = 75.9%, CI = (53.8%, 87.5%); Supplementary Figs. 1–3). Concurrently, the pooled effect size exhibited enhanced statistical significance with more precise CIs (OR = 1.2795, P = 0.0023, CI = (1.242, 1.4562); Table 3 and Fig. 3b). Subgroup analysis by country did not show significant differences (P = 0.2399), and effect sizes ranged from 1.07 to 1.3861 (Extended Data Table 4). Egger’s test (P = 0.1884) did not indicate asymmetry (Fig. 3b and Extended Data Table 5). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −8.425, P < 0.001, z half = −10.228, P < 0.001, power estimate = 99%, CI = (99%, 99%); Fig. 3b and Extended Data Table 6).
Extended Data Table 4.
Meta-analyses of functional ability by country
Extended Data Table 5.
Publication bias test (Egger’s test) of functional ability
Extended Data Table 6.
Analyses of p-curve in functional ability
Demographic factors
A meta-analysis on the demographic factors associated with functional ability (age and sex, k = 9) did not reach significance (OR = 1.1232, P = 0.5132, CI = (0.7593, 1.6613)). High heterogeneity was detected (I2 = 95.9%, CI = (93.4%, 97.1%)). The pooled effect size without outliers presented high heterogeneity (I2 = 94.8%, CI = (91.6%, 96.8%)) and non-statistical significance (OR = 1.137, P = 0.2887, CI = (0.8681, 1.4891); Table 3, Extended Data Fig. 2a and Supplementary Figs. 1–3). Subgroup analysis showed significant differences between countries (P < 0.0001), and effect sizes ranged from 0.6633 to 2.5641 (Extended Data Table 4). Egger’s test (P = 0.5791) did not indicate asymmetry (Extended Data Table 5 and Extended Data Fig. 2a), and p-curve analysis showed significant results for the half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −11.177, P < 0.001, z half = −12.303, P < 0.001, power estimate = 99%, CI = (99%, 99%); Extended Data Table 6 and Extended Data Fig. 2a).
Extended Data Fig. 2. Metanalysis across different risk factors for functional ability.
Each panel illustrates the major effects for each factor analyzed: panel a focuses on demographics; panel b on health; panel c on mental health; panel d on lifestyle; and panel e on Social Determinants of Health (SDH). The forest plot shows k studies using the random effects model (first author, odds ratio, confidence interval, and weights). Information on the P-curve and GOSH plot was not displayed in panel c due to a reduced number of studies available for conducting specific analyses. The random effects model results (Demographics: k = 9, n = 99120, OR = 1.1232, p-value = 0.5132, CI = [0.7593; 1.6613]; Health: k = 8, n = 98992, OR = 1.4634, p-value = 0.1264, CI = [0.8708; 2.4595]; Mental health symptoms: k = 2, n = 49257, OR = 1.083, p-value = 0.0577, CI = [0.9878; 1.874]; Lifestyle: k = 4, n = 80130, OR = 1.3086, p-value = 0.2756, CI = [0.6875; 2.4906]; SDH: k = 8, n = 98793, OR = 1.2273, p-value = 0.1144, CI = [0.9382; 1.6055]) are reported with Knapp-Hartung correction for false discovery rate, the prediction interval, and heterogeneity values (I², tau²). Contour-enhanced funnel plot showing effect sizes, standard errors, and significance. P-curve analysis, showing the accumulation of p-values over the significant studies (observed p-curve), the no-effect curve, and the 33% power curve. GOSH (Graphic Display of Heterogeneity) shows the distribution for all 2k-1 possible study combinations (1 million randomly selected models when 2k-1 > 106) in blue and leaving out the most negatively influential study in green. Blank panels indicate that P-Curves and GOSH cannot be estimated due to insufficient studies.
Health status
A meta-analysis on the health factors associated with functional ability (cardiometabolic diseases and other, k = 8) did not reach significance (OR = 1.4634, P = 0.1264, 95% CI = (0.8708, 2.4595)) and exhibited high heterogeneity (I2 = 79.6%, CI = (60.2%, 89.5%)). After excluding outliers, heterogeneity decreased, although it remained high (I2 = 58.5%, CI = (4.3%, 82%)). Concurrently, the pooled effect size exhibited statistical significance with narrower CIs (OR = 1.1921, P < 0.0003, 95% CI = (1.1260, 1.2622); Table 3, Extended Data Fig. 2b and Supplementary Figs. 1–3). Subgroup analysis showed significant country differences (P = 0.0288), and effect sizes ranged from 0.9227 to 1.9227 (Extended Data Table 1). Egger’s test (P = 0.0926) did not indicate asymmetry (Extended Data Table 2 and Extended Data Fig. 2c). p-curve analysis showed significant results for half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −11.004, P < 0.001, z half = –10.804, P < 0.001, power estimate = 96%, CI = (83.1%, 99%); Extended Data Table 3 and Extended Data Fig. 2b).
Mental health symptoms
A meta-analysis on the role of mental health symptoms on functional ability (depression, anxiety and other, k = 2) did not show significant effects (OR = 1.083, P = 0.0577, CI = (0.9878, 1.874)). The I2 and other statistics were not calculated due to insufficient studies (Extended Data Table 4 and Extended Data Fig. 2c).
Lifestyle
The lifestyle factors meta-analysis (physical activity, nutrition, alcohol consumption and smoking, k = 4) did not reach significance (OR = 1.3086, P = 0.2756, 95% CI = (0.6875, 2.4906)) and showed high heterogeneity (I2 = 99.4%, CI = (99.2%, 99.6%)). No outliers were detected (Extended Data Table 4, Extended Data Fig. 2d and Supplementary Figs. 1–3). Subgroup analysis did not show significant country differences (P = 0.7242), and effect sizes ranged from 1.1597 to 1.9564 (Extended Data Table 4). Egger’s test (P = 0.3858) did not indicate asymmetry (Extended Data Table 5 and Extended Data Fig. 2d). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −12.412, P < 0.001, z half = −12.246, P < 0.001, power estimate = 99%, CI = (97.9%, 99%); Extended Data Table 6 and Extended Data Fig. 2d).
SDH
The SDH factors meta-analysis (educational attainment and socioeconomic status, k = 8) did not reach significant effects on functional ability (OR = 1.2273, P = 0.1144, 95% CI = (0.9382, 1.6055)) and showed high heterogeneity (I2 = 99%, CI = (98.7%, 99.2%)). After excluding outliers, the random effects model showed a statistically significant effect (OR = 1.3401, P = 0.0328, CI = (1.0338, 1.7371)), and heterogeneity remained high (I2 = 99.1%, CI = (98.8%, 99.3%); Extended Data Table 4, Extended Data Fig. 2e and Supplementary Figs. 1–3). Subgroup analysis did not show significant differences between countries (P = 0.7294), and effect sizes ranged from 1.0586 to 1.3086 (Extended Data Table 4). Egger’s test (P = 0.8271) did not indicate asymmetry (Extended Data Table 5 and Extended Data Fig. 2e). p-curve analysis showed significant results for both half and full p-curve tests, indicating the presence of a true non-zero effect (z full = −10.614, P < 0.001, z half = −10.099, P < 0.001, power estimate = 99%, CI = (99%, 99%); Extended Data Table 6 and Extended Data Fig. 2e).
Scoping review results
A complete list of studies included in all analyses for cognition1,23–49 and functional ability1,28,50–57 is included here and further described in Supplementary Table 1. This table includes the review, citations, sample sizes, methods employed, effect sizes, results and methodological gaps identified. Most of the studies (96%) did not use data-driven approaches to avoid a priori bias in the selection of predictors from other regions. Similarly, most of the studies (94%) involved a single country from Latin America with no country comparisons; used no machine learning with standard procedures to assess overfitting and generalization; and did not combine cross-sectional and longitudinal studies, nor did they use any multi-method complementary analysis for confirmation. In most of the studies (92%), there was a lack of training and test partition, and they relied on a single data source for training and testing. Similarly, most of the studies did not use heterogeneity-robust methodologies (80%). Also, some of these studies focused on patients without considering healthy aging (32%) and did not assess multiple potential predictors (28%).
Discussion
The meta-analyses and review reveal significant heterogeneity in the relationship between various risk factors and both cognition and functional ability in Latin America. In high-income countries, meta-analyses and systematic reviews have demonstrated associations between healthy aging and most of the factors addressed in this work11–13,15,16. The identification of underlying true non-zero effects across many analyses suggests that these areas are rich grounds for further research. However, the presence of high heterogeneity, the impact of outliers and publication bias all stress weakness of the available evidence. The review of methodological procedures indicates multiple flaws in the literature. Thus, our results undermine the reliability and generalizability of the available research, limiting the ability to develop tailored prevention and intervention programs for healthy aging in the region.
Considering all factors, the meta-analyses showed true non-zero effects. For cognition, robust effects were observed only after excluding outliers. The heterogeneity and significant differences between countries point to inconsistent effects9,10,20,19. For functional ability, small but significant effects were observed but with narrow CIs after excluding outliers. A minor number of reports showed stable effects, contrasting with other meta-analyses in the United States and Europe11–16. Overall, although significantly influencing aging, the main risk factors of healthy aging exhibit large heterogeneity and lack specificity.
The effects of specific factors were even less robust. Demographics significantly influence cognition, albeit with substantial heterogeneity and publication bias. For functional ability, the evidence was weaker, although an underlying true non-zero effect exists. An association between health status and both cognition and functional ability was demonstrated but with high heterogeneity. Mental health symptoms influence cognition, with significant heterogeneity and potential publication bias, and non-significant effects were observed on functional ability. For both cognition and functional ability, the primary analyses for SDH were non-significant. However, p-curve analyses indicated an underlying true non-zero effect, suggesting a minor effect. Conservatively, ranking the effects by CI without outliers yields demographics > mental health > health status effects, with lifestyle and SDH being not significant. This sequence contradicts previous reports1. Lifestyle and SDH were less frequently assessed, and measures varied across studies. Just a few studies evidenced significant effects of SDH. Most of the reports used disparate measures to track SDH. When studies include a few countries with harmonized measures, significant effects arise1. The lack of systematization in SDH across the region may lessen their importance as predictors. Additionally, an intrinsic heterogeneity of SDH is present within LACs1,66,67. This variation arises from differences in socioeconomic development, healthcare infrastructure, education access, ethnic and racial diversity and public health initiatives across countries, among other factors66,67. Consequently, the inherent diversity within LACs and the challenges in measuring regional social risks could skew our understanding of SDH in aging. The prediction of functional ability was weaker than cognition, likely due to fewer studies and less direct and objective assessment methods1. Overall, the evidence regarding specific factors associated with healthy aging appears inconclusive.
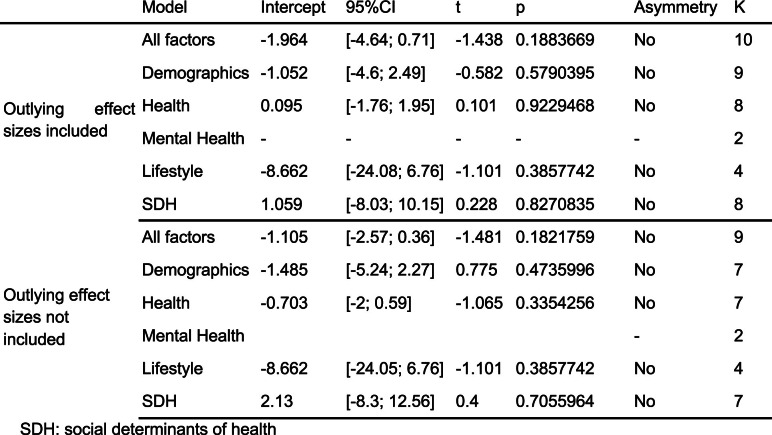
Some methodological factors extended the last conclusion. To address outliers, we implemented a procedure designed to reduce heterogeneity. The significance of the procedure lies in the fact that outliers often stem from atypical values, not from a broader distribution of values that indicate more significant inequalities. However, despite excluding outliers, significant heterogeneity persisted in most cases. This implies that high heterogeneity is a fundamental attribute of the datasets. The high I2 values showcase a considerable variation across studies, and exclusion of outliers was required to maintain data integrity, raising concerns about the representativeness of the findings61. Additionally, Egger’s test58 indicated asymmetry in some analyses, hinting at potential publication biases59. This test may lack statistical power to detect bias when the number of studies is small (k < 10), and, therefore, the results provided for functional ability must be read with caution. Different statistical constrains, including Knapp–Hatung adjustments65 and the Paule–Mandel estimator64, were used to try to reduce false discovery rates. Although theoretically sound, these measures might add layers of complexity and potential sources of error.
The scoping review provided additional caveats. Considerable research was concentrated on individual nations, neglecting the opportunity to address the regional population dynamic. Moreover, a lack of data-driven approaches that can avoid predefined biases from other regions constitutes a substantial barrier to the development of representative insights. With some exceptions, limited applications of machine learning techniques with data-driven feature selection, k-fold cross-validation and out-of-sample testing68 were used in the revised studies. These methods are instrumental in overcoming methodological biases associated with assuming a priori theoretical hierarchies in studying factors associated with clinical outcomes from other populations that are not necessarily equivalent1,5,22. Furthermore, these methods can better assess complex interactions between multiple variables related to an outcome while controlling for overfitting and ensuring out-of-sample validation68. However, machine learning algorithms are not free of other biases regarding the type of variables, missing data and contextual interpretation69. To determine unbiased predictors, non-data-driven domain expertise is important in aging research, and tailored programs would require causal methodology69. Combining cross-sectional and longitudinal studies can introduce biases related to survival and attrition. Longitudinal studies might overrepresent some positive factors associated with healthy aging, as they often include only older individuals who demonstrate high survival outcomes. These differences can profoundly affect the interpretation of results, potentially leading to a skewed representation of health outcomes. However, due to the unbalanced nature and scarcity of studies in this field, conducting sufficiently robust separate analyses is challenging. In any case, the currently available data in the region must be interpreted with caution. These results point to the necessity for a critical reassessment of the current approaches2,10,17 and the development of a more robust, tailored and inclusive research landscape69.
Results underscore the urgent need for systematic assessments of geographical variation and improved harmonization. These improvements will enable us to distinguish between components related to the intrinsic variability of populations, influenced by diverse genetic–environmental interactions18, and those arising from methodological limitations in current datasets. The substantial impact of outliers and publication bias highlights the weaknesses in the available evidence. Furthermore, our review of methodological procedures has uncovered several shortcomings in the existing literature. These shortcomings include inconsistent methods for assessing risk factors, focusing on individual predictors rather than their interactions, inadequate harmonization in assessing predictors and outcomes related to healthy aging and reliance on weak predictive models. Additionally, most studies of aging predictors do not employ data-driven approaches, thus avoiding potential biases associated with including pre-defined theoretical categories from other regions70.
Our study has limitations that underscore the need for further research. Most of the reports assessed had relatively small sample sizes compared to other regions11–16. This was particularly evident in the analysis of functional ability and the use of specific predictors, such as lifestyle and SDH. Significant discrepancies in sample sizes among studies may have skewed results, with larger samples reducing statistical uncertainty and, therefore, having a greater influence on the pooled effect size determined by the random effects models. The use of non-harmonized measures for risk factors, combined with the absence of interactions among variables, might have increased heterogeneity and diminished the prominence of specific factors. Including dissimilar studies, exposures and outcomes in a meta-analysis could partially explain heterogeneity. However, other meta-analyses from other regions followed this approach and found consistent results11–13,15,16. The pooled effect might not faithfully represent the actual effect size, which could differ across populations, contexts or study designs. This limits the generalizability of our findings and their overall statistical significance. The observed heterogeneity could also be influenced by factors not covered in the studies, such as genetics, population admixture, healthcare infrastructure and others1,2,9,10,17,19–21. Some reports have highlighted the premature mortality in LACs71, associated with socioeconomic disparities71. However, there is contrasting information when compared to populations in the United States. A lower rate of premature mortality among both male and female Latinos living in the United States has been called ‘the Latin American paradox’72. Future studies should adjust for regional mortality to better reflect the unique sociodemographic dynamics in the region. Addressing the effects according to World Bank categorization would provide useful information. However, when segmenting by domain and income, an unbalanced and small number of studies are observed in several categories, hindering the feasibility of such analyses. Similarly, risk factors in high-income countries within Latin America do not follow standard trends according to countries’ income seen in other regions5,22. Moreover, our search and meta-analysis analyzed various studies, including population-representative research, clinical samplings of participants visiting clinics or healthcare providers and community-dwelling studies (Supplementary Table 1). The diversity of these studies could partially explain the heterogeneity effects observed in the meta-analysis. Future research should control for the type and source of participants to assess the impact of risk factors on healthy aging across Latin America. Finally, although our scoping review focused on methodological gaps, future reviews should consider other potential aspects.
In conclusion, our results indicate a lack of robust healthy aging risk effects in Latin America, highlighting the need for more studies and better-quality research methods. The inherent limitations of current databases in LACs, along with the urgent need for harmonized assessment protocols, highlight the critical need for governments, stakeholders and researchers to collaborate in supporting the development of open, shareable, harmonized and multicentric data science initiatives dedicated to aging research in the region. The observed large heterogeneous effects, disparate results across countries, small effect sizes and publication bias all emphasize the urgent need for more systematic research. The inherent limitations of current databases in LACs, along with the urgent need for harmonized assessment protocols, highlight the critical need for governments, stakeholders and researchers to collaborate in supporting the development of open, shareable, harmonized and multicentric data science initiatives dedicated to aging research in the region. The findings advocate for developing more research to promptly address healthy aging and its multiple risk factors in Latin America, enabling governments to craft truly evidence-based initiatives for prevention and intervention.
Methods
Search and selection criteria
In this meta-analysis and review, we searched MEDLINE, PsycINFO and Virtual Health Library databases for studies published between the database inception and 30 March 2023. We contacted the corresponding authors to obtain the required data when they were not reported. Studies were excluded if the required data were not obtained after at least two attempts. Figure 1 shows the study design. We used a combined set of keywords related to aging, SDH, health status, mental health symptoms, lifestyle, demographics and Latin America (Supplementary File 1) to identify human studies reporting factors associated with cognition and functional ability in the region (Fig. 2). We also checked the reference lists of the retrieved studies for relevant reports that could be included in the current review. Randomized controlled trials, cohort studies and cross-sectional studies that met the eligibility criteria were included. Studies had to be (1) published in a peer-reviewed journal, (2) written in English, Spanish, Portuguese or French and (3) reporting on data collected from humans. A set of 38 studies was used for both the scoping review and the meta-analysis. These comprised 28 studies on cognition and 10 on functional ability. Independent assessments were conducted by reviewers for both the scoping review and the meta-analysis. We adhered to PRISMA guidelines for scoping reviews, ensuring that our methodology was transparent and replicable. Our study did not include randomized clinical trials or case–control studies, as we focused on identifying factors associated with healthy aging instead of those linked to aging-related diseases.
Ethics and inclusion statement
This work involved a collaboration among scientists in multiple countries, including Argentina, Chile, Colombia and Ireland. Contributors from all sites are included as coauthors or in acknowledgements according to their contributions. Researchers residing in LACs were involved in study design, study implementation, methodological procedure and writing and reviewing processes. Roles and responsibilities were agreed among collaborators ahead of the research. Local ethics committees of each database approved this research. To prevent any stigmatization, all identifying information was removed to preserve the privacy of individuals. Each country included in this study retained ownership of all human material shared for research purposes. We endorse the Nature Portfolio journals’ guidance on low- and middle-income country (LMIC) authorship and inclusion. Authorship was based on the intellectual contribution, commitment and involvement of each researcher in this study. We included authors born in LMICs and other underrepresented countries in this study. This study holds local relevance for each investigated country by presenting disaggregated findings, thereby offering country-specific risk factors of healthy aging. The selection of variables was informed by previous research and in accordance with established guidelines for global aging studies.
Meta-analysis
Clinical outcomes
Different meta-analyses were run for risk factors of cognition and functional ability in Latin American populations. A list of variables used to track each outcome is provided in Supplementary Table 2. For each outcome (cognition and functional ability), we ran six meta-analyses, including a global meta-analysis studying all risk factors and five independent meta-analyses for each factor studied independently. Summarized results can be found in Tables 1, 2 and 3.
Risk factors
Due to the significant heterogeneity of the predictors observed by the studies, they were grouped into five categories: demographics (age and sex), health (cardiometabolic risks and other somatic diseases), mental health symptoms (depression and anxiety), lifestyle (physical activity, nutritional habits, alcohol consumption and smoking) and SDH (educational attainment and socioeconomic status). Educational attainment refers to the highest level of education that an individual has completed. This can include levels such as a high school diploma, vocational training, undergraduate degrees or advanced degrees such as master’s or doctoral degrees. Some studies assessed education by determining the years of completed formal education1. This work includes studies that assessed education using education attainment measures and years of formal education. Socioeconomic status refers to the individual’s or group’s level in society, typically determined by a combination of factors, including educational background, income level and occupation73. A list of variables used to track educational attainment and socioeconomic status is provided in Supplementary Table 3. Other variables, such as ethnicity, health access and social adversities, were not included in further analyses because few studies incorporated these measures, and there was no systematic or reproducible approach across the studies.
Data analysis
A group of three reviewers extracted information on authors, year of publication, effect size, CI, type of effect size, predictors, outcomes, population, country study design and analyses and control variables of all articles. These were verified for accuracy by another independent reviewer. Any missing fields were left blank. For assessing publication bias, we used Egger’s test58 and enhanced funnel plots59. Additionally, we assessed the strength and possible bias of the empirical evidence we found as source of this meta-analysis with the p-curve test60. However, this approach is not robust when high heterogeneity exists between studies. For this reason, we also included the I2 index61, a measure of the proportion of the estimates of variance due to heterogeneity. This metric is independent of the number of studies and can be compared across meta-analyses with a different number of studies and metrics. Values above 35% are considered as reflecting high heterogeneity in the group of studies assessed. Heterogeneity was further explored with I2 GOSH plots62. To explore heterogeneity even further, subgroup analyses were performed between countries. When outlying effect sizes were identified based on previous criteria, we conducted subsequent analyses by pooling effects, excluding outliers63.
We employed random effects models to analyze all combined factors as well as each factor individually. The Paule–Mendel estimator was used, given its suitability for scenarios with a limited number of studies. Furthermore, we incorporated Knapp–Hartung adjustments to account for false discovery65. Only one article did not report adequate statistics (logistic regression models), and we transformed the reported Cohen’s f for every model using Cohen’s d transformations to log OR using the following formula74:
For each model in this study, we first converted Cohen’s f to Cohen’s d (f = d/2; ref. 75). Afterwards, we converted it to the log OR74 (log OR = d × phi/sqrt). This conversion allowed us to achieve a consistent analytical framework across studies.
Following previous classifications76, we define an outlier when the CIs do not overlap with those of the pooled effect size, indicating that its effect size is extreme and differs significantly from the overall effect76. All analyses were conducted in R (version 4.1.2) using additional packages specifically developed for meta-analyses.
Our approach involves harmonizing the values of predictors and their effects on outcomes74,76 by converting them into ORs and additional steps. We identified the most analogous variables across each study and incorporated those variables into our analyses, following established procedures74,76 and previous meta-analyses77–79. This enabled us to obtain specific ORs for each factor in every study and transform their effect sizes74,76, making the different variables comparable. We also provided the individual effects at the study level, highlighting the unique effects. Such procedures allowed us to assess the heterogeneity across studies, achieving partial harmonization and providing individual report metrics. However, the inherent limitations of current databases in LACs, along with the urgent need for harmonized assessment protocols, highlight the critical need for governments, stakeholders and researchers to collaborate in supporting the development of open, shareable, harmonized and multicentric data science initiatives dedicated to aging research in the region. All models and statistical analyses were run using Python version 3.9.13.
Scoping review on methodological approaches
Following the criteria detailed in the search and selection section (Supplementary File 1), we initially identified 455 studies. After a detailed review of the titles and abstracts, we narrowed down to 38 studies that fully met the criteria for our proposed scoping review. We analyzed several methodological aspects across studies, including (1) evaluating simultaneous associations and the interplay between various potential factors that could be related to healthy aging; (2) determining whether the studies used cross-sectional, longitudinal or combined methods; (3) analyzing the use of data-driven approaches to mitigate potential biases arising from theoretical assumptions; (4) assessing the representation of countries with different socioeconomic backgrounds; and (5) examining the deployment of robust methodological approaches for predictions, which included measures to control overfitting, out-of-sample validation, cross-validation and other adequate standards of analysis. This approach ensured a comprehensive and thorough analysis, facilitating a robust review grounded in stringent criteria that allowed for the inclusion of a variety of perspectives and methodologies.
Statistics and reproducibility
In this meta-analysis and scoping review, no statistical method was used to pre-determine sample size. No data were excluded from the analyses. The procedures implemented for meta-analysis and scoping review followed international guidelines. The investigators assessed independently the group of studies to the meta-analysis and the scoping review. No experimental or blinded procedures were used in this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Tables 1–3. Table 1. Main results of studies assessing risk factors of functional ability and cognition. Table 2. Measures used to assess cognition and functionality across studies. Table 3. Measures used to track social determinants of health and socioeconomic disparities across studies.
Acknowledgements
A.I. is partially supported by grants from ANID/FONDECYT Regular (1210195, 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152 and ID22I10029; ANID/FONDAP 15150012; Takeda CW2680521; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat, supported by the Fogarty International Center; the National Institutes of Health, National Institute on Aging (R01 AG057234, R01 AG075775, R01 AG021051, R01 AG083799 and CARDS-NIH); the Alzheimer’s Association (SG-20–725707); the Rainwater Charitable foundation–Tau Consortium; the Bluefield Project to Cure Frontotemporal Dementia; and the Global Brain Health Institute). The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Extended data
Author contributions
A.I. and H.S.-G. developed the study concept and the study design. A.I., M.M. and H.S.-G. performed testing and data curation. M.M. performed data analysis, under the supervision of A.I. A.I., H.S.-G. and M.M. interpreted the results and drafted the manuscript. All authors provided critical revisions, participated sufficiently in the work and approved the final version of the manuscript for submission.
Peer review
Peer review information
Nature Aging thanks Anja Leist, Sarah Tom and Xiaolin Xu for their contribution to the peer review of this work.
Data availability
Data included in this study are available on GitHub at https://github.com/AI-BrainLat-team/Latam-Aging-Meta-Analysis/tree/main/Data. Data from studies analyzed in the meta-analysis and scoping review are summarized in the Results section. Furthermore, all data are reported in Supplementary Table 1 and Supplementary File 1.
Code availability
All code for data analysis associated with this manuscript is available for download on GitHub at https://github.com/AI-BrainLat-team/Latam-Aging-Meta-Analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Agustin Ibanez, Email: agustin.ibanez@gbhi.org.
Hernando Santamaria-Garcia, Email: hernando.santamaria@gbhi.org.
Extended data
is available for this paper at 10.1038/s43587-024-00648-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s43587-024-00648-6.
