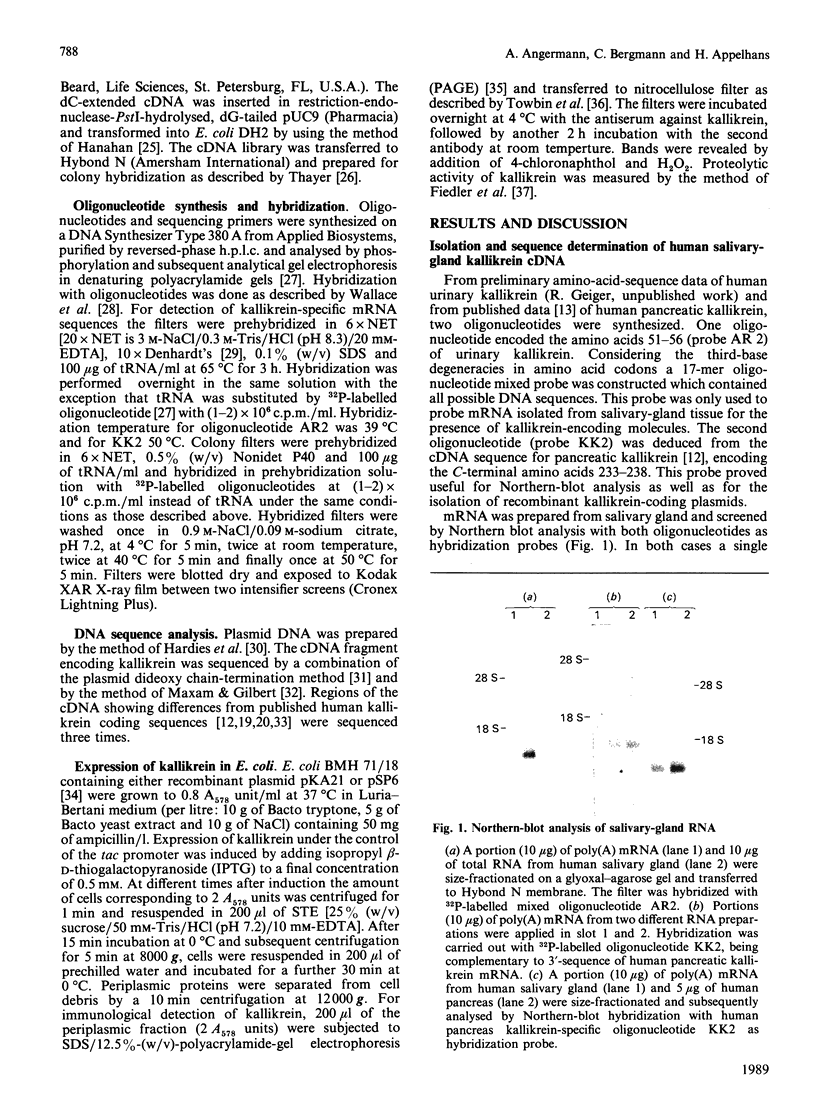
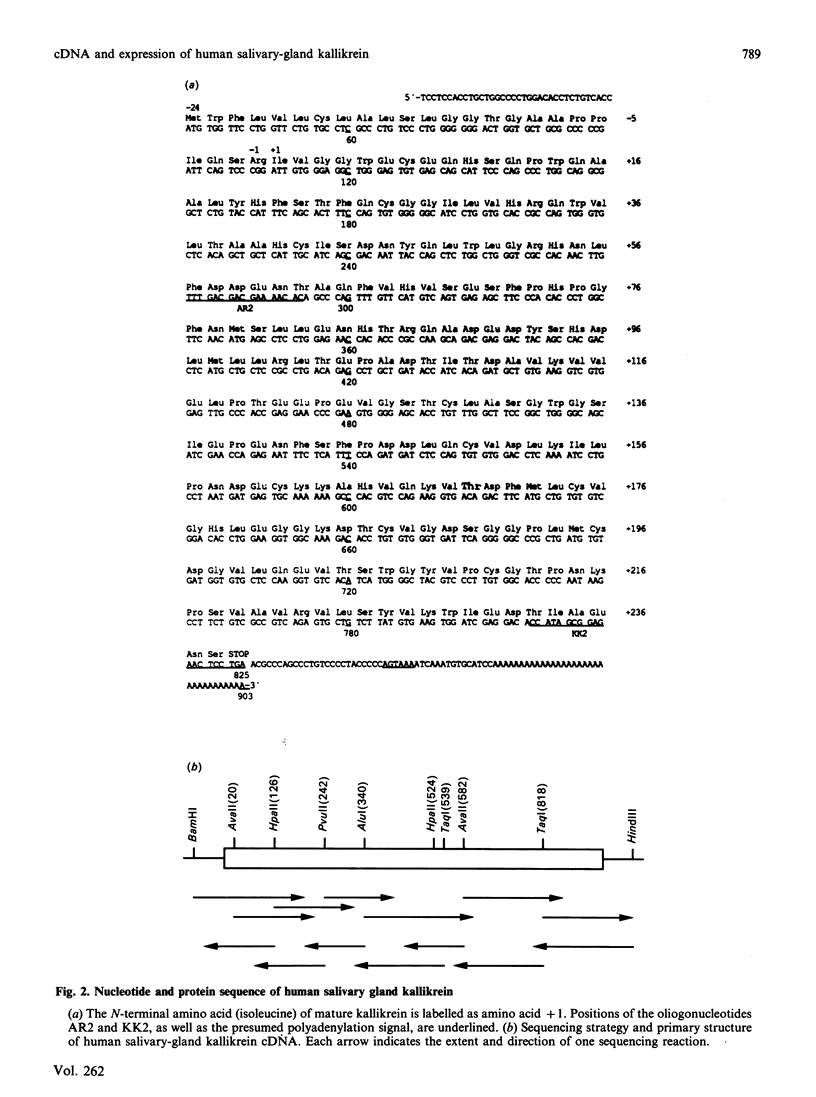
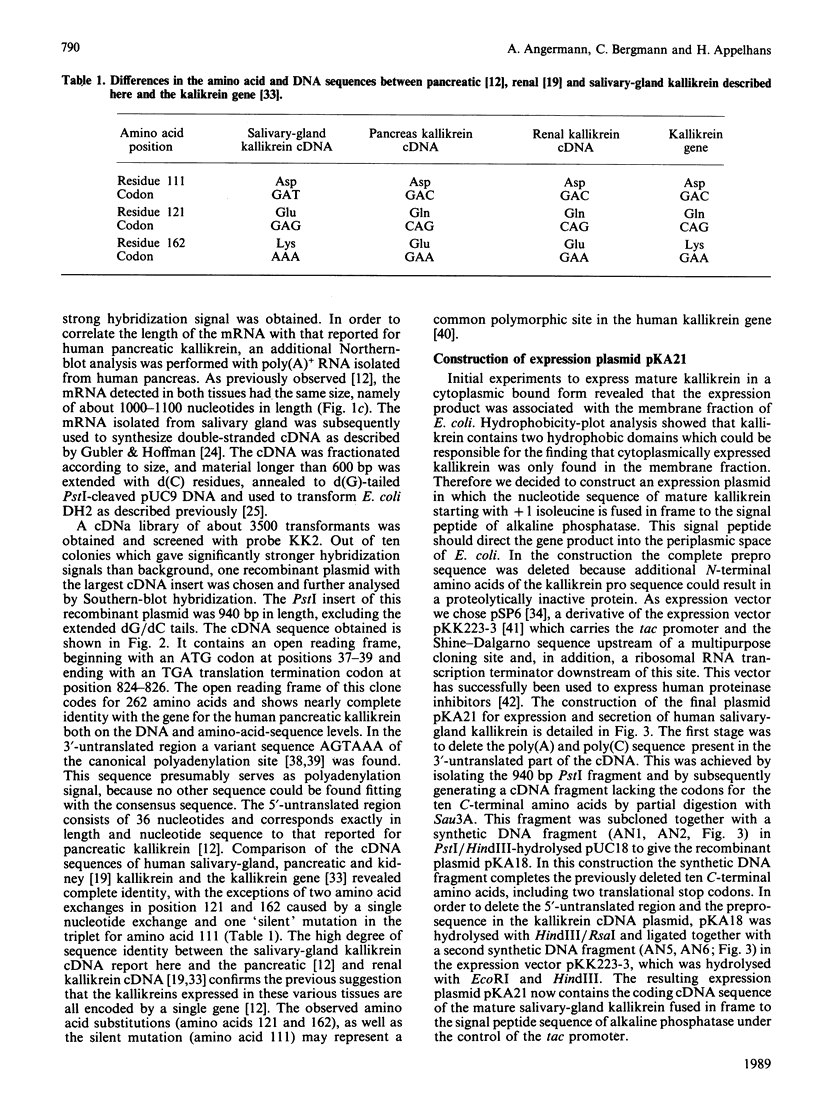
Abstract
CDNA clones for human kallikrein have been identified in a cDNA library constructed from mRNA of human salivary gland. The entire coding sequence for preprokallikrein and for the 5'- and 3'-untranslated regions were isolated by using a mixture of oligonucleotides corresponding to amino acids 51-56 of human urinary kallikrein and one oligonucleotide corresponding to amino acids 233-238 of human pancreatic kallikrein. The DNA sequence proved that, with the exception of two amino acid exchanges, kallikrein of the human salivary gland is identical with pancreatic kallikrein. Salivary gland and renal kallikrein was expressed in Escherichia coli from plasmid pKK223-3 under the control of the tac promoter. The protein was identified by Western-blot analysis and by demonstration of its specific proteolytic activity.
Full text
PDF
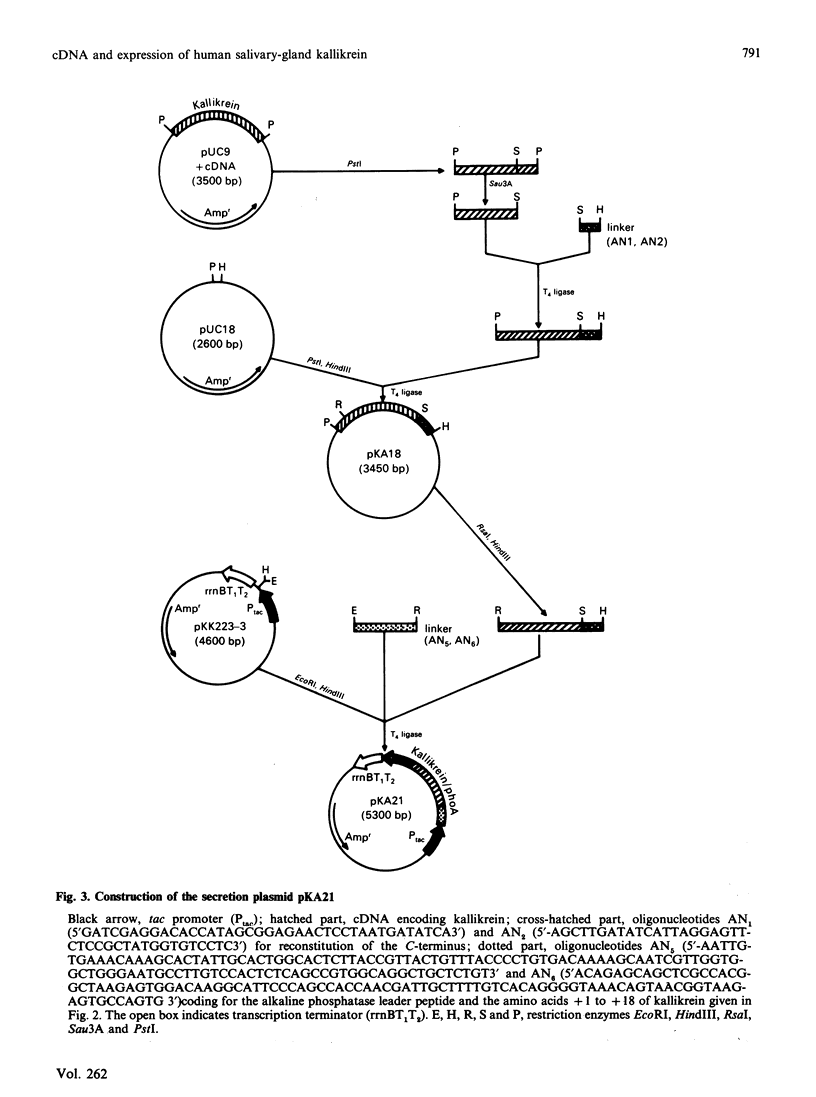
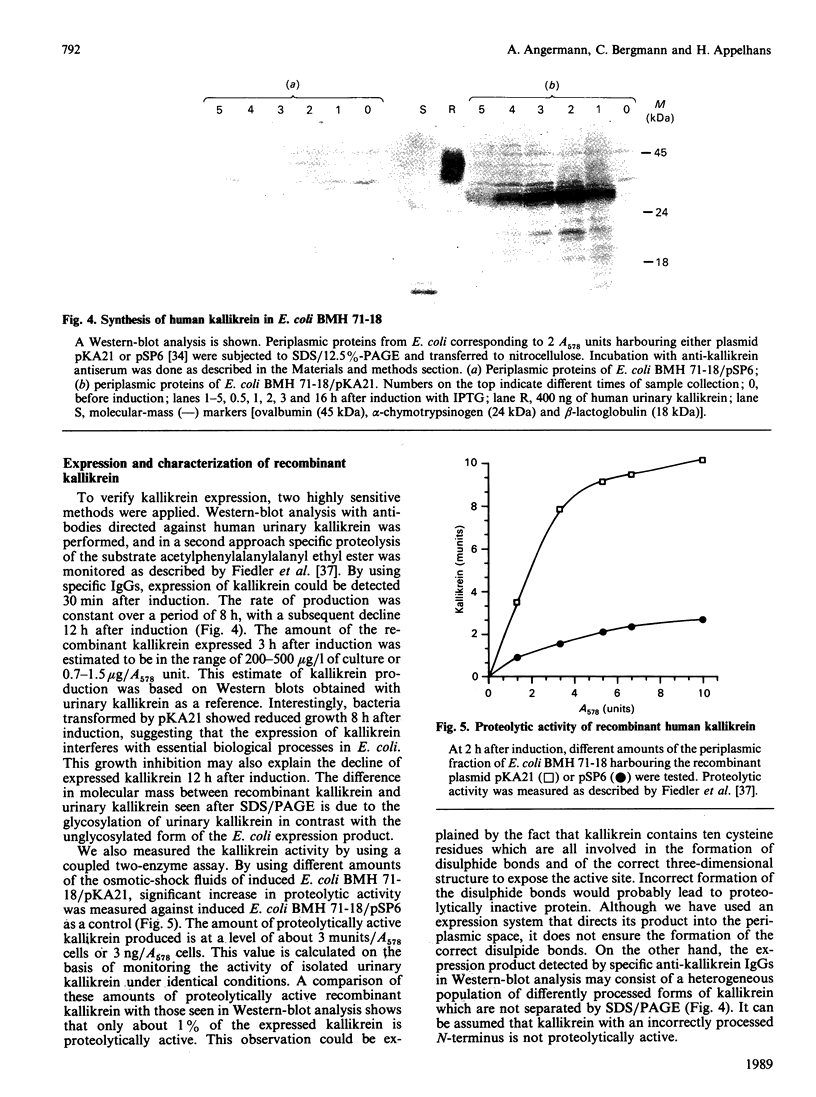

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. R., Shine J. Human kidney kallikrein: cDNA cloning and sequence analysis. DNA. 1985 Dec;4(6):445–450. doi: 10.1089/dna.1985.4.445. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J., Chao L., Margolius H. S. Isolation of tissue kallikrein in rat spleen by monoclonal antibody-affinity chromatography. Biochim Biophys Acta. 1984 Sep 28;801(2):244–249. doi: 10.1016/0304-4165(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Chao J., Woodley C., Chao L., Margolius H. S. Identification of tissue kallikrein in brain and in the cell-free translation product encoded by brain mRNA. J Biol Chem. 1983 Dec 25;258(24):15173–15178. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dodt J., Schmitz T., Schäfer T., Bergmann C. Expression, secretion and processing of hirudin in E. coli using the alkaline phosphatase signal sequence. FEBS Lett. 1986 Jul 7;202(2):373–377. doi: 10.1016/0014-5793(86)80721-9. [DOI] [PubMed] [Google Scholar]
- Ekfors T. O., Hopsu-Havu V. K. Immunofluorescent localization of trypsin-like esteropeptidases in the mouse submandibular gland. Histochem J. 1971 Nov;3(6):415–420. doi: 10.1007/BF01014779. [DOI] [PubMed] [Google Scholar]
- Evans B. A., Drinkwater C. C., Richards R. I. Mouse glandular kallikrein genes. Structure and partial sequence analysis of the kallikrein gene locus. J Biol Chem. 1987 Jun 15;262(17):8027–8034. [PubMed] [Google Scholar]
- Evans B. A., Yun Z. X., Close J. A., Tregear G. W., Kitamura N., Nakanishi S., Callen D. F., Baker E., Hyland V. J., Sutherland G. R. Structure and chromosomal localization of the human renal kallikrein gene. Biochemistry. 1988 May 3;27(9):3124–3129. doi: 10.1021/bi00409a003. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Geiger R., Hirschauer C., Leysath G. Peptide esters and nitroanilides as substrates for the assay of human urinary kallikrein. Hoppe Seylers Z Physiol Chem. 1978 Dec;359(12):1667–1673. doi: 10.1515/bchm2.1978.359.2.1667. [DOI] [PubMed] [Google Scholar]
- Frey P., Forand R., Maciag T., Shooter E. M. The biosynthetic precursor of epidermal growth factor and the mechanism of its processing. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6294–6298. doi: 10.1073/pnas.76.12.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima D., Kitamura N., Nakanishi S. Nucleotide sequence of cloned cDNA for human pancreatic kallikrein. Biochemistry. 1985 Dec 31;24(27):8037–8043. doi: 10.1021/bi00348a030. [DOI] [PubMed] [Google Scholar]
- Gerald W. L., Chao J., Chao L. Immunological identification of rat tissue kallikrein cDNA and characterization of the kallikrein gene family. Biochim Biophys Acta. 1986 Feb 24;866(1):1–14. doi: 10.1016/0167-4781(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Howles P. N., Dickinson D. P., DiCaprio L. L., Woodworth-Gutai M., Gross K. W. Use of a cDNA recombinant for the gamma-subunit of mouse nerve growth factor to localize members of this multigene family near the TAM-1 locus on chromosome 7. Nucleic Acids Res. 1984 Mar 26;12(6):2791–2805. doi: 10.1093/nar/12.6.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lottspeich F., Geiger R., Henschen A., Kutzbach C. N-Terminal amino acid sequence of human urinary kallikrein homology with other serine proteases. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1947–1950. [PubMed] [Google Scholar]
- Lundwall A., Lilja H. Molecular cloning of human prostate specific antigen cDNA. FEBS Lett. 1987 Apr 20;214(2):317–322. doi: 10.1016/0014-5793(87)80078-9. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Margolius H. S., Erdös E. G. Molecular biology of tissue kallikrein. Biochem J. 1988 Jul 15;253(2):313–321. doi: 10.1042/bj2530313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nolly H., Scicli A. G., Scicli G., Carretero O. A. Characterization of a kininogenase from rat vascular tissue resembling tissue kallikrein. Circ Res. 1985 Jun;56(6):816–821. doi: 10.1161/01.res.56.6.816. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message and beyond. Nature. 1984 Feb 2;307(5950):412–413. doi: 10.1038/307412a0. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Schedlich L. J., Bennetts B. H., Morris B. J. Primary structure of a human glandular kallikrein gene. DNA. 1987 Oct;6(5):429–437. doi: 10.1089/dna.1987.6.429. [DOI] [PubMed] [Google Scholar]
- Schill W. B., Preissler G., Dittmann B., Muller W. P. Effect of pancreatic kallikrein, sperm acrosin and high molecular weight (HMW) kininogen on cervical mucus penetration ability of seminal plasma-free human spermatozoa. Adv Exp Med Biol. 1979;120B:305–310. [PubMed] [Google Scholar]
- Strauss M., Bartsch F. O., Stollwerk J., Trstenjak M., Böhning A., Gassen H. G., Machleidt W., Turk V. Chemical synthesis of a gene for human cystatin C and its expression in E. coli. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):209–218. [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt K. W., Lee P. J., M'Timkulu T., Chan W. P., Loor R. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A. 1986 May;83(10):3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklmayr M., Dietze G., Mayer L., Böttger I., Grunst J. Evidence for an involvement of kinin liberation in the priming action of insulin on glucose uptake into skeletal muscle. FEBS Lett. 1979 Feb 1;98(1):61–65. doi: 10.1016/0014-5793(79)80152-0. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]