Abstract
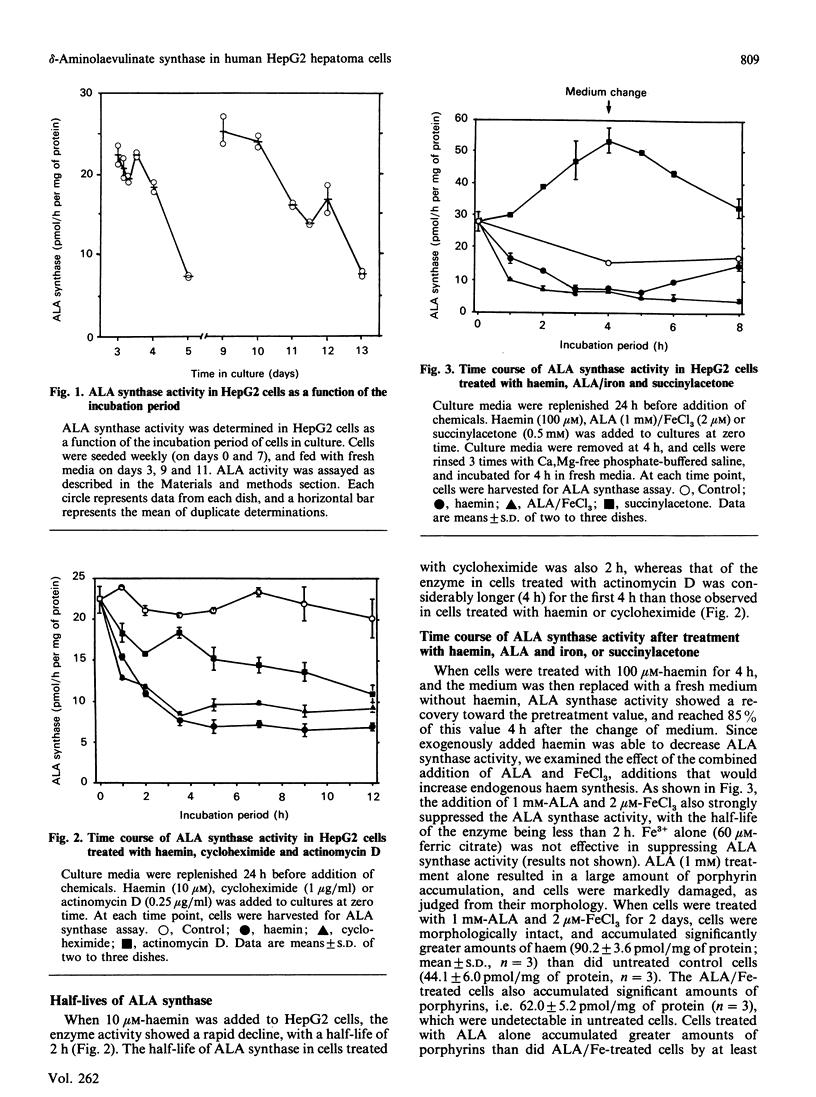
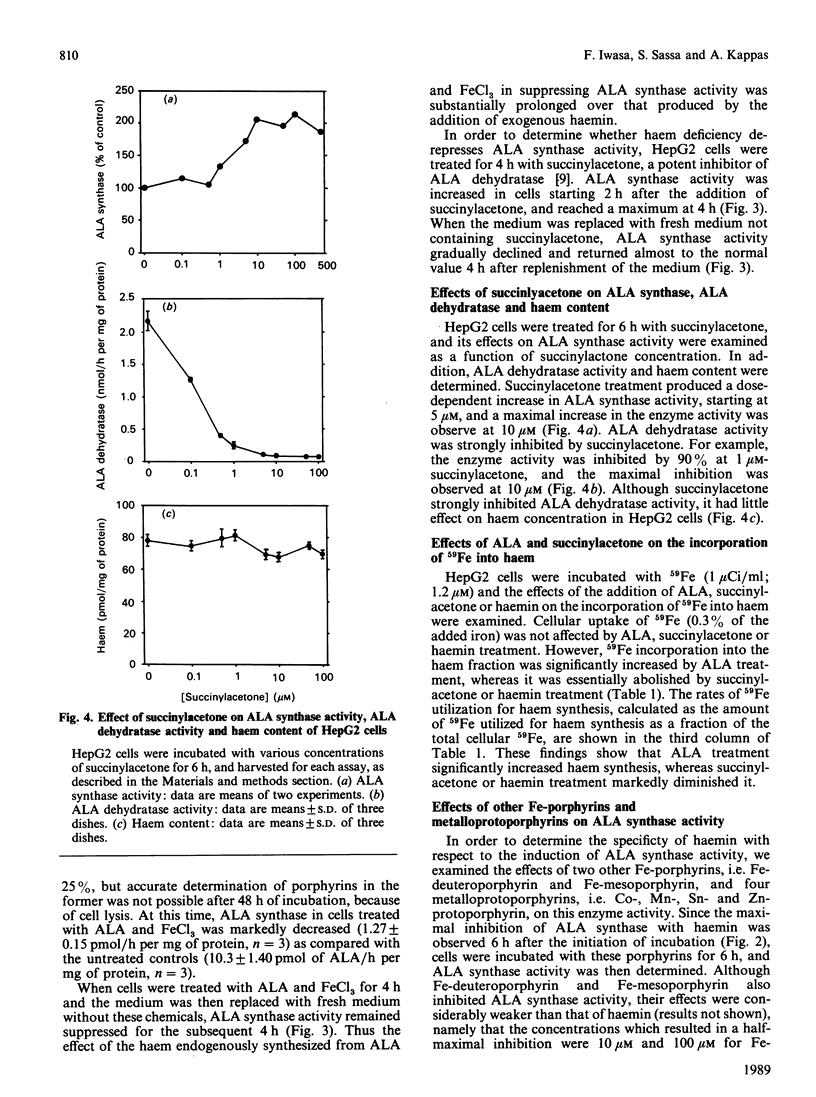
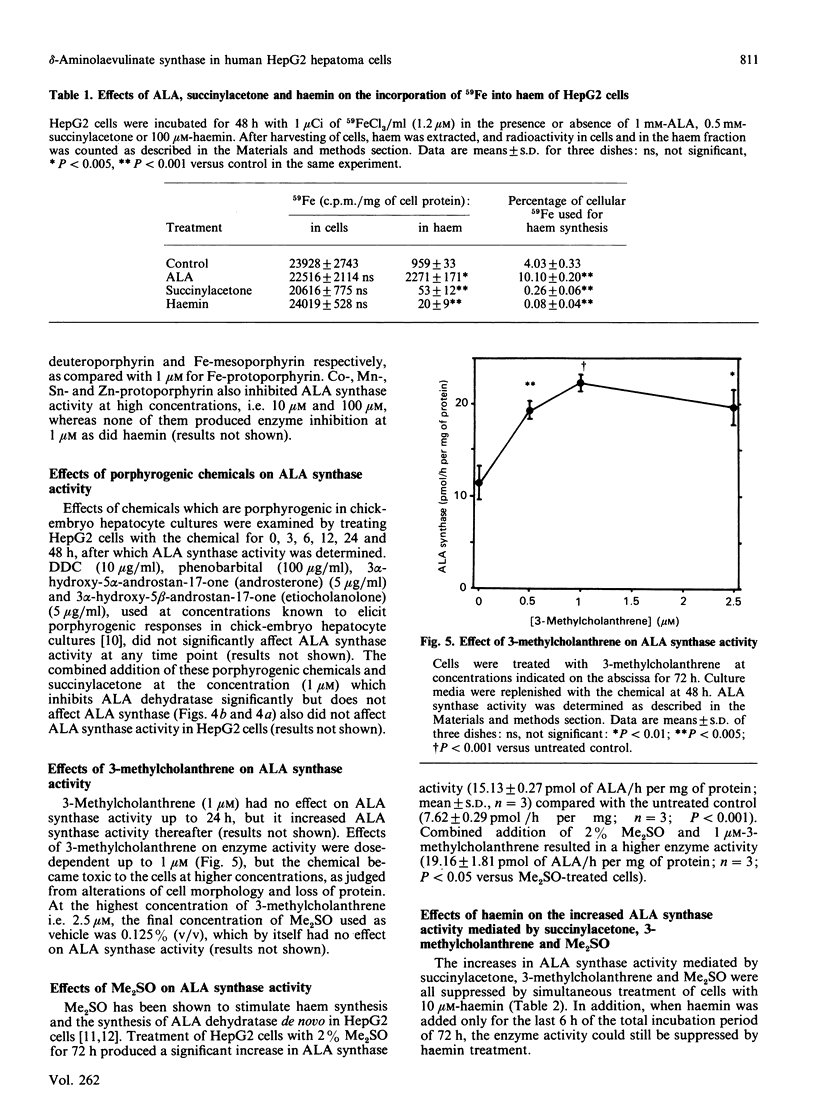
delta-Aminolaevulinate (ALA) synthase, the rate-limiting enzyme in haem biosynthesis in the normal liver, was examined in human HepG2 hepatoma cells. Haemin, up to 100 microM, had no effect on ALA synthase activity in vitro; it did, however, exhibit a dose-dependent inhibitory action when added to cells growing in culture (half-maximal inhibition at 1 microM). The half-life of ALA synthase activity after haemin treatment was 2 h, which was similar to that found after treatment with cycloheximide. Cells treated with actinomycin D showed a longer half-life of the enzyme activity, i.e. 4 h, compared with haemin or cycloheximide treatment. Treatment of cells with succinylacetone markedly inhibited the activity of ALA dehydratase and 59Fe incorporation into haem, but in increased ALA synthase activity. Both the haemin-induced repression and the succinylacetone-mediated de-repression of ALA synthase activity were reversible within 4 h after replacing the medium with fresh medium without the chemical. In addition to succinylacetone, dimethyl sulphoxide and 3-methylcholanthrene induced the enzyme. Induction of ALA synthase by these chemicals was also suppressed by treatment of cells with haemin. These findings indicate that the level of ALA synthase in HepG2 cells is maintained by both synthesis and degradation of the enzyme, and that the synthesis of the enzyme is regulated by the concentration of regulatory free haem in the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker J. D., Srivastava G., May B. K., Elliott W. H. Radiochemical assay for gamma-aminolevulinate synthase. Enzyme. 1982;28(2-3):109–119. doi: 10.1159/000459095. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Galbraith R. A., Sassa S., Fujita H. Regulation of heme synthesis in HepG2 human hepatoma cells by dimethyl sulfoxide. Biochem Biophys Res Commun. 1988 Jun 16;153(2):869–874. doi: 10.1016/s0006-291x(88)81176-8. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Sassa S., Kappas A. Induction of haem synthesis in Hep G2 human hepatoma cells by dimethyl sulphoxide. A transcriptionally activated event. Biochem J. 1986 Jul 15;237(2):597–600. doi: 10.1042/bj2370597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick J. L., Sassa S. Hemin control of heme biosynthesis in mouse Friend virus-transformed erythroleukemia cells in culture. J Biol Chem. 1978 Aug 10;253(15):5402–5406. [PubMed] [Google Scholar]
- Guzelian P. S., O'Connor L., Fernandez S., Chan W., Giampietro P., Desnick R. Rapid loss of delta-aminolevulinic acid dehydratase activity in primary cultures of adult rat hepatocytes: a new model of zinc deficiency. Life Sci. 1982 Sep 13;31(11):1111–1116. doi: 10.1016/0024-3205(82)90084-4. [DOI] [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Gillette P. N., Song C. S., Kappas A. Drug stimulation of -aminolevulinic acid synthetase and cytochrome P-450 in vivo in chick embryo liver. J Pharmacol Exp Ther. 1973 May;185(2):214–225. [PubMed] [Google Scholar]
- Sassa S., Bradlow H. L., Kappas A. Steroid induction of delta-aminolevulinic acid synthase and porphyrins in liver. Structure-activity studies and the permissive effects of hormones on the induction process. J Biol Chem. 1979 Oct 25;254(20):10011–10020. [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983 Mar;71(3):625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Sassa S., Schwartz S., Ruth G. Accumulation of protoporphyrin IX from delta-aminolevulinic acid in bovine skin fibroblasts with hereditary erythropoietic protoporphyria. A gene-dosage effect. J Exp Med. 1981 May 1;153(5):1094–1101. doi: 10.1084/jem.153.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Sugita O., Galbraith R. A., Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987 Feb 27;143(1):52–57. doi: 10.1016/0006-291x(87)90628-0. [DOI] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Poh-Fitzpatrick M. B., Anderson K. E., Kappas A. Studies in porphyria: functional evidence for a partial deficiency of ferrochelatase activity in mitogen-stimulated lymphocytes from patients with erythropoietic protoporphyria. J Clin Invest. 1982 Apr;69(4):809–815. doi: 10.1172/JCI110520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Poh-Fitzpatrick M. B., Kappas A. Studies in porphyria IX: Detection of the gene defect of erythropoietic protoporphyria in mitogen-stimulated human lymphocytes. Trans Assoc Am Physicians. 1979;92:268–276. [PubMed] [Google Scholar]
- Siepker L. J., Kramer S. Protoporphyrin accumulation by mitogen stimulated lymphocytes and protoporphyrinogen oxidase activity in patients with porphyria variegata and erythropoietic protoporphyria: evidence for deficiency of protoporphyrinogen oxidase and ferrochelatase in both diseases. Br J Haematol. 1985 May;60(1):65–74. doi: 10.1111/j.1365-2141.1985.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Kurata N., Kohno M., Yoshida T., Kuroiwa Y. Induction of cytochrome P-450 and related drug-oxidizing activities in muscone (3-methylcyclopentadecanone)-treated rats. Biochem Pharmacol. 1987 Dec 15;36(24):4263–4267. doi: 10.1016/0006-2952(87)90668-x. [DOI] [PubMed] [Google Scholar]
- Wada O., Sassa S., Takaku F., Yano Y., Uratta G., Nakao K. Different responses of the hepatic and erythropoietic delta-aminolevulinic acid synthetase of mice. Biochim Biophys Acta. 1967 Nov 28;148(2):585–587. doi: 10.1016/0304-4165(67)90165-1. [DOI] [PubMed] [Google Scholar]
- Woods J. S., Murthy V. V. Delta-aminolevulinic acid synthetase from fetal rat liver: studies on the partially purified enzyