Abstract
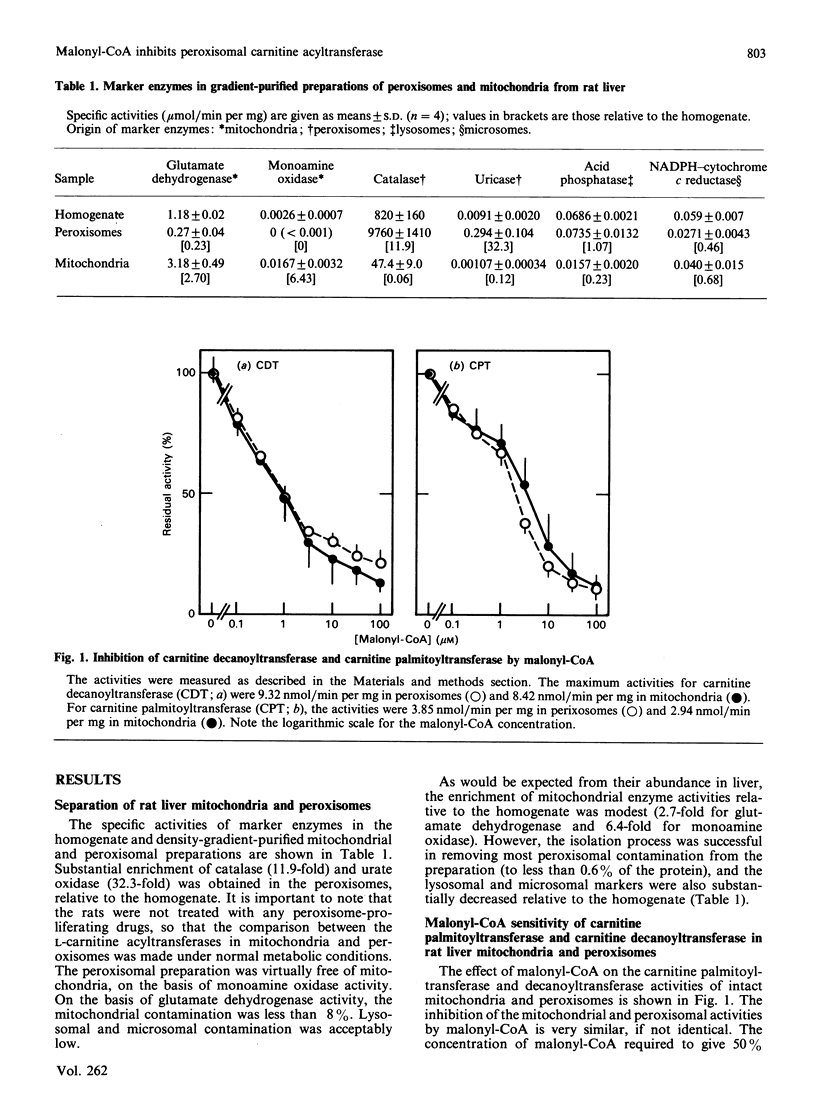
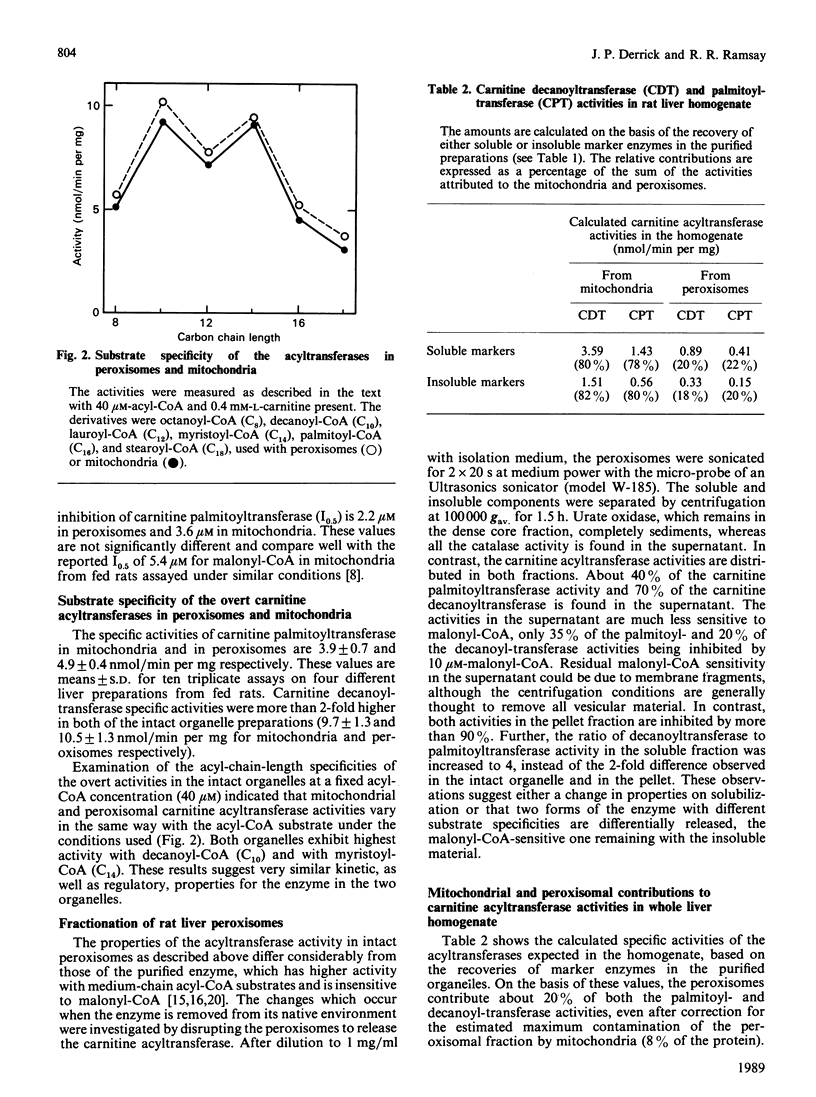
Inhibition of the overt mitochondrial carnitine palmitoyltransferase by malonyl-CoA is important in the regulation of fatty acid oxidation. In the past, the contribution of peroxisomal carnitine acyltransferase activity to the generation of medium- and long-chain acylcarnitines in the cytoplasm has been ignored. On the basis of marker enzyme levels, we now estimate that peroxisomal palmitoyltransferase activity constitutes about 20% of the peroxisomal plus overt-mitochondrial pool in fed rat liver. When assayed in situ, both the palmitoyltransferase and decanoyltransferase activities of gradient-purified peroxisomes are sensitive to malonyl-CoA, with up to 90% inhibition reached at less than 10 microM-malonyl-CoA. Very similar results were obtained with intact gradient-purified mitochondria from the same livers. In addition, the acyl-CoA substrate chain-length specificity was identical in both the peroxisomes and the mitochondria, with a decanoyltransferase/palmitoyltransferase ratio of 2. Thus the overt carnitine acyltransferase activities in peroxisomes and mitochondria have the same properties. Further, the malonyl-CoA sensitivity of the peroxisomal activity is lost on solubilization, as has been observed for the overt mitochondrial enzyme. It is suggested that malonyl-CoA inhibition of the peroxisomal enzyme as well as of the mitochondrial enzyme is important for the regulation of mitochondrial fatty acid oxidation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L., Krahling J. B., Clarke P. R., Valkner K. J., Tolbert N. E. Carnitine acyltransferases in rat liver peroxisomes. Arch Biochem Biophys. 1981 Oct 15;211(2):599–604. doi: 10.1016/0003-9861(81)90494-x. [DOI] [PubMed] [Google Scholar]
- Brady P. S., Marine K. A., Brady L. J., Ramsay R. R. Co-ordinate induction of hepatic mitochondrial and peroxisomal carnitine acyltransferase synthesis by diet and drugs. Biochem J. 1989 May 15;260(1):93–100. doi: 10.1042/bj2600093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Cox K. A. Hysteretic behaviour of carnitine palmitoyltransferase. The effect of preincubation with malonyl-CoA. Biochem J. 1986 Jun 15;236(3):917–919. doi: 10.1042/bj2360917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S. O., Bieber L. L. Carnitine octanoyltransferase of mouse liver peroxisomes: properties and effect of hypolipidemic drugs. Arch Biochem Biophys. 1983 Apr 1;222(1):123–132. doi: 10.1016/0003-9861(83)90509-x. [DOI] [PubMed] [Google Scholar]
- Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J Biol Chem. 1984 Nov 10;259(21):13089–13095. [PubMed] [Google Scholar]
- Ghosh M. K., Hajra A. K. A rapid method for the isolation of peroxisomes from rat liver. Anal Biochem. 1986 Nov 15;159(1):169–174. doi: 10.1016/0003-2697(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Lloyd A. C., Carpenter C. A., Saggerson E. D. Intertissue differences in the hysteretic behaviour of carnitine palmitoyltransferase in the presence of malonyl-CoA. Biochem J. 1986 Jul 1;237(1):289–291. doi: 10.1042/bj2370289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Bieber L. L., Tolbert N. E. Differential increase of hepatic peroxisomal, mitochondrial and microsomal carnitine acyltransferases in clofibrate-fed rats. Biochem Pharmacol. 1977 Sep 15;26(18):1697–1702. doi: 10.1016/0006-2952(77)90147-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., McGroarty E. J., Bieber L. L., Tolbert N. E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973 May 25;248(10):3426–3432. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Furuta S., Osumi T., Hashimoto T. Purification and properties of carnitine acetyltransferase from rat liver. J Biochem. 1983 Feb;93(2):439–451. doi: 10.1093/oxfordjournals.jbchem.a134198. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Krahling J. B., Gee R., Kirk J. R., Tolbert N. E. Enzyme activities of isolated hepatic peroxisomes from genetically lean and obese male mice. Arch Biochem Biophys. 1979 Mar;193(1):179–185. doi: 10.1016/0003-9861(79)90021-3. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neat C. E., Thomassen M. S., Osmundsen H. Induction of peroxisomal beta-oxidation in rat liver by high-fat diets. Biochem J. 1980 Jan 15;186(1):369–371. doi: 10.1042/bj1860369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. C., Buege J. A., Aust S. D. Microsomal electron transport. The role of reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase in liver microsomal lipid peroxidation. J Biol Chem. 1973 Oct 25;248(20):7134–7141. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Derrick J. P., Friend A. S., Tubbs P. K. Purification and properties of the soluble carnitine palmitoyltransferase from bovine liver mitochondria. Biochem J. 1987 Jun 1;244(2):271–278. doi: 10.1042/bj2440271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R. The soluble carnitine palmitoyltransferase from bovine liver. A comparison with the enzymes from peroxisomes and from the mitochondrial inner membrane. Biochem J. 1988 Jan 1;249(1):239–245. doi: 10.1042/bj2490239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R., Tubbs P. K. The mechanism of fatty acid uptake by heart mitochondria: an acylcarnitine-carnitine exchange. FEBS Lett. 1975 Jun 1;54(1):21–25. doi: 10.1016/0014-5793(75)81059-3. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salach J. I., Weyler W. Preparation of the flavin-containing aromatic amine oxidases of human placenta and beef liver. Methods Enzymol. 1987;142:627–637. doi: 10.1016/s0076-6879(87)42075-2. [DOI] [PubMed] [Google Scholar]
- Vamecq J. Chlorpromazine and carnitine-dependency of rat liver peroxisomal beta-oxidation of long-chain fatty acids. Biochem J. 1987 Feb 1;241(3):783–791. doi: 10.1042/bj2410783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. Carnitine palmitoyltransferase activities (EC 2.3.1.-) of rat liver mitochondria. Biochem J. 1970 Sep;119(3):547–552. doi: 10.1042/bj1190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. The partial latency and intramitochondrial distribution of carnitine-palmitoyltransferase (e.c.2.3.1.-), and the CoASH and carnitine permeable space of rat liver mitochondria. Biochem Biophys Res Commun. 1966 May 25;23(4):460–465. doi: 10.1016/0006-291x(66)90750-9. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. Reversible sensitization and desensitization of carnitine palmitoyltransferase I to inhibition by malonyl-CoA in isolated rat liver mitochondria. Significance for the mechanism of malonyl-CoA-induced sensitization. Biochem J. 1983 Sep 15;214(3):1027–1030. doi: 10.1042/bj2141027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


