Abstract
Atherosclerotic cardiovascular disease (ASCVD) remains a leading cause of morbidity and mortality despite effective low-density lipoprotein cholesterol-targeted therapies. This review explores the crucial role of inflammation in the residual risk of ASCVD, emphasizing its impact on atherosclerosis progression and plaque stability. Evidence suggests that high-sensitivity C-reactive protein (hsCRP), and potentially other inflammatory biomarkers, can be used to identify the inflammatory residual ASCVD risk phenotype and may serve as future targets for the development of more efficacious therapeutic approaches. We review the biological basis for the association of inflammation with ASCVD, propose new therapeutic strategies for the use of inflammation-targeted treatments, and discuss current challenges in the implementation of this new treatment paradigm for ASCVD.
Keywords: Inflammation, Atherosclerotic cardiovascular disease, NLRP3 inflammasome, Interleukins, LDL-C, Triglyceride-rich lipoproteins, Atherosclerosis, Canakinumab, Colchicine, Ziltivekimab
Key Summary Points
| Atherosclerotic cardiovascular events continue to occur despite maximal lipid-lowering therapy (LLT). |
| High-sensitivity C-reactive protein (hsCRP) has been shown to predict atherosclerotic cardiovascular disease (ASCVD) risk. |
| hsCRP remains a useful tool to identify an inflammatory residual risk phenotype and may be used to tailor therapy in patients with low-grade chronic inflammation. |
| To date, repurposed drugs such as colchicine and canakinumab have been shown to significantly reduce the relative risk of major cardiovascular events. |
| Future trials may indicate that drugs that target interleukin-6, such as ziltivekimab and tocilizumab, could be useful therapies for reducing ASCVD risk. |
| In the future, combined LLT and anti-inflammatory targeted drug therapies may be used to reduce ASCVD. |
Introduction
It is now well understood that cardiovascular events can still repeatedly occur in high-risk and even low-risk patients despite achieving effective lowering of low-density lipoprotein cholesterol (LDL-C) using statins and other drugs [1]. This residual risk of atherosclerotic cardiovascular disease (ASCVD) can be affected by a wide variety of pathophysiological processes, but inflammation is likely to play a key role.
Uncontrolled persistent inflammation underlies all phases of atherogenesis. Early stages of atherosclerosis are marked by endothelial dysfunction and increased vascular permeability, which facilitate infiltration of plasma LDL and triglyceride-rich lipoprotein (TRL) remnants into the subendothelial intimal matrix. LDL and TRL particles in the intimal layer are exposed to oxidative or other types of conformational modifications that may increase their atherogenic potential. These modified lipoproteins initiate subsequent stages of atherosclerosis, including triggering macrophages and other cells to express adhesion molecules and to secrete inflammatory cytokines. The balance between inflammatory and anti-inflammatory activity, in part, modulates the progression of atherosclerosis. Numerous studies suggest that the inflammatory burden is a key factor in plaque stability [2]. Indeed, stable plaques are characterized by a chronic inflammatory infiltrate [3]. Therefore, early detection of atherosclerosis using inflammatory biomarkers could play an important role in treatment decision-making.
Traditional biomarkers for ASCVD include LDL-C, TRL, lipoprotein(a), and glucose. However, approximately 20% of all cases of myocardial infarction (MI) and stroke occur in patients with no evident common risk factors [4]. Hence, there is great interest in identifying additional pathophysiological biomarkers of inflammation as well as an inflammatory coronary plaque imaging phenotype. The clinical utility of these other biomarkers and the need for their further clinical evaluation will be highlighted in the current review. We will also discuss new lipid-lowering therapies as well as alternative therapeutic approaches that predominantly target inflammation underlying cardiovascular disease (CVD).
Cardiovascular Residual Risk Biomarkers
High-Sensitivity C-Reactive Protein (hsCRP)
The majority of studies have focused on well-established hsCRP as an ASCVD inflammation marker. The current US 2018 multi-society guidelines in ASCVD risk assessment use hsCRP as a risk enhancer [5]. For example, patients with an intermediate 10-year risk score (7.5–20%) and hsCRP > 2 mg/L are considered to be at increased risk. This recommendation comes from several primary prevention trials, including the Physicians Health Study [6], where the highest quartile of hsCRP was associated with a 2.9-fold greater risk for MI and 1.9-fold greater risk for stroke (p < 0.001, p = 0.02, respectively). Subsequently, the Emerging Risk Factors Collaboration [7] meta-analysis of 54 prospective cohorts of primary prevention showed that log-transformed hsCRP was associated with 1.23-fold increased risk for coronary heart disease. Similar outcomes for ischemic stroke and vascular-associated risk were found (relative risk [RR] of 1.32; 95% confidence interval [CI], 1.18–1.49 and 1.34; 95% CI 1.20–1.50). These associations were further strengthened by a meta-analysis of > 160,000 individuals in a primary prevention study that identified an adjusted RR for coronary heart disease of 1.37 (95% CI 1.27–1.48), which is comparable to traditional risk factors, such as non-high-density lipoprotein cholesterol (HDL-C) and systolic blood pressure. These associations were also strongly predictive of ischemic stroke (RR 1.27, 95% CI 1.15–1.40) and ASCVD mortality (RR 1.55, 95% CI 1.37–1.76), even after adjustment for traditional risk factors [8]. These findings demonstrate that hsCRP, as a surrogate marker of the interleukin (IL)-1β-to-IL-6-to-hsCRP pathway, is a reliable and important risk marker, but not a risk factor, for ischemic heart disease.
Triglyceride-Rich Lipoproteins
For decades, triglycerides (TGs) have been considered a risk factor for ASCVD, but the relationship between TG and ASCVD events remains controversial. Two key questions arise: Is it the TG itself or the lipoprotein which transports it (TRL or TRL remnants) that is the causal factor? Secondly, is this risk association with TRL due to its cholesterol or its TG content, or both? To date, epidemiological and Mendelian randomization studies have demonstrated a causal relationship of TRL to ASCVD risk [9, 10].
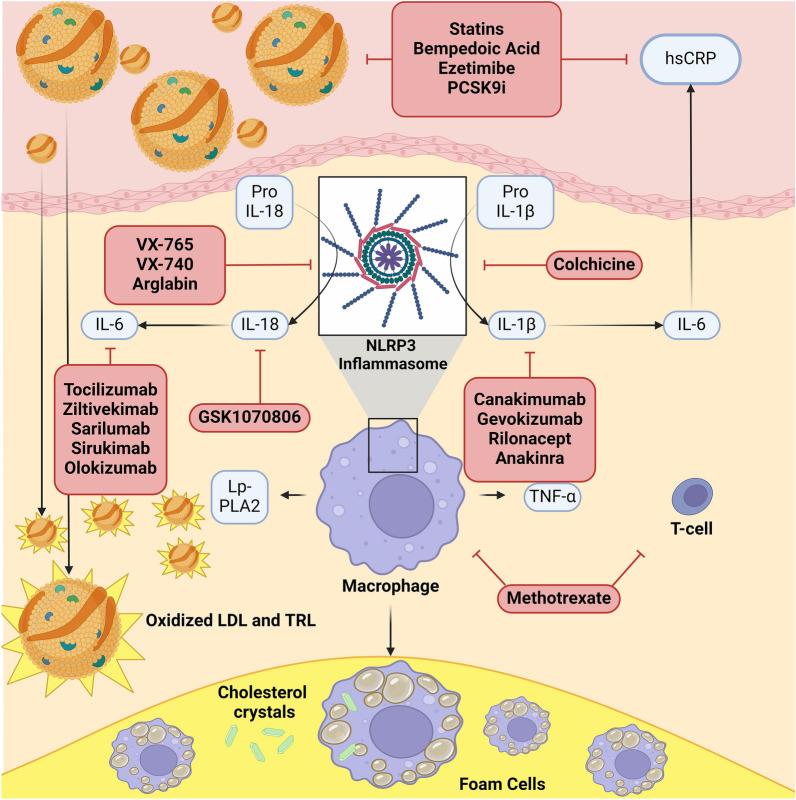
TRLs are considered to be more atherogenic per particle than LDL [11]. Due to their size (< 70 nm), they can only be transported through the artery wall in one direction (Fig. 1) [12]. TRL biochemical composition is also critical for arterial retention and subsequent events, since larger TRL particles can contain up to four times the cholesterol content per particle as LDL particles, even though TGs are their main component [13]. Additionally, TRLs are enriched in apoC-III and apoE, which are both implicated in TRL binding and retention in the arterial wall. TRL uptake by macrophages activates the NLRP3 inflammasome [14–16]. Recent genetic research supports the causality of TRLs in cardiovascular (CV) risk, implicating various genes related to TG metabolism such as APOA5, APOC3, ANGPTL3, ANGPTL4, and lipoprotein lipase (LPL) [17]. Additional studies have shown that an increase in TG levels is accompanied by increased levels of hsCRP [18].
Fig. 1.
Inflammatory signaling pathways and targeted therapeutic interventions. LDL low-density lipoprotein, TRL triglyceride-rich lipoprotein, hsCRP high-sensitivity C-reactive protein, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor, BA bempedoic acid, IL-6 interleukin 6, IL-1β interleukin 1 beta, IL-18 interleukin-18, TNF-α tumor necrosis factor alpha, NLRP3 nucleotide-binding oligomerization domain-like receptor 3, Lp-PLA2 lipoprotein phospholipase A2. This original figure was created using BioRender
The uptake of unmodified TRL in the arterial wall can induce macrophage foam cell formation [19]. Free fatty acids and other bioactive lysolipids generated during TRL-TG lipolysis can exert proinflammatory effects in macrophages and smooth muscle (SM) cells [20]. Lipid-laden foam cells and SM cells in lesions are abundant in LPL and promote endothelial dysfunction [21]. TG-laden macrophages also stimulate proinflammatory cytokine release via the polarization of macrophages to the M1 phenotype, thereby increasing the inflammatory burden of arterial lesions [22]. Cholesterol crystal formation may also occur upon very-low-density lipoprotein (VLDL) or remnant uptake by macrophages, with activation of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome and an inflammatory response [16, 23, 24].