References
- 1.Santamaria-Garcia, H. et al. Factors associated with healthy aging in Latin American populations. Nat. Med.29, 2248–2258 (2023). 10.1038/s41591-023-02495-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risk factors related to population diversity and disparity determine healthy aging. Nat. Med.29, 2183–2184 (2023). [DOI] [PubMed]
- 3.Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet396, 413–446 (2020). 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baez, S., Alladi, S. & Ibanez, A. Global South research is critical for understanding brain health, ageing and dementia. Clin. Transl. Med.13, e1486 (2023). 10.1002/ctm2.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters, H. Diverse factors shape healthy aging in Latin America. Nat. Aging3, 1175 (2023). 10.1038/s43587-023-00508-9 [DOI] [Google Scholar]
- 6.Fujiwara, Y. et al. Predictors of improvement or decline in instrumental activities of daily living among community-dwelling older Japanese. Gerontology54, 373–380 (2008). 10.1159/000151221 [DOI] [PubMed] [Google Scholar]
- 7.Hoogerduijn, J. G., Schuurmans, M. J., Duijnstee, M. S., de Rooij, S. E. & Grypdonck, M. F. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J. Clin. Nurs.16, 46–57 (2007). 10.1111/j.1365-2702.2006.01579.x [DOI] [PubMed] [Google Scholar]
- 8.Tucker-Drob, E. M. Cognitive aging and dementia: a life-span perspective. Annu. Rev. Dev. Psychol.1, 177–196 (2019). 10.1146/annurev-devpsych-121318-085204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan, B. C., Kurth, T., Matthews, F. E., Brayne, C. & Dufouil, C. Dementia risk prediction in the population: are screening models accurate? Nat. Rev. Neurol.6, 318–326 (2010). 10.1038/nrneurol.2010.54 [DOI] [PubMed] [Google Scholar]
- 10.Stephan, B. C. M. et al. Prediction of dementia risk in low-income and middle-income countries (the 10/66 Study): an independent external validation of existing models. Lancet Glob. Health8, e524–e535 (2020). 10.1016/S2214-109X(20)30062-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dregan, A., Stewart, R. & Gulliford, M. C. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing42, 338–345 (2013). 10.1093/ageing/afs166 [DOI] [PubMed] [Google Scholar]
- 12.Pignatti, F., Rozzini, R. & Trabucchi, M. Physical activity and cognitive decline in elderly persons. Arch. Intern. Med.162, 361–362 (2002). 10.1001/archinte.162.3.361 [DOI] [PubMed] [Google Scholar]
- 13.Mourao, R. J., Mansur, G., Malloy-Diniz, L. F., Castro Costa, E. & Diniz, B. S. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry31, 905–911 (2016). 10.1002/gps.4406 [DOI] [PubMed] [Google Scholar]
- 14.Lo, J. C., Groeger, J. A., Cheng, G. H., Dijk, D. J. & Chee, M. W. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med.17, 87–98 (2016). 10.1016/j.sleep.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 15.Anstey, K. J., Mack, H. A. & Cherbuin, N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry17, 542–555 (2009). 10.1097/JGP.0b013e3181a2fd07 [DOI] [PubMed] [Google Scholar]
- 16.Evans, I. E. M., Martyr, A., Collins, R., Brayne, C. & Clare, L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J. Alzheimers Dis.70, S119–S144 (2019). 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fittipaldi, S. et al. Heterogeneous factors influence social cognition across diverse settings in brain health and age-related diseases. Nat. Mental Health2, 63–75 (2024).
- 18.Ibanez, A., Legaz, A. & Ruiz-Adame, M. Addressing the gaps between socioeconomic disparities and biological models of dementia. Brain2023, 3561–3564 (2023). 10.1093/brain/awad236 [DOI] [Google Scholar]
- 19.Parra, M. A. et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology90, 222–231 (2018). 10.1212/WNL.0000000000004897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parra, M. A. et al. Dementia in Latin America: paving the way toward a regional action plan. Alzheimers Dement.17, 295–313 (2021). 10.1002/alz.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols, E. et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health7, e105–e125 (2022). 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santamaria-Garcia, H. et al. Risk factors associated with aging in Latin American populations. Nat. Med.29, 2248–2258 (2023). [DOI] [PMC free article] [PubMed]
- 23.O’Donovan, G. et al. The burden of mild cognitive impairment attributable to physical inactivity in Colombia. Eur. Rev. Aging Phys. Act.19, 28 (2022). 10.1186/s11556-022-00307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, L. et al. Social participation and mild cognitive impairment in low-and middle-income countries. Prev. Med.164, 107230 (2022). 10.1016/j.ypmed.2022.107230 [DOI] [PubMed] [Google Scholar]
- 25.García-Chanes, R. E., Gutiérrez-Robledo, L. M., Álvarez-Cisneros, T. & Roa-Rojas, P. Predictors of successful memory aging in older Mexican adults. Behav. Neurol.2022, 9045290 (2022). 10.1155/2022/9045290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa, A. R. et al. The paradoxical effect of living alone on cognitive reserve and mild cognitive impairment among women aged 60+ in Mexico City. Int. J. Environ. Res. Public Health18, 10939 (2021). [DOI] [PMC free article] [PubMed]
- 27.Jacob, L. et al. Sarcopenia and mild cognitive impairment in older adults from six low- and middle-income countries. J. Alzheimers Dis.82, 1745–1754 (2021). 10.3233/JAD-210321 [DOI] [PubMed] [Google Scholar]
- 28.Borda, M. G. et al. Body mass index, performance on activities of daily living and cognition: analysis in two different populations. BMC Geriatr.21, 177 (2021). 10.1186/s12877-021-02127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters, G. et al. Risk factors for incident dementia among older Cubans. Front. Public Health8, 481 (2020). 10.3389/fpubh.2020.00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochar-Soares, N. et al. Does undiagnosed diabetes mitigate the association between diabetes and cognitive impairment? Findings from the ELSI-Brazil study. J. Diabetes12, 834–843 (2020). 10.1111/1753-0407.13074 [DOI] [PubMed] [Google Scholar]
- 31.Solis-Urra, P. et al. The mediation effect of self-report physical activity patterns in the relationship between educational level and cognitive impairment in elderly: a cross-sectional analysis of Chilean Health National Survey 2016–2017. Int. J. Environ. Res. Public Health17, 2619 (2020). [DOI] [PMC free article] [PubMed]
- 32.Poblete-Valderrama, F. et al. [Physical activity and sedentary behaviours are associated with cognitive impairment in Chilean older adults]. Rev. Med. Chil.147, 1247–1255 (2019). 10.4067/s0034-98872019001001247 [DOI] [PubMed] [Google Scholar]
- 33.Santos, C. D. S. D., Bessa, T. A. D. & Xavier, A. J. Factors associated with dementia in elderly. Cien. Saude Colet.25, 603–611 (2020). 10.1590/1413-81232020252.02042018 [DOI] [PubMed] [Google Scholar]
- 34.Vancampfort, D. et al. Associations between handgrip strength and mild cognitive impairment in middle‐aged and older adults in six low‐and middle‐income countries. Int. J. Geriatr. Psychiatry34, 609–616 (2019). 10.1002/gps.5061 [DOI] [PubMed] [Google Scholar]
- 35.Bello-Chavolla, O. Y., Aguilar-Salinas, C. A. & Avila-Funes, J. A. Geriatric syndromes and not cardiovascular risk factors are associated with cognitive impairment among Mexican community-dwelling elderly with type 2 diabetes. Rev. Invest. Clin.69, 166–172 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Zamora-Macorra, M. et al. The association between social support and cognitive function in Mexican adults aged 50 and older. Arch. Gerontol. Geriatr.68, 113–118 (2017). 10.1016/j.archger.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 37.Silva, H. S. D. et al. Correlates of above-average cognitive performance among older adults: the SABE study. Cad. Saude Publica30, 1977–1986 (2014). 10.1590/0102-311X00131913 [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Sanguinetti, M. A. et al. [Factors associated with cognitive impairment in older adults in Chile]. Rev. Med. Chil.147, 1013–1023 (2019). 10.4067/S0034-98872019000801013 [DOI] [PubMed] [Google Scholar]
- 39.Aguilar-Navarro, S. G. et al. Association between ApoE ε4 carrier status and cardiovascular risk factors on mild cognitive impairment among Mexican older adults. Brain Sci.11, 68 (2021). 10.3390/brainsci11010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.León-Ortiz, P., Ruiz-Flores, M. L., Ramírez-Bermúdez, J. & Sosa-Ortíz, A. L. [Lifestyle and probability of dementia in the elderly]. Gac. Med. Mex.149, 36–45 (2013). [PubMed] [Google Scholar]
- 41.de León-Arcila, R., Milián-Suazo, F., Camacho-Calderón, N., Arévalo-Cedano, R. E. & Escartín-Chávez, M. [Risk factors for cognitive and functional impairment in the elderly]. Rev. Med. Inst. Mex. Seguro Soc.47, 277–284 (2009). [PubMed] [Google Scholar]
- 42.Mendes-Chiloff, C. L., Torres, A. R., Lima, M. C. P. & Ramos-Cerqueira, A. T. A. Prevalence and correlates of cognitive impairment among the elderly in a general hospital. Dement. Geriatr. Cogn. Disord.28, 427–433 (2009). 10.1159/000255512 [DOI] [PubMed] [Google Scholar]
- 43.Valle, E. A., Castro-Costa, E., Firmo, J. O. A., Uchoa, E. & Lima-Costa, M. F. Estudo de base populacional dos fatores associados ao desempenho no Mini Exame do Estado Mental entre idosos: Projeto Bambuí [A population-based study on factors associated with performance on the Mini-Mental State Examination in the elderly: the Bambuí Study]. Cad. Saude Publica25, 918–926 (2009). 10.1590/S0102-311X2009000400023 [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez, A. F. U., Linde, J. M. M., Barcoa, M. & Londoño, L. G. [The relationship between cognitive impairment and depression in older Colombian women]. Rev. Esp. Geriatr. Gerontol.43, 85–89 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Scazufca, M. et al. Risk factors across the life course and dementia in a Brazilian population: results from the Sao Paulo Ageing & Health Study (SPAH). Int. J. Epidemiol.37, 879–890 (2008). 10.1093/ije/dyn125 [DOI] [PubMed] [Google Scholar]
- 46.Matallana, D. et al. The relationship between education level and Mini-Mental State Examination domains among older Mexican Americans. J. Geriatr. Psychiatry Neurol.24, 9–18 (2011). 10.1177/0891988710373597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llibre, J. J. et al. [Dementia syndrome and risk factors in adults older than 60 years old residing in Habana]. Rev. Neurol.29, 908–911 (1999). [PubMed] [Google Scholar]
- 48.Passos, V. M. A., Raymundo, C. E., Bezerra, F. F. & Faerstein, E. Diabetes and hypertension are associated with lowered cognitive performance among middle-aged Brazilian adults: cross-sectional analyses nested in the longitudinal Pró-Saúde study. Sao Paulo Med. J.139, 46–52 (2021). 10.1590/1516-3180.2020.0269.r1.30102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Confortin, S. C. et al. Anthropometric indicators associated with dementia in the elderly from Florianópolis–SC, Brazil: EpiFloripa Ageing Study. Cien. Saude Colet.24, 2317–2324 (2019). 10.1590/1413-81232018246.20492017 [DOI] [PubMed] [Google Scholar]
- 50.Marquez, I. et al. Motoric cognitive risk syndrome: prevalence and cognitive performance. A cross-sectional study. Lancet Reg. Health Am.8, 100162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fallaci, I. V., Fabrício, D. M., Alexandre, T. D. S. & Chagas, M. H. N. Association between falls and cognitive performance among community-dwelling older people: a cross-sectional study. Sao Paulo Med. J.140, 422–429 (2022). 10.1590/1516-3180.2021.0180.r1.15092021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez, F., Osorio-García, D., Panesso, L. & Curcio, C. L. Healthy aging determinants and disability among older adults: SABE Colombia. Rev. Panam. Salud Publica45, e98 (2021). 10.26633/RPSP.2021.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parodi, J. F. & Runzer-Colmenares, F. M. [Impact of social support on limited mobility in older people in high Andean communities in Peru]. Rev. Panam. Salud Publica45, e88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brito, T. R. P., Nunes, D. P., Duarte, Y. A. O. & Lebrão, M. L. [Social network and older people’s functionality: Health, Well-being, and Aging (SABE) study evidences]. Rev. Bras. Epidemiol.21, e180003 (2019). 10.1590/1980-549720180003.supl.2 [DOI] [PubMed] [Google Scholar]
- 55.Mattos, I. E., do Carmo, C. N., Santiago, L. M. & Luz, L. L. Factors associated with functional incapacity in elders living in long stay institutions in Brazil: a cross-sectional study. BMC Geriatr.14, 47 (2014). 10.1186/1471-2318-14-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdés-Badilla, P. et al. Factors associated with poor health-related quality of life in physically active older people. Int. J. Environ. Res. Public Health19, 13799 (2022). [DOI] [PMC free article] [PubMed]
- 57.Castro, K. C. M. & Guerra, R. O. Impact of cognitive performance on the functional capacity of an elderly population in Natal, Brazil. Arq. Neuropsiquiatr.66, 809–813 (2008). 10.1590/S0004-282X2008000600006 [DOI] [PubMed] [Google Scholar]
- 58.Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ315, 629–634 (1997). 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R. & Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol.61, 991–996 (2008). 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 60.Simonsohn, U., Nelson, L. D. & Simmons, J. P. P-curve: a key to the file-drawer. J. Exp. Psychol.143, 534–547 (2014). 10.1037/a0033242 [DOI] [PubMed] [Google Scholar]
- 61.Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med.21, 1539–1558 (2002). 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 62.Olkin, I., Dahabreh, I. J. & Trikalinos, T. A. GOSH—a graphical display of study heterogeneity. Res. Synth. Methods.3, 214–223 (2012). 10.1002/jrsm.1053 [DOI] [PubMed] [Google Scholar]
- 63.Viechtbauer, W. & Cheung, M. W.-L. Outlier and influence diagnostics formeta-analysis. Res. Synth. Methods.1, 112–125 (2010). 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 64.Paule, R. C. & Mandel, J. Consensus values and weighting factors. J. Res. Natl Bur. Stand. (1977)87, 377–385 (1982). 10.6028/jres.087.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knapp, G. & Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med.22, 2693–2710 (2003). 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 66.Robledo, L. M. G., Cano-GutiéRrez, C. & Garcia, E. V. Healthcare for older people in Central and South America. Age Ageing51, afac017 (2022). 10.1093/ageing/afac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.da Silva Jr, J. B., Rowe, J. W. & Jauregui, J. R. Healthy aging in the Americas. Rev. Panam. Salud Publica45, e116 (2021). 10.26633/RPSP.2021.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin, S. A., Townend, F. J., Barkhof, F. & Cole, J. H. Interpretable machine learning for dementia: a systematic review. Alzheimers Dement.19, 2135–2149 (2023). 10.1002/alz.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leist, A. K. et al. Mapping of machine learning approaches for description, prediction, and causal inference in the social and health sciences. Sci. Adv.8, eabk1942 (2022). 10.1126/sciadv.abk1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maito, M. A. et al. Classification of Alzheimer’s disease and frontotemporal dementia using routine clinical and cognitive measures across multicentric underrepresented samples: a cross sectional observational study. Lancet Reg. Health Am.17, 100387 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bilal, U. et al. Life expectancy and mortality in 363 cities of Latin America. Nat. Med.27, 463–470 (2021). 10.1038/s41591-020-01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraído-Lanza, A. F., Mendoza-Grey, S. & Flórez, K. R. A commentary on the Latin American paradox. JAMA Network Open3, e1921165 (2020). 10.1001/jamanetworkopen.2019.21165 [DOI] [PubMed] [Google Scholar]
- 73.Kim, J. & Durden, E. Socioeconomic status and age trajectories of health. Soc. Sci. Med.65, 2489–2502 (2007). 10.1016/j.socscimed.2007.07.022 [DOI] [PubMed] [Google Scholar]
- 74.Sánchez-Meca, J., Marín-Martínez, F. & Chacón-Moscoso, S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol. Methods8, 448–467 (2003). 10.1037/1082-989X.8.4.448 [DOI] [PubMed] [Google Scholar]
- 75.Selya, A. S., Rose, J. S., Dierker, L. C., Hedeker, D. & Mermelstein, R. J. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Front. Psychol.3, 111 (2012). 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrer, M., Cuijpers, P., Furukawa, T. & Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide (Chapman and Hall/CRC, 2021).
- 77.Samtani, S. et al. Associations between social connections and cognition: a global collaborative individual participant data meta-analysis. Lancet Healthy Longev.3, e740–e753 (2022). 10.1016/S2666-7568(22)00199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wallace, E. R., Segerstrom, S. C., van Horne, C. G., Schmitt, F. A. & Koehl, L. M. Meta-analysis of cognition in Parkinson’s disease mild cognitive impairment and dementia progression. Neuropsychol. Rev.32, 149–160 (2021). [DOI] [PubMed]
- 79.Sulaiman, S. K., Musa, M. S., Tsiga-Ahmed, F. I. I., Sulaiman, A. K. & Bako, A. T. A systematic review and meta-analysis of the global prevalence and determinants of COVID-19 vaccine acceptance and uptake in people living with HIV. Nat. Hum. Behav.8, 100–114 (2023). 10.1038/s41562-023-01733-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–3. Table 1. Main results of studies assessing risk factors of functional ability and cognition. Table 2. Measures used to assess cognition and functionality across studies. Table 3. Measures used to track social determinants of health and socioeconomic disparities across studies.
Data Availability Statement
Data included in this study are available on GitHub at https://github.com/AI-BrainLat-team/Latam-Aging-Meta-Analysis/tree/main/Data. Data from studies analyzed in the meta-analysis and scoping review are summarized in the Results section. Furthermore, all data are reported in Supplementary Table 1 and Supplementary File 1.
All code for data analysis associated with this manuscript is available for download on GitHub at https://github.com/AI-BrainLat-team/Latam-Aging-Meta-Analysis.