Lipoprotein(a)
Similar to LDL, lipoprotein(a) [Lp(a)] is an apoB-containing lipoprotein with apolipoprotein(a) [apo(a)] covalently bound. Lp(a) has been identified as a causal factor for coronary heart disease based on epidemiological and genetic studies [25–27]. Although the circulating levels of Lp(a) are much lower than other lipoproteins, it has been shown that the per-particle ASCVD risk of Lp(a) is six times that of LDL (point estimate of 6.6; 95% CI 5.1–8.8) [28]. It is estimated that up to 1% of the population has extreme levels of Lp(a) above 430 nmol/L (180 mg/dL), which is associated with a more than threefold increased risk of ASCVD—the same lifetime risk for ASCVD as untreated heterozygous familial hypercholesterolemia [26].
The relationship between Lp(a) and inflammation is complex. The presence of oxidized phospholipids (OxPLs) in Lp(a) increases arterial wall inflammation (and also initiates aortic valve calcification) by activating monocytes and endothelial cells. Furthermore, Lp(a) has a direct link to IL-6, since the LPA gene contains a class II IL-6 response element (CTGGGA) which upregulates apo(a) expression [29]. In one study, serological testing of 1153 patients revealed that Lp(a) levels were higher in patients with elevated IL-6 (> 6 pg/mL) [30]. These authors also found that LPA gene expression in the liver was influenced by IL-6 response genes. In other in vitro experiments, human peripheral blood monocytes stimulated with Lp(a) had significantly increased IL-6 secretion [31]. This is supported by the finding that IL-6 SNP–174G/C was associated with increased odds of having elevated Lp(a) [32]. This is also true for IL-1, as individuals with a proinflammatory interleukin-1 genotype have higher CV risk associated with Lp(a) [33, 34].
Several clinical studies have reported that hsCRP also affects Lp(a) levels. A large study conducted in a Danish population showed that Lp(a) levels increased with increasing CRP levels (e.g., median Lp(a) was 18.0 mg/dL with CRP < 1 mg/L and 21.1 mg/dL with CRP > 10 mg/L) [35]. This is also supported by the ACCELERATE and MESA trials, where the former showed that when hsCRP was above 2 mg/dL, each unit increase in log of Lp(a) was associated with 13% greater risk of CV death, nonfatal MI, or stroke [36], with the latter study also showing that only individuals with hsCRP > 2 mg/dL and Lp(a) > 50 mg/dL demonstrated a risk associated with ASCVD. This association was not shown in an analysis of the JUPITER trial, where Lp(a) was a significant determinant of residual risk in both treatment groups where LDL-C < 130 mg/dL and hsCRP was either less than or greater than 2 mg/dL. Interestingly, in this study, when patients were stratified by the median of CRP values (4 mg/L), a significant association was observed between Lp(a) levels and the incident primary endpoint in the group with hsCRP below the median, but not in the group with hsCRP above the median [37]. Conversely, it has also been shown that rheumatoid arthritis (RA) treatment with IL-6 inhibitors (tocilizumab) results in a 50% reduction in Lp(a) [37]. Overall, treatments that block IL-6 reduced Lp(a) by 16–41% [38]. However, more recent data from the Copenhagen General Population study showed that high levels of Lp(a) were associated with ASCVD and aortic valve stenosis irrespective of whether hsCRP levels were above or below 2 mg/L. Taken together, these studies indicate that hsCRP has limited ability for identifying a higher CV risk [39].
Interleukin-1 (IL-1)
The IL-1 family is composed of 11 proteins which include IL-1α, IL-1β, IL-1Ra, IL-18, IL-33, IL-36α, IL-36β, IL-36γ, IL-36Ra IL-37, and IL-38. Both IL-1α and IL-1β bind to the IL-1 receptor and are the most potent proinflammatory cytokines implicated in the development of chronic inflammatory diseases such as RA. However, they have also been described to play a role in other diseases, such as type 2 diabetes (T2D). IL-1 signaling also leads to upregulation of other inflammatory markers, including IL-6, hsCRP, fibrinogen, and plasminogen activator inhibitor [40].
There is strong evidence to support the involvement of IL-1 in SM cell mitogenesis and extracellular matrix metabolism. This mechanism has been corroborated by studies of gene polymorphism of IL-1 receptor agonists associated with lower incidence of stent restenosis [41]. Moreover, extended direct exposure of IL-1β in porcine coronary arteries was found to induce intimal thickening and vasospasm, which was attenuated by an IL-1β-neutralizing antibody [42].
Interleukin 18 (IL-18) is a proinflammatory cytokine that has been implicated in several disease states. Canonical NLRP3 inflammasome activation leads to IL-1β and pro-IL-18 cleavage [43]. In animal models of acute MI, pressure overload, and lipopolysaccharide (LPS)-induced dysfunction, IL-18 regulates cardiomyocyte hypertrophy and induces cardiac contractile dysfunction and extracellular matrix remodeling. In humans, high IL-18 levels correlate with increased risk of developing ASCVD [44].
Interleukin-6
Interleukin-6 (IL-6) is a type 2 cytokine (i.e., activates humoral immune response) that generates diffusible molecules with autocrine, paracrine, or endocrine functions. IL-6 and IL-1 receptors trigger downstream transduction pathways, such as JAK/STAT, mitogen-activated protein (MAP) kinases, and Pi3k-Akt. IL-6 originates from different sources in the human body, but is mainly produced by macrophages, T helper cells, B cells, vascular endothelial cells, SM cells, and fibroblasts [45]. Mendelian randomization studies have demonstrated a link between genetic variation associated with plasma IL-6 levels and coronary events, suggesting a causal relationship for the IL-6 pathway [45].
IL-6 was found to be strongly associated with incident MI in the Physicians Health Study. Those in the highest quartile had a 2.3-fold greater risk of MI than those in the lower quartile (p = 0.03). This was also explored in the Women’s Health study, which found the same risk association, where the highest quartiles of IL-6 had 2.2-fold greater risk of CV events than the lowest quartile [46]. Furthermore, a meta-analysis of 29 trials found that for each standard deviation increase in IL-6, there was a 25% increase in nonfatal MI or coronary heart disease death [47].
Despite its association with ASCVD, however, there have not been a sufficient number of studies to confirm IL-6 as a risk enhancer, most likely due to the lack of access to reliable IL-6 assays.
Lp-PLA2
Lipoprotein-associated phospholipase-A2 (Lp-PLA2), a plasma biomarker of inflammation, is primarily produced by macrophages and macrophage-derived foam cells within atherosclerotic plaques. This enzyme plays a key role in the breakdown of oxidized phospholipids, releasing pro-atherogenic and proinflammatory oxidized fatty acids and lysophosphatidylcholine, thereby inducing oxidative stress and promoting atherosclerotic plaque progression [48]. Lp-PLA2 is primarily associated with LDL, and studies have shown that higher plasma levels are independently associated with the risk of developing coronary artery disease (CAD) events, regardless of non-HDL-C levels. Despite promising results in reducing plaque volume using darapladib, a selective Lp-PLA2 inhibitor, the Integrated Biomarker and Imaging Study 2 failed to demonstrate clear CV benefits [49]. As a result, the practical utility of measuring Lp-LPA2 remains uncertain in the absence of an effective targeted therapeutic with proven ASCVD benefits.
NLRP3 Inflammasome
The inflammasome is a macromolecular complex that is triggered by injury which amplifies the inflammatory response [50]. The pyrin-containing NLRP is a family of intracellular receptors that sense patterns of pathogens of injury. The NLRP3 inflammasome is the most extensively analyzed and has been shown to play a role in multiple autoimmune diseases. Its activation leads to production of caspases 1, 4, 5, and 11, and promotes the maturation and secretion of proinflammatory cytokines such as IL-1β and IL-18. This complex biochemical cascade activation culminates in cell death, called “pyroptosis” [51].
Atherosclerotic lesions contain deposits of cholesterol crystals in the necrotic core that induce inflammation by stimulation of the NLRP3 inflammasome. It was found that in ApoE-deficient mice fed a high-cholesterol diet, the formation of cholesterol crystals induced acute inflammation, whereas in mouse models deficient in NLRP3 inflammasome components, no inflammation was observed [16]. Moreover, deletion of the Nlrp3, Asc, and Il-1 alpha (IL1A) and beta (IL1B) genes in mice with hypercholesterolemia prevented the development of atherosclerosis [16, 52, 53]. An association between the NLRP3 inflammasome and other CVDs such as myocarditis, sarcoidosis, septic cardiomyopathy, chronic non-ischemic cardiomyopathy, and pericarditis has also been described [54].
A number of potential surrogate markers for inflammation in ASCVD currently under investigation, including fibrinogen, uric acid, midkine, pentraxins, cystatin C, microRNA (miRNA), and matrix metalloproteinases (MMPs), are beyond the scope of this review, and have been described in other recent reviews [55].
Imaging and Inflammation
Recent advances in imaging modalities, in particular cardiac computed tomography angiography (CCTA), have made this visualization technique a sensitive tool for monitoring vascular inflammation and atherosclerotic coronary plaque characterization. During atherosclerotic plaque buildup, the arterial wall microenvironment includes metabolically active cells which release proinflammatory mediators. The latter induce alterations in both luminal and perivascular morphological adipose tissue. This complex inter-tissue relationship can be evaluated by CCTA as perivascular adipose tissue (PVAT) attenuation, also known as the perivascular fat attenuation index (FAI) [56]. FAI around the proximal segment of the left anterior descending (LAD) artery or right coronary artery (RCA) was a strong predictor of all-cause and cardiac mortality in the CRISP-CT study, involving 4000 individuals undergoing diagnostic CCTA [57]. The PVAT concept has been effectively applied in a recently developed algorithm, namely CaRi-HEART (Caristo Diagnostics, Oxford, UK), that enables a quantitative analysis of vascular inflammation [58]. Moreover, FAI is differentially associated with the coronary plaque phenotype, which can be determined by CCTA. Indeed, detecting high-risk or vulnerable plaque features not only improves clinical decision-making but also aids in validating novel biomarkers and CAD drug development [59]. It has been shown that other sources of fat depots besides perivascular fat might contribute to cardio-metabolic disease progression [60]. For example, a CCTA-based approach for assessing epicardial and thoracic (pericardial) adipose tissue was found to be longitudinally associated with coronary and cardiac characteristics in psoriasis [60]. Moreover, by applying the same imaging modality for detecting visceral adipose tissue (VAT), it was revealed that a reduction in psoriatic inflammation was associated with lower VAT and improved longitudinal coronary plaque burden [61].
Although CCTA-derived imaging markers have proved its sensitivity for ASCVD risk prediction, a spatial resolution similar to that of invasive coronary angiography or intravascular ultrasound is still a matter of concern. As an alternative imaging technique, positron emission tomography/computed tomography (PET/CT) has been used less extensively due to its high cost and limited availability [62]. One of the most commonly utilized tracers for assessing inflammation is 18F-fluorodeoxyglucose (FDG) [63]. In atherosclerotic plaque, macrophages have higher metabolic activity due to hypoxia-induced anaerobic glycolysis as compared to the residing cell milieu. Thus, levels of FDG uptake by plaque macrophages represent its activity related to the degree of inflammation, whereas 18F-FDG uptake by other metabolically active organs, such as bone marrow, spleen, and liver, was associated with systemic inflammation and subclinical atherosclerosis in patients with psoriasis and RA. Despite its diagnostic utility, 18F-FDG PET-CT lacks cell specificity and needs to be further technically advanced for wider routine use [64].
Lipid-Lowering Therapies and Inflammation
Statins, Ezetimibe, and PCSK9 Inhibitors
Several clinical trials have shown the potential for statins to reduce markers of inflammation. For example, in the PRINCE study [65] and in a subset of patients from the CARE study, it was found that treatment with pravastatin 40 mg daily reduced hsCRP levels longitudinally by 16.9% and 21.6%, respectively [66].
Statin therapy intensity has also been shown to play a role in the hsCRP reduction. For example, in the JUPITER trial, patients on 20 mg of rosuvastatin lowered their levels of hsCRP by 37% [67]. The magnitude of reduction in hsCRP achieved in the treatment group of the JUPITER trial was proportional to the reduction in CV risk. Furthermore, those that achieved hsCRP levels of < 2 mg/L had a 55% reduction in the primary endpoint compared with those that did not [67]. In line with these findings, treatment with atorvastatin 80 mg was shown to achieve hsCRP of < 2 mg/L in 57.5% of patients in the PROVE-IT TIMI 22 study [68]. It is also important to note that those patients who achieved hsCRP levels of < 2 mg/L had fewer CV events independently of LDL-C.
There are limited data in the assessment of inflammation markers with ezetimibe or PCSK9 inhibitor treatment. For example, in the IMPROVE-IT study, a 14% reduction in hsCRP was demonstrated in the combined simvastatin and ezetimibe treatment group [69]. In this study, patients treated with a statin alone or in combination with ezetimibe who achieved both LDL-C < 70 mg/dL and hsCRP < 2 mg/L had better CV outcomes (CV death, major coronary event, or stroke) than patients with higher LDL-C and hsCRP levels (adjusted hazard ratio [HR] of 0.73 [95% CI 0.66–0.81]) [70]. Despite the considerable reduction in LDL-C, PCSK9 inhibitor trials have provided scant evidence for an improvement in hsCRP. In the FOURIER trial, only 30% of patients had hsCRP < 3 mg/L, and in the follow-up period, the median reduction in hsCRP was 0.2 mg/L [71]. Despite this nonsignificant improvement in hsCRP levels, however, the absolute relative risk reduction (RRR) in those with high levels of hsCRP was 2.6% for the primary endpoint.
PCSK9i also lowers Lp(a) to some extent. Several studies have shown that Lp(a) can be lowered by 24–29% with the PCSK9 inhibitor evolocumab [72, 73]. However, a study by Stiekema et al. found that while evolocumab effectively lowered LDL-C levels and achieved a slight decrease in Lp(a) levels, there was no significant change in arterial wall inflammation among patients who underwent 18F-FDG PET/CT to assess carotid or thoracic aortic artery wall inflammation before and after treatment with evolocumab [74].
Bempedoic Acid
Bempedoic acid (BA) has consistently been shown to lower hsCRP independently of statins. A pooled analysis of the four phase 3 studies of BA (CLEAR Harmony, CLEAR Wisdom, CLEAR Serenity, and CLEAR Tranquility) showed that in those with baseline hsCRP of > 2 mg/L, the percentage changes were essentially the same for those on statins and those with low or no statins (41.9 vs. 43.3%, respectively) [75]. It was also shown that those who at baseline had hsCRP > 2 mg/L, 38.7% and 40.7% treated with ASCVD/HeFH or with low-dose or no statin, respectively, achieved levels of hsCRP < 2 mg/L at 12 weeks of treatment. Overall, among all of those treated with BA with baseline high hsCRP > 2 mg/L, 68.6% achieved low levels at 12 weeks. In these studies, a total of 20.8% in the ASCVD and/or HeFH pool receiving both statins and BA attained the dual goal of LDL-C < 70 mg/dL and hsCRP < 2 mg/L, compared to 4.3% of those receiving statins and placebo, regardless of baseline hsCRP levels. Additionally, more patients in the low-dose or no statin pool achieved both LDL-C < 100 mg/dL and hsCRP < 2 mg/dL than those receiving placebo (32% vs. 5.3%, respectively). In the CLEAR Outcomes trial, at 6 months of BA treatment, hsCRP was reduced by 21.6% and LDL-C by 21.1%. A sub-study within this trial found that BA had similar efficacy in reducing CV events across all levels of hsCRP and LDL-C. Further studies are needed to determine whether the reduction in hsCRP levels with BA contributed to the observed benefits [76].
Lp(a)-Lowering Medications
Several Lp(a)-lowering therapies are currently in phase II/III studies. Antisense oligonucleotides (pelacarsen), inhibitors of apo(a) binding to LDL (muvalaplin), and small interfering RNA (olpasiran, SLN360 and lepodisiran)-based therapies have shown an impressive 65–98% reduction in plasma Lp(a) levels. However, the effect of these drugs on inflammation has only been partially explored with pelacarsen. One study, based on transcriptome analysis in circulating monocytes of healthy individuals, showed that pelacarsen lowered Lp(a) by 47% and concomitantly reduced the proinflammatory gene expression in monocytes of patients with ASCVD with elevated Lp(a) and demonstrated a functional reduction in the transendothelial migration capacity of monocytes. [74].
New Anti-inflammatory Therapies
IL-1β-Targeted Therapy
Canakinumab, a monoclonal antibody (mAb) targeting IL-1β, demonstrated its efficacy in the CANTOS phase III randomized placebo-controlled clinical trial involving 10,061 participants with a history of MI and hsCRP levels exceeding 2.0 mg/L. Canakinumab at a dose of 150 mg led to a 15% reduction in the primary composite endpoint of MI, stroke, and CV death, with an HR of 0.85 (95% CI 0.74–0.98) when compared to placebo [77]. Key secondary endpoints, including MI, unstable angina necessitating urgent revascularization, and any coronary revascularization, were also diminished. However, no significant reduction was observed in CV mortality or stroke. Notably, CANTOS marked a groundbreaking moment by demonstrating the clinical benefits of targeting inflammation. Despite an elevated risk of fatal infection associated with canakinumab therapy, there was a reduction in cancer-related deaths.
Moreover, individuals achieving hsCRP < 2 mg/L with canakinumab treatment exhibited a 25% reduction in CV events and a 31% decrease in all-cause mortality, with only a 5% reduction in those who did not achieve hsCRP < 2 mg/L [78]. Similarly, another post hoc analysis of this trial showed that with treatment with canakinumab, those who achieved on-treatment IL-6 levels below the study median value of 1.65 ng/L experienced a 32% reduction in major adverse cardiovascular events (MACE) and a 52% reduction in CV mortality. In contrast, for those who did not achieve this, there was no significant benefit for any endpoint.
However, the true effect on lipids in this trial still needs to be studied in detail. In the CANTOS trial, no difference in LDL-C levels was observed, as 90% of the population were on statins, and the median LDL-C between groups was ≈ 80 mg/dL [40]. Because of different IL-1 phenotypes, and furthermore because IL-6-modulating therapies can lower Lp(a), canakinumab could have potentially affected Lp(a) levels in the CANTOS trial.
To our knowledge, only a single study has explored the effects of canakinumab treatment on Lp(a) levels. In this study, 189 patients with ASCVD and T2D were evaluated and randomized to receive 150 mg of canakinumab for 12 months. After 3 months, the treatment group had a significant 27% reduction in Lp(a), which persisted for the 12-month duration of the trial (a reduction of 4.30 mg/dl [range −8.5 to −0.55 mg/dl]; p = 0.025) [79]. Consistent with these findings, an analysis of the CANTOS trial presented at the 2021 European Atherosclerosis Society found that treatment with canakinumab lowered Lp(a) by 23%. This analysis suggests that the Lp(a) lowering by canakinumab accounted for over 40% of the favorable results in CANTOS [80]. Further studies are needed to confirm these findings. In summary, the CANTOS trial demonstrated, for the first time, the proof of principle that therapeutic targeting of the immune system can be beneficial for CV outcomes.
Translating these findings to clinical practice can be challenging, as this post hoc analysis often raises more questions than it answers. It is currently not known whether patients should be treated to target IL-1β, or whether hsCRP and IL-6 should be monitored in order to stop treatment in those that cannot achieve a significant reduction in inflammation, as determined by hsCRP levels.
Anakinra, a recombinant interleukin-1 receptor antagonist, has also been tested in acute myocardial infarction (AMI). The VCU-ART [81–85] phase II clinical trials program included three sequential studies (n = 139) which analyzed hsCRP levels in patients with acute ST-segment elevation MI (STEMI). In each study, patients were randomized to receive anakinra or placebo. Treatment with anakinra was well tolerated and led to a decrease in the hsCRP area under the curve (AUC) in the 14-day period after the AMI. It also showed a significantly lower incidence of new-onset heart failure (HF) and HF-related hospitalization in the 1-year follow-up. The MRC-ILA-Heart study [86] (n = 182) studied patients with AMI, but without STEMI. In this study, anakinra also reduced hsCRP levels at 7 days but failed to improve clinical outcomes. These studies show that NLRP3 inflammasome activation with enhanced IL-1 activity during and after AMI is also a contributor to HF risk. Additional IL-1 modulators have been approved for indications other than ASCVD. Rilonacept (IL-1 trap) and gevokizumab (IL-1β binding protein) have been tested for inflammatory diseases, but to date not for ASCVD.
IL-6-Targeted Therapy
Tocilizumab, a humanized recombinant mAb targeting the IL-6 receptor, has demonstrated its efficacy and safety in several clinical trials. It has been approved for the treatment of several autoimmune diseases such as giant cell arteritis and RA, and has been considered for secondary prevention of ASCVD and MI. Recently, the randomized, double-blind, placebo-controlled phase III ASSAIL-MI trial [87] showed a larger myocardial salvage index in the tocilizumab group than under placebo. Also, microvascular obstruction was less extensive in the tocilizumab arm, but there was no significant difference in the final infarct size.
In a recent double-blind placebo-controlled trial in 117 patients with non-ST segment elevation myocardial infarction (NSTEMI), 2.1-fold lower hsCRP levels were reported in the tocilizumab group compared to placebo (2.0 vs. 4.2 mg/L/h, p < 0.001). Tocilizumab also reduced troponin levels (234 vs. 159 ng/L/h, placebo versus tocilizumab, respectively, p = 0.007), suggesting that treated patients sustained less myocardial damage than those on placebo [88].
A newer drug, ziltivekimab, a fully human mAb directed against the IL-6 ligand, has shown safety and efficacy in reducing biomarkers of inflammation and thrombosis in patients with high ASCVD risk. Recently, the RESCUE trial [89] confirmed that the use of ziltivekimab reduced hsCRP in a dose-dependent manner, from 77% to 93%, and other markers including fibrinogen, serum amyloid A (SAA), haptoglobin sPLA2, and Lp(a) in patients with ASCVD and chronic kidney disease (CKD) [90]. Currently, the ZEUS trial will compare ziltivekimab to placebo in 6200 randomized patients with stage 3–4 CKD or ASCVD and with elevated hsCRP, to formally test whether reducing circulating IL-6 reduces CV event rates. An important secondary aim to address is whether chronic IL-6 inhibition slows the progression of renal disease. This ambitious trial represents the next step toward understanding the benefits of targeting residual inflammatory risk (NCT05021835).
Sarilumab, a recently approved human mAb directed against both soluble and membrane-bound interleukin 6 receptor-α, shown to be effective in RA, could be also promising for managing ASCVD [91]. Currently there are only trials for the interaction with simvastatin registered in ClinicalTrials.gov (NCT02017639).
IL-18-Targeted Therapy
Currently, IL-18 blockers are under investigation. The role of IL-18 in inflammasome activation suggests inhibitors may target ASCVD. Recently, a novel IgG mAb that neutralizes IL-18 (GSK1070806) [92] has been reported to be well tolerated in healthy individuals, those with T2D, and obese subjects [93].
Methotrexate
Methotrexate (MTX) is a Food and Drug Administration (FDA)-approved folic acid antagonist which reduces T-cell proliferation and secretion of cytokines and MMPs, and thus is a potential treatment for atherosclerotic disease. MTX is indicated for the treatment of RA, juvenile idiopathic arthritis, and certain types of cancer. Its use for atherosclerosis has been shown in mouse models. In mice fed a high-cholesterol diet, MTX reduced both the size of the lesion areas of by 75% and the intima–media ratio by twofold. The drug inhibited macrophage migration into the intima by 50% and the presence of apoptotic cells by 84%, but did not inhibit the intimal proliferation of SM cells [94]. In observational studies of patients with RA, those treated with MTX had a 15% reduction in CV events [95], and in patients with psoriasis, a 50% reduction in the composite of ASCVD-related death, stroke, or MI was reported. A meta-analysis of 10 observational studies also showed that in patients with RA, psoriasis, or polyarthritis, treatment with MTX was associated with 21% lower risk for total ASCVD and 18% lower risk for MI. These studies prompted the Cardiovascular Reduction Trial (CIRT), a randomized double-blind, placebo-controlled trial looking to determine whether low doses of MTX could reduce CV events. This study was stopped early for futility, as MTX neither decreased MACE over 5 years (HR 1.01; 95% CI [0.82–1.25]; p = 0.91 versus placebo) nor lowered the concentration of inflammatory markers, particularly hsCRP [96]. Although patients included in the trial did not have residual inflammatory risk at baseline, this could not fully account for the trial results. Thus, only patients with CVD with rheumatological disease appear to benefit from low-dose MTX treatment.
Colchicine
Colchicine inhibits cytoskeletal reorganization, phagocytosis, mitosis, and other intracellular activity and is commonly prescribed for gout, familial Mediterranean fever, and off-label for pericarditis. Within its myriad effects, it also inhibits NLRP3 inflammasome-driven inflammation. Small studies have shown that in patients with stable CAD under statin treatment, the introduction of colchicine reduced hsCRP levels by 60%. Other studies have shown that in patients with acute coronary syndrome (ACS) and stable CAD randomized to receive either colchicine (1 mg, followed by 0.5 mg 1 h later) or no colchicine therapy prior to coronary angiography, those treated had significantly reduced transcoronary gradients of IL-1β, IL-18, and IL-6 cytokines by 40–88% (p = 0.028, 0.032, and 0.032, respectively) [97].
More recently, the LODOCO2 [98] and the COLCOT [99] studies demonstrated the utility of low-dose colchicine to reduce the risk of recurrent ischemic CV events. The COLCOT study involving 4745 post-MI patients demonstrated a remarkable 23% reduction in MACE, including CV death, cardiac arrest, MI, stroke, or urgent revascularization, over 22.6 months. This trial establishes the role of an anti-inflammatory agent in post-MI risk reduction. Subsequently, the LODOCO2 trial, which included 2762 patients with chronic coronary disease to receive low doses of colchicine, demonstrated a 31% reduction in MACE compared to placebo. Neither of the trials used hsCRP as an inclusion criterion. Given these results, in 2023 the FDA granted colchicine a broad label to reduce the risk of CV events in secondary prevention, or with multiple CV risk factors (https://us.agephapharma.com/blog/2023/06/20/us-fda-approves-first-anti-inflammatory-drug-for-cardiovascular-disease/).
Not all colchicine clinical trials, however, have been positive. In the COPS trial, the addition of colchicine to standard medical therapy in patients hospitalized with ACS did not affect CV outcomes at 12 months (HR 0.65, 95% CI 0.38–1.09, p = 0.10) [100]. Post hoc analysis demonstrated a significant reduction in the primary outcome in favor of colchicine. Unfortunately, this study was underpowered, thereby limiting interpretation of the mortality data.
Clinical trials are underway to investigate the use of colchicine in several different clinical settings. For example, the use of colchicine in ACS (COACS) (NCT01906749) trial examines vascular inflammation assessed with PET (COLPET) (NCT02162303). The CONVINCE trial, a large trial in phase 3, is planned to study the role of low-dose colchicine in adults who have suffered a stroke. This trial will evaluate patients (n = 2623) with ischemic stroke or transient ischemic attack, who will be followed though 60 months, and will assess the recurrence of stroke or nonfatal major cardiac events (NCT02898610). The most anticipated trial is CLEAR SYNERGY, the largest ongoing trial (n = 7000), which will be performed over 2 years in patients with MI. This study contains four arms (colchicine 0.5 mg twice daily and/or spironolactone 25 mg daily and/or SYNERGY stent and/or colchicine/spironolactone-placebo), providing the best approach for assessing the utility of colchicine in secondary ASCVD prevention (NCT03048825). This last trial is expected to be completed by 2025.
NLRP3 Inflammasome Inhibition Targeted Therapy
To date, the interest in NLRP3 inflammasome inhibition has led to the development of both direct and indirect inhibitors. Related to CVD, two direct inhibitors, MCC950 and VX-765, have been reported to prevent atherosclerosis in animal models [101–103]. Unfortunately, after the promising results of MCC950 in apoE-knockout (KO) mice in preventing atherosclerosis, its development was stopped due to its hepatotoxicity [104]. VX-740 and its analog VX-765, which are peptidomimetic inhibitors of caspase-1, have been reported to reduce oxidized LDL-induced vascular SM cell apoptosis in vitro and to prevent the development of atherosclerosis in apoE-KO mice [103]. Indirect inhibition of NLRP3 inflammasome can also be achieved by arglabin treatment, which has been shown to reduce both inflammation and plasma lipids by increasing autophagy and by directing macrophages to an anti-inflammatory phenotype in ApoE2-knock-in (KI) mice [105].
New glucose-lowering therapies have also been shown to indirectly target the inflammasome. In particular, sodium–glucose cotransporter-2 inhibitors and GLP-1 receptor agonists directly reduce CV risk [106, 107]. Several studies which evaluated the effects of these dugs on dysfunctional PVAT and diabetic mouse models have indicated that they may indirectly inhibit the NLRP3 inflammasome [108, 109]. Currently, there is growing interest in the evaluation of the ability of these treatments to reduce inflammation in clinical trials.
Other direct or indirect inhibitors of the NLRP3 inflammasome are currently under development for non-CVD inflammatory diseases, and their potential benefit in ASCVD risk reduction will be explored in the near future.
Corticosteroids
Corticosteroids are widely known as powerful anti-inflammatory agents and have been used to treat several autoimmune diseases. However, long-term use, especially at high doses, is associated with an increase in other ASCVD risk factors (hypertension, T2D, obesity, insulin resistance, and dyslipidemia) and a significant increase in ASCVD risk [110]. More recently, meta-analyses have shown that even low-dose corticosteroid treatment can increase CV events in RA. Therefore, corticosteroid appears to be of little use in treating low-grade inflammation for the prevention of ASCVD [111].
The mechanism of action and trials for all drugs discussed herein are summarized in Table 1.
Table 1.
Summary table of current drugs in trials or development for treatment of cardiovascular inflammation
| Drug | Mechanism of action | Study name | Patient cohort | Primary endpoint | Outcome | References |
|---|---|---|---|---|---|---|
| IL-1-targeted therapy | ||||||
| Canakinumab | Fully human monoclonal antibody against IL-1β | CANTOS | 10,061 individuals with a history of MI and elevated plasma CRP levels | Nonfatal MI, nonfatal stroke, or cardiovascular death | 25% reduction in cardiovascular events and a 31% decrease | [78] |
| Anakinra | Humanized monoclonal antibody against IL-1 | MRC-IL1-HEART | 182 individuals with non-ST elevation acute coronary syndrome | The hsCRP AUC value was reduced by nearly 50% in anakinra group versus placebo group in the first 7 days | hsCRP levels were lower after 14 days of anakinra medication; the risk of MACE was similar at 30 days and 3 months but significantly higher at 1 year in the anakinra group compared to the placebo group | [85, 117] |
| VCUART3 | 99 patients with STEMI | AUC for hsCRP, measured at baseline, 72 h, and day 14 | hsCRP AUC was significantly lower in patients receiving anakinra versus placebo (median, 67 [interquartile range, 39–120] versus 214 [interquartile range, 131–394] mg/day/L; P < 0.001) | |||
| IL-6-targeted therapy | ||||||
| Tocilizumab | Monoclonal antibody against IL-6 | Kleveland |
117 NSTEMI patients were randomized at a median of 2 days after the beginning of symptoms |
The hsCRP AUC at 1–3 days of treatment initiation |
Tocilizumab reduced hsCRP and hsTnT levels compared with placebo | [87, 88] |
| ASSAIL-MI | 117 NSTEMI patients scheduled for angiography | AUC for hsCRP | Tocilizumab reduced hsCRP levels and troponin T release after PCI | |||
| ENTRACTE | 3080 patients with RA and 1 CV risk factor | Comparison of time to first occurrence of MACE |
Non-inferior to TNF-α inhibitor in reducing cardiovascular events |
|||
| Ziltivekimab | Monoclonal antibody against IL-6 ligand | RESCUE | 264 patients with chronic kidney disease and hsCRP > 2 mg/L | The primary outcome was the percentage change in hsCRP vs. the baseline levels after 12 weeks of ziltivekimab vs. placebo treatment |
77–93% hsCRP reduction Reduction also of fibrinogen, SAA, haptoglobin sPLA2 and Lp(a) |
[89] |
| ZEUS | 6200 patients with chronic kidney disease and CRP ≥ 2 mg/L | The occurrence of MACE including MI, stroke and cardiovascular death | Not yet completed | |||
| Sarilumab | Monoclonal antibody directed against both soluble and membrane-bound interleukin 6 receptor-α | SARIPET | 20 Patients | Effect of sarilumab on carotid atheroma plaque (effect on the inflammatory component) using ultrasound in patients with RA | Not yet recruiting | [91] |
| IL-18-targeted therapy | ||||||
| GSK1070806 | Humanized IgG1 antibody that binds to human IL-18 | McKie | 37 patients with obesity and T2DM | To assess the efficacy of two | Inhibition of IL-18 did not lead to any improvements in glucose control | [92] |
| NLRP3 inflammasome-targeted therapy | ||||||
| VX-765 | Peptidomimetic inhibitors of caspase-1 inhibitor | Animal studies have shown prevention of atherosclerosis | [103] | |||
| VX-740 | Peptidomimetic inhibitors of caspase-1 inhibitor | Animal studies have shown prevention of atherosclerosis | [105] | |||
| Arglabin | Inhibition of farnesyl transferase | To reduce both inflammation and plasma lipids by increasing autophagy and by directing macrophages to an anti-inflammatory phenotype in ApoE2.KO mice | [105] | |||
| Other anti-inflammatory therapeutics | ||||||
| Methotrexate |
Folic acid antagonist Reduced T-cell proliferation Reduced cytokine release Reduced expression of cell-surface adhesion molecules |
CIRT | 4786 patients with previous MI or multivessel coronary disease who additionally had either type 2 diabetes or the metabolic syndrome | Nonfatal MI, nonfatal stroke, or cardiovascular death | No reduction in hsCRP, IL-1β, or IL-6; no reduction in cardiovascular events | [96] |
| Colchicine |
Inhibits microtubule polymerization Prevents activation of NLRP3 inflammasome Reduces release of IL-1β |
COLCOT | 4745 patients with MI within 30 days before enrolment | Death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or hospitalization for angina leading to coronary revascularization | 23% reduction in major cardiovascular events | [98, 99] |
| LoDoCo2 | 5522 patients with chronic coronary artery disease | Death from cardiovascular causes, spontaneous MI, ischemic stroke or ischemia-driven coronary revascularization | 31% reduction of major cardiovascular events | |||
| CLEAR Synergy | 7063 patients with STEMI | MACE (defined as the composite of death, recurrent target vessel MI, stroke, or ischemia-driven target vessel revascularization) compared to performance goal | Not yet published | |||
| Statins | Hydroxymethylglutaryl-CoA reductase inhibitors | CARE | 4159 patients with MI | Fatal coronary event or nonfatal MI | 24% reduction in primary outcome and 21.6% hsCRP reduction | [67] |
| JUPITER | 15,548 healthy patients | Nonfatal MI, nonfatal stroke, admission for unstable angina, arterial revascularization, or cardiovascular death | Achieving hsCRP < 2 mg/dL associated with a 62% reduction in primary event; also 37% hsCRP reduction | |||
| Ezetimibe | Inhibits Niemann-Pick C1-like 1 protein | IMPROVE-IT (post hoc) | 18,144 patients stabilized after acute coronary syndrome to simvastatin or ezetimibe/simvastatin | MACE outcomes assessed with prespecified targets of LDL-C < 70 mg/dL and hsCRP < 2 mg/L versus achieving neither target | Those achieving dual targets had lower primary endpoint rates than those meeting neither target (cardiovascular death, major coronary event, or stroke; 38.9% versus 28.0%; adjusted hazard ratio, 0.73; 0.66–0.81; p < 0.001) | [69] |
| PCSK9i | Monoclonal antibodies against PCSK9 | FOURIER AND ODYSSEY | Neutral over hsCRP | [71] | ||
| Bempedoic Acid | Inhibits adenosine triphosphate-citrate lyase | CLEAR-Outcomes | 13,970 patients with either increased CV risk or secondary prevention | Four-component MACE | 13% lower MACE and 21.6% hsCRP reduction | [75] |
| MLN1202 | Neutralizing monoclonal antibody against CC-chemokine receptor 2 (CCR2) | MLN1202 | 112 patients with 2 risk factors for ASCVD and CRP > 3 m/dL | Median change in CRP | 26.6% reduction in hsCRP | [118] |
LDL low-density lipoprotein, TRL triglyceride-rich lipoprotein, hsCRP high-sensitivity C-reactive protein, CRP C-reactive protein, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor, BA bempedoic acid, IL-6 interleukin 6, IL-1β interleukin 1 beta, IL-18 interleukin-18, TNF-α tumor necrosis factor alpha, NLRP3 nucleotide-binding oligomerization domain-like receptor 3, Lp-PLA2 lipoprotein phospholipase A2, MI myocardial infarction, STEMI ST-elevation myocardial infarction, NSTEMI non-ST elevation myocardial infarction, ASCVD atherosclerotic cardiovascular disease, MACE major adverse cardiovascular event, AUC area under the curve, HsTnT high-sensitivity troponin T, PCI percutaneous coronary intervention, RA rheumatoid arthritis, CV cardiovascular, SAA serum amyloid A, T2D type 2 diabetes, ApoE apolipoprotein E, KO knockout
Current Challenges and Future Direction
The individualized approach in immunotherapy has a promising future in ASCVD treatment. A major challenge is in tailoring therapy based on distinct characteristics of patients, such as immune profile or genetic background, and by correctly identifying the clinical ASCVD phenotype.
Identifying individuals with high residual cholesterol continues to be the main focus in ASCVD risk assessment, following the “lower-the-better” concept for LDL-C. This has resulted in the development of several lipid-lowering therapies that further reduce ASCVD risk when added to treatment with a statin. The detection of an inflammatory phenotype (measured mainly by hsCRP) and data collected over the past 30 years on the fundamental role inflammation plays in atherosclerosis provide the basis for therapies targeting residual inflammatory risk. Moreover, the impact of inflammation on the progression of ASCVD events no longer remains a theory insofar as the CANTOS trial showed proof of the clinical impact of residual inflammatory risk.
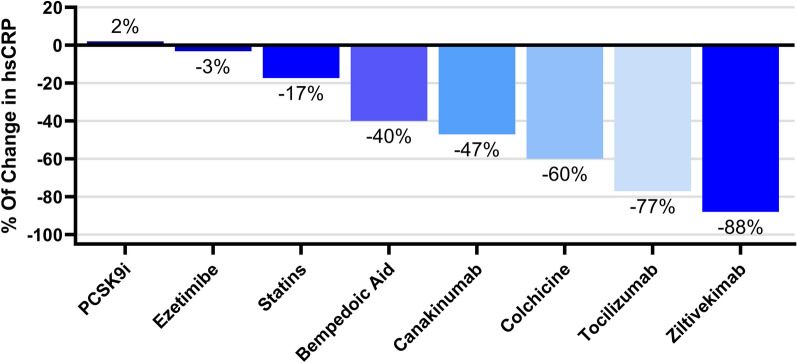
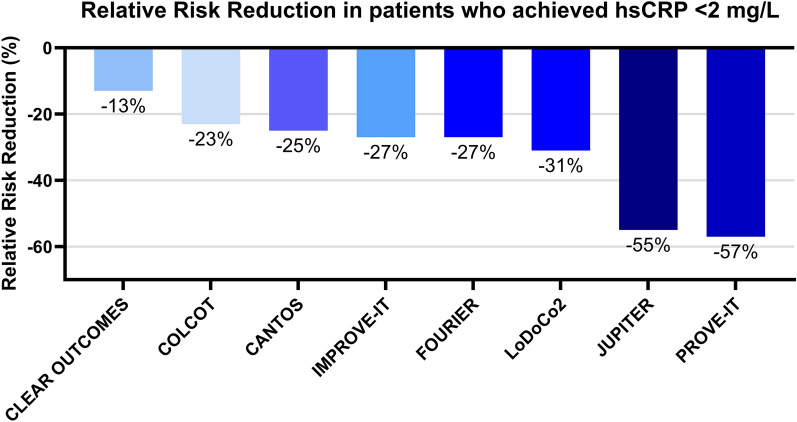
While defining residual inflammatory risk continues to be based solely on hsCRP levels (as a surrogate marker of the IL-1β-to-IL-6-to-hsCRP pathway), it is now clear that treatment that achieves lowering of hsCRP < 2 mg/L results in RRR for MACE (as shown in Fig. 2). Nevertheless, there are discrepancies in the degree of hsCRP-lowering potential and RRR between different drugs as shown in Fig. 3. It appears that a residual inflammatory phenotype, irrespective of LDL-C, confers a higher risk for MACE [112]. The hypothesis that other biomarkers may further aid in clinical decision-making is based on the evidence that incorporating IL-6 as a measurement of response to therapy may play a role in tailoring immunotherapy to reduce residual risk. The variable disease settings and inclusion criteria among clinical trials targeting inflammation in ASCVD challenges the implementation in a clinical setting. The small number of ongoing trials in this setting is surprising, given the success of immunotherapy in atherosclerosis. Currently, inflammation can be detected via imaging modalities, but its application in drug development is still limited.
Fig. 2.
Percentage reduction in hsCRP with different therapies. hsCRP high-sensitivity C-reactive protein, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor. This original figure was created using GraphPad Prism
Fig. 3.
Relative risk reduction for major cardiovascular events in trials in a population who achieved high-sensitivity C-reactive protein (hsCRP) < 2 mg/L. This original figure was created using GraphPad Prism
One essential need is the early identification of patients who would most benefit from a specific treatment. This was clearly demonstrated in the CANTOS trial, as those who did not show a decrease in IL-6 did not have ASCVD benefits, compared to those who showed a decrease. Perhaps those who do not show a decrease in IL-6 may benefit more from other therapies, such as tocilizumab. Figure 4 presents a potential guideline for clinical decision-making and precision medicine when considering treatment for inflammatory states that may increase ASCVD risk.
Fig. 4.
Potential assessment of secondary prevention phenotype. Dotted lines indicate unanswered questions. LDL-C low-density lipoprotein cholesterol, hsCRP high-sensitivity C-reactive protein, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor, BA bempedoic acid, IL-6 interleukin 6. This original figure was created using BioRender
As we gain further information and insights, the clinical picture may become clearer. Nevertheless, several questions arise from the clinical trials. Even though colchicine has been approved for secondary prevention, we still do not know exactly when to initiate or how long to maintain treatment. It is yet to be determined whether hsCRP is a useful tool for decisions regarding add-on treatment or use as a therapeutic goal, and if so, what target level. Would adding other biomarkers help to make better decisions regarding the benefit of the therapy? Ridker et al. [112] showed that identifying inflammatory residual risk (hsCRP > 2 mg/dL), independently of LDL-C, was associated with an 18–20% greater risk of MACE, 83–91% greater risk of CV mortality, and 75–83% greater risk of all-cause mortality. This post hoc analysis implies that further lowering of LDL-C to < 70 mg/dL may be beneficial. Although the American College of Cardiology/American Heart Association/multi-society cholesterol guidelines [113] still endorse an LDL-C threshold of ≥ 70 mg/dL for adding non-statin treatment in secondary prevention, other guidelines such as the 2019 European Society of Cardiology/European Atherosclerosis Society Guidelines for Management of Dyslipidemia [114] go further and recommend < 55 mg/dL and even < 40 mg/dL for patients with multiple CV events within 2 years despite optimal statin therapy. However, post hoc analysis of the FOURIER trial showed that there is continued effectiveness, even with LDL-C < 40 mg/dL, for patients with high ASCVD risk, further validating the European guidelines [115]. Thus, as shown in Fig. 4, both lipid residual risk and inflammatory risk should be addressed in all high-risk patients with ASCVD.
Additional challenges that should be carefully considered in implementing anti-inflammatory therapies include the secondary effects of such therapies. For example, in the CANTOS trial, even though the use of canakinumab was associated with a lower incidence of lung cancer compared to placebo [116], it was associated with a small but statistically significant increase in non-opportunistic fatal infection. Another example is the CIRT trial, where the use of methotrexate was linked to a small increase in the incidence of skin cancer [96]. Finally, all of these trials are focused on secondary and not primary prevention. Identifying an inflammatory phenotype in primary prevention could help target immunotherapy in those at higher risk for ASCVD events.
Conclusion
Despite achieving low levels of LDL-C through guideline-proven therapies, residual risk from low chronic inflammation continues to drive cardiovascular events and therefore should potentially be targeted. The abovementioned studies provide proof of principle for reducing ASCVD risk by treating inflammation. New drugs targeting either interleukins or the NLRP3 inflammasome may be the most promising in managing recurrent ASCVD events. The future of these clinical trials and the implementation of new biomarkers may improve the scope of cardiovascular drug development and precision medicine approaches. Currently, however, there are many more questions than answers on how to implement these new drugs in cardiovascular medicine.
Although the role of inflammation is clear and several biomarkers are available, hsCRP is the most commonly studied and used clinically. Based on the reviewed literature, identifying a low-grade inflammation phenotype using hsCRP may be a reasonable tool for reclassifying ASCVD risk. Growing evidence supports its use to tailor therapy, but research is ongoing to determine whether an alternative anti-inflammatory biomarker may be superior. The use of imaging in inflammation detection and response to therapy, though promising, still requires further investigation. Development of new lipid- and inflammation-targeted therapies should rely on the concept of an individualized, tailored approach for efficiently addressing all forms of residual risk in ASCVD.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Acknowledgements
The authors wish to thank Drs. Gretell Henriquez-Santos and Pablo Corral for their insightful comments and revision of the manuscript.
Author Contributions
Conceptualization: Rafael Zubirán and Alexander V. Sorokin; Methodology: Rafael Zubirán performed the literature search; Writing—original draft preparation: Rafael Zubirán, Amaury Dasseux; Writing: Rafael Zubirán, Review and editing: Rafael Zubirán, Edward B. Neufeld, Alan T. Remaley and Alexander V. Sorokin.
Funding
Funding was provided by intramural DIR research funds from the National Heart, Lung, and Blood Institute. No funding or sponsorship was received for the publication of this article.
Declarations
Conflict of Interest
Alan T. Remaley, Edward B Neufeld and Rafael Zubirán are full-time US Government employees. Amaury Dasseux has declared that no potential conflict of interest exists. Alexander V. Sorokin is a full-time employee of Regeneron Pharmaceuticals Inc.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, Nordestgaard BG, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376(9738):333–9. 10.1016/S0140-6736(10)60713-1 [DOI] [PubMed] [Google Scholar]
- 2.Antonopoulos AS, Angelopoulos A, Papanikolaou P, Simantiris S, Oikonomou EK, Vamvakaris K, et al. Biomarkers of vascular inflammation for cardiovascular risk prognostication: a meta-analysis. JACC Cardiovasc Imaging. 2022;15(3):460–71. 10.1016/j.jcmg.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007;48(11):1800–15. 10.2967/jnumed.107.038661 [DOI] [PubMed] [Google Scholar]
- 4.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8 Suppl):C19-31. 10.1016/j.jacc.2005.10.066 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007–11. 10.1161/01.CIR.97.20.2007 [DOI] [PubMed] [Google Scholar]
- 7.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–20. [DOI] [PMC free article] [PubMed]
- 8.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–40. [DOI] [PMC free article] [PubMed]
- 9.Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornson E, Packard CJ, Adiels M, Andersson L, Matikainen N, Soderlund S, et al. Investigation of human apoB48 metabolism using a new, integrated non-steady-state model of apoB48 and apoB100 kinetics. J Intern Med. 2019;285(5):562–77. 10.1111/joim.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tybjaerg-Hansen A, Nordestgaard BG, Christoffersen M. Triglyceride-rich remnant lipoproteins are more atherogenic than LDL per particle: is this important? Eur Heart J. 2023;44(39):4196–8. 10.1093/eurheartj/ehad419 [DOI] [PubMed] [Google Scholar]
- 12.Balling M, Afzal S, Varbo A, Langsted A, Davey Smith G, Nordestgaard BG. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J Am Coll Cardiol. 2020;76(23):2725–35. 10.1016/j.jacc.2020.09.610 [DOI] [PubMed] [Google Scholar]
- 13.Johansen MO, Vedel-Krogh S, Nielsen SF, Afzal S, Davey Smith G, Nordestgaard BG. Per-particle triglyceride-rich lipoproteins imply higher myocardial infarction risk than low-density lipoproteins: Copenhagen general population study. Arterioscler Thromb Vasc Biol. 2021;41(6):2063–75. 10.1161/ATVBAHA.120.315639 [DOI] [PubMed] [Google Scholar]
- 14.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117(1):94–8. 10.1172/JCI30889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PH, Wight TN, Chait A. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res. 2002;43(11):1969–77. 10.1194/jlr.M200322-JLR200 [DOI] [PubMed] [Google Scholar]
- 16.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–63. 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 18.Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem. 2019;65(2):321–32. 10.1373/clinchem.2018.294926 [DOI] [PubMed] [Google Scholar]
- 19.Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30. 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman MJ, Orsoni A, Tan R, Mellett NA, Nguyen A, Robillard P, et al. LDL subclass lipidomics in atherogenic dyslipidemia: effect of statin therapy on bioactive lipids and dense LDL. J Lipid Res. 2020;61(6):911–32. 10.1194/jlr.P119000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta. 2012;1821(5):858–66. 10.1016/j.bbalip.2011.09.021 [DOI] [PubMed] [Google Scholar]
- 22.Wu HM, Ni XX, Xu QY, Wang Q, Li XY, Hua J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-kappaB signaling pathway. J Gastroenterol Hepatol. 2020;35(11):1998–2008. 10.1111/jgh.15025 [DOI] [PubMed] [Google Scholar]
- 23.Tangirala RK, Jerome WG, Jones NL, Small DM, Johnson WJ, Glick JM, et al. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J Lipid Res. 1994;35(1):93–104. 10.1016/S0022-2275(20)40131-2 [DOI] [PubMed] [Google Scholar]
- 24.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60(3):473–85. 10.1161/01.CIR.60.3.473 [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–46. 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronenberg F, Mora S, Stroes ESG. Consensus and guidelines on lipoprotein(a)—seeing the forest through the trees. Curr Opin Lipidol. 2022;33(6):342–52. 10.1097/MOL.0000000000000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–60. 10.1161/ATV.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornson E, Adiels M, Taskinen MR, Burgess S, Chapman MJ, Packard CJ, Boren J. Lipoprotein(a) is markedly more atherogenic than LDL: an apolipoprotein B-based genetic analysis. J Am Coll Cardiol. 2024;83(3):385–95. 10.1016/j.jacc.2023.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wade DP, Clarke JG, Lindahl GE, Liu AC, Zysow BR, Meer K, et al. 5’ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. Proc Natl Acad Sci USA. 1993;90(4):1369–73. 10.1073/pnas.90.4.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller N, Schulte DM, Turk K, Freitag-Wolf S, Hampe J, Zeuner R, et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res. 2015;56(5):1034–42. 10.1194/jlr.P052209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buechler C, Ullrich H, Aslanidis C, Bared SM, Lingenhel A, Ritter M, Schmitz G. Lipoprotein (a) downregulates lysosomal acid lipase and induces interleukin-6 in human blood monocytes. Biochim Biophys Acta. 2003;1642(1–2):25–31. 10.1016/S0167-4889(03)00083-1 [DOI] [PubMed] [Google Scholar]
- 32.Berthold HK, Laudes M, Krone W, Gouni-Berthold I. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS ONE. 2011;6(9): e24719. 10.1371/journal.pone.0024719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsimikas S, Duff GW, Berger PB, Rogus J, Huttner K, Clopton P, et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol. 2014;63(17):1724–34. 10.1016/j.jacc.2013.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naka KK, Bechlioullis A, Marini A, Sionis D, Vakalis K, Triantis G, et al. Interleukin-1 genotypes modulate the long-term effect of lipoprotein(a) on cardiovascular events: the Ioannina Study. J Clin Lipidol. 2018;12(2):338–47. 10.1016/j.jacl.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 35.Langsted A, Kamstrup PR, Nordestgaard BG. Lipoprotein(a): fasting and nonfasting levels, inflammation, and cardiovascular risk. Atherosclerosis. 2014;234(1):95–101. 10.1016/j.atherosclerosis.2014.01.049 [DOI] [PubMed] [Google Scholar]
- 36.Puri R, Nissen SE, Arsenault BJ, St John J, Riesmeyer JS, Ruotolo G, et al. Effect of C-reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. 2020;5(10):1136–43. 10.1001/jamacardio.2020.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129(6):635–42. 10.1161/CIRCULATIONAHA.113.004406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makris A, Barkas F, Sfikakis PP, Liberopoulos E, Filippatos TD, Ray KK, Agouridis AP. Lipoprotein(a), Interleukin-6 inhibitors, and atherosclerotic cardiovascular disease: is there an association? Atheroscler Plus. 2023;54:1–6. 10.1016/j.athplu.2023.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas PE, Vedel-Krogh S, Kamstrup PR, Nordestgaard BG. Lipoprotein(a) is linked to atherothrombosis and aortic valve stenosis independent of C-reactive protein. Eur Heart J. 2023;44(16):1449–60. 10.1093/eurheartj/ehad055 [DOI] [PubMed] [Google Scholar]
- 40.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70(18):2278–89. 10.1016/j.jacc.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kastrati A, Koch W, Berger PB, Mehilli J, Stephenson K, Neumann FJ, et al. Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J Am Coll Cardiol. 2000;36(7):2168–73. 10.1016/S0735-1097(00)01014-7 [DOI] [PubMed] [Google Scholar]
- 42.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97(3):769–76. [DOI] [PMC free article] [PubMed]
- 43.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun W, Han Y, Yang S, Zhuang H, Zhang J, Cheng L, Fu L. The assessment of interleukin-18 on the risk of coronary heart disease. Med Chem. 2020;16(5):626–34. 10.2174/1573406415666191004115128 [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–15. 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 47.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–89. 10.1093/eurheartj/eht367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25(5):923–31. 10.1161/01.ATV.0000160551.21962.a7 [DOI] [PubMed] [Google Scholar]
- 49.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–82. 10.1161/CIRCULATIONAHA.108.771899 [DOI] [PubMed] [Google Scholar]
- 50.Napodano C, Carnazzo V, Basile V, Pocino K, Stefanile A, Gallucci S, et al. NLRP3 inflammasome involvement in heart, liver, and lung diseases—a lesson from cytokine storm syndrome. Int J Mol Sci. 2023;24(23):16556. 10.3390/ijms242316556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toldo S, Abbate A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. 2023;21:219. 10.1038/s41569-023-00946-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122(1):70–9. 10.1172/JCI43713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hettwer J, Hinterdobler J, Miritsch B, Deutsch MA, Li X, Mauersberger C, et al. Interleukin-1beta suppression dampens inflammatory leucocyte production and uptake in atherosclerosis. Cardiovasc Res. 2022;118(13):2778–91. 10.1093/cvr/cvab337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28(1):51. 10.1186/s11658-023-00462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adam CA, Salaru DL, Prisacariu C, Marcu DTM, Sascau RA, Statescu C. Novel biomarkers of atherosclerotic vascular disease-latest insights in the research field. Int J Mol Sci. 2022;23(9):4998. 10.3390/ijms23094998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398). [DOI] [PubMed]
- 57.Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392(10151):929–39. 10.1016/S0140-6736(18)31114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikonomou EK, Antonopoulos AS, Schottlander D, Marwan M, Mathers C, Tomlins P, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res. 2021;117(13):2677–90. [DOI] [PubMed] [Google Scholar]
- 59.Sorokin AV, Patel N, Abdelrahman KM, Ling C, Reimund M, Graziano G, et al. Complex association of apolipoprotein E-containing HDL with coronary artery disease burden in cardiovascular disease. JCI Insight. 2022;7(10). [DOI] [PMC free article] [PubMed]
- 60.O’Hagan R, Hsu LY, Li H, Hong CG, Parel PM, Berg AR, et al. Longitudinal association of epicardial and thoracic adipose tissues with coronary and cardiac characteristics in psoriasis. Heliyon. 2023;9(10): e20732. 10.1016/j.heliyon.2023.e20732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sajja A, Abdelrahman KM, Reddy AS, Dey AK, Uceda DE, Lateef SS, et al. Chronic inflammation in psoriasis promotes visceral adiposity associated with noncalcified coronary burden over time. JCI Insight. 2020;5(22). [DOI] [PMC free article] [PubMed]
- 62.Bhambhvani P. Challenges of cardiac inflammation imaging with F-18 FDG positron emission tomography. J Nucl Cardiol. 2017;24(1):100–2. 10.1007/s12350-016-0508-1 [DOI] [PubMed] [Google Scholar]
- 63.Patel NH, Osborne MT, Teague H, Parel P, Svirydava M, Sorokin AV, et al. Heightened splenic and bone marrow uptake of (18)F-FDG PET/CT is associated with systemic inflammation and subclinical atherosclerosis by CCTA in psoriasis: an observational study. Atherosclerosis. 2021;339:20–6. 10.1016/j.atherosclerosis.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwartz DM, Parel P, Li H, Sorokin AV, Berg AR, Chen M, et al. PET/CT-based characterization of 18F-FDG uptake in various tissues reveals novel potential contributions to coronary artery disease in psoriatic arthritis. Front Immunol. 2022;13: 909760. 10.3389/fimmu.2022.909760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. 10.1001/jama.286.1.64 [DOI] [PubMed] [Google Scholar]
- 66.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9. [DOI] [PubMed]
- 67.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–82. 10.1016/S0140-6736(09)60447-5 [DOI] [PubMed] [Google Scholar]
- 68.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45(10):1644–8. 10.1016/j.jacc.2005.02.080 [DOI] [PubMed] [Google Scholar]
- 69.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114(4):281–8. 10.1161/CIRCULATIONAHA.106.628909 [DOI] [PubMed] [Google Scholar]
- 70.Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–33. 10.1161/CIRCULATIONAHA.115.018381 [DOI] [PubMed] [Google Scholar]
- 71.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 72.O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–92. 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 73.Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J. 2017;38(20):1584–93. 10.1093/eurheartj/ehx002 [DOI] [PubMed] [Google Scholar]
- 74.Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, et al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J. 2020;41(24):2262–71. 10.1093/eurheartj/ehaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stroes ESG, Bays HE, Banach M, Catapano AL, Duell PB, Laufs U, et al. Bempedoic acid lowers high-sensitivity C-reactive protein and low-density lipoprotein cholesterol: analysis of pooled data from four phase 3 clinical trials. Atherosclerosis. 2023;373:1–9. 10.1016/j.atherosclerosis.2023.03.020 [DOI] [PubMed] [Google Scholar]
- 76.Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64. 10.1056/NEJMoa2215024 [DOI] [PubMed] [Google Scholar]
- 77.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 78.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Group CT. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–28. 10.1016/S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 79.Choudhury RP, Birks JS, Mani V, Biasiolli L, Robson MD, L’Allier PL, et al. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J Am Coll Cardiol. 2016;68(16):1769–80. 10.1016/j.jacc.2016.07.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schrock CG. Lipoprotein(a) reduction by canakinumab, a monoclonal antibody targeting interleukin-1Beta, explains a significant portion of the favorable outcomes of the cantos trial. Atherosclerosis. 2021;331:e36. 10.1016/j.atherosclerosis.2021.06.103 [DOI] [Google Scholar]
- 81.Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115(3):288–92. 10.1016/j.amjcard.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 82.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105(10):1371–7e1. [DOI] [PubMed]
- 83.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111(10):1394–400. 10.1016/j.amjcard.2013.01.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Tassell BW, Lipinski MJ, Appleton D, Roberts CS, Kontos MC, Abouzaki N, et al. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): A randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol. 2018;41(8):1004–8. 10.1002/clc.22988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9(5): e014941. 10.1161/JAHA.119.014941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36(6):377–84. 10.1093/eurheartj/ehu272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Broch K, Anstensrud AK, Woxholt S, Sharma K, Tollefsen IM, Bendz B, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77(15):1845–55. 10.1016/j.jacc.2021.02.049 [DOI] [PubMed] [Google Scholar]
- 88.Kleveland O, Ueland T, Kunszt G, Bratlie M, Yndestad A, Broch K, et al. Interleukin-6 receptor inhibition with tocilizumab induces a selective and substantial increase in plasma IP-10 and MIP-1beta in non-ST-elevation myocardial infarction. Int J Cardiol. 2018;271:1–7. 10.1016/j.ijcard.2018.04.136 [DOI] [PubMed] [Google Scholar]
- 89.Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397(10289):2060–9. 10.1016/S0140-6736(21)00520-1 [DOI] [PubMed] [Google Scholar]
- 90.Pergola PE, Devalaraja M, Fishbane S, Chonchol M, Mathur VS, Smith MT, et al. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. J Am Soc Nephrol. 2021;32(1):211–22. 10.1681/ASN.2020050595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gossec L, Strand V, Proudfoot C, Chen CI, Guillonneau S, Kimura T, et al. Effects of sarilumab on rheumatoid arthritis as reported by patients using the rheumatoid arthritis impact of disease scale. J Rheumatol. 2019;46(10):1259–67. 10.3899/jrheum.180904 [DOI] [PubMed] [Google Scholar]
- 92.Mistry P, Reid J, Pouliquen I, McHugh S, Abberley L, DeWall S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin-18 mAb GSK1070806 in healthy and obese subjects. Int J Clin Pharmacol Ther. 2014;52(10):867–79. 10.5414/CP202087 [DOI] [PubMed] [Google Scholar]
- 93.McKie EA, Reid JL, Mistry PC, DeWall SL, Abberley L, Ambery PD, Gil-Extremera B. A study to investigate the efficacy and safety of an anti-interleukin-18 monoclonal antibody in the treatment of type 2 diabetes mellitus. PLoS ONE. 2016;11(3): e0150018. 10.1371/journal.pone.0150018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bulgarelli A, Martins Dias AA, Caramelli B, Maranhao RC. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59(4):308–14. 10.1097/FJC.0b013e318241c385 [DOI] [PubMed] [Google Scholar]
- 95.Naranjo A, Sokka T, Descalzo MA, Calvo-Alen J, Horslev-Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. 10.1186/ar2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–62. 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, et al. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc. 2015;4(8): e002128. 10.1161/JAHA.115.002128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–47. 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 99.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505. 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 100.Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS Randomized Clinical Trial. Circulation. 2020;142(20):1890–900. 10.1161/CIRCULATIONAHA.120.050771 [DOI] [PubMed] [Google Scholar]
- 101.van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol. 2017;37(8):1457–61. 10.1161/ATVBAHA.117.309575 [DOI] [PubMed] [Google Scholar]
- 102.Ren P, Wu D, Appel R, Zhang L, Zhang C, Luo W, et al. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J Am Heart Assoc. 2020;9(7): e014044. 10.1161/JAHA.119.014044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Niu X, Xu H, Li Q, Meng L, He M, et al. VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp Cell Res. 2020;389(1): 111847. 10.1016/j.yexcr.2020.111847 [DOI] [PubMed] [Google Scholar]
- 104.Shah F, Greene N. Analysis of Pfizer compounds in EPA’s ToxCast chemicals-assay space. Chem Res Toxicol. 2014;27(1):86–98. 10.1021/tx400343t [DOI] [PubMed] [Google Scholar]
- 105.Abderrazak A, Couchie D, Mahmood DF, Elhage R, Vindis C, Laffargue M, et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation. 2015;131(12):1061–70. [DOI] [PubMed]
- 106.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33(2):187–215. 10.1210/er.2011-1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaneto H, Obata A, Kimura T, Shimoda M, Kinoshita T, Matsuoka TA, Kaku K. Unexpected pleiotropic effects of sglt2 inhibitors: pearls and pitfalls of this novel antidiabetic class. Int J Mol Sci. 2021;22(6). [DOI] [PMC free article] [PubMed]
- 108.Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. 10.1038/s41467-020-15983-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X, Huang Q, Feng J, Xiao Z, Zhang X, Zhao L. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-kappaB signalling pathway. J Int Med Res. 2021;49(2):300060521992981. 10.1177/0300060521992981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 2020;17(12): e1003432. 10.1371/journal.pmed.1003432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruyssen-Witrand A, Fautrel B, Saraux A, Le Loet X, Pham T. Cardiovascular risk induced by low-dose corticosteroids in rheumatoid arthritis: a systematic literature review. Joint Bone Spine. 2011;78(1):23–30. 10.1016/j.jbspin.2010.02.040 [DOI] [PubMed] [Google Scholar]
- 112.Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–301. 10.1016/S0140-6736(23)00215-5 [DOI] [PubMed] [Google Scholar]
- 113.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 115.Marston NA, Giugliano RP, Park JG, Ruzza A, Sever PS, Keech AC, Sabatine MS. Cardiovascular benefit of lowering low-density lipoprotein cholesterol below 40 mg/dL. Circulation. 2021;144(21):1732–4. 10.1161/CIRCULATIONAHA.121.056536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Group CT. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–42. 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- 117.Corna G, Golino M, Moroni F, Del Buono MG, Talasaz AH, Decotto S, et al. Response to interleukin-1 blockade with Anakinra in Black and White Americans with ST-segment elevation myocardial infarction. Am J Cardiol. 2023;207:336–8. 10.1016/j.amjcard.2023.08.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol. 2011;107(6):906–11. 10.1016/j.amjcard.2010.11.005 [DOI] [PubMed] [Google Scholar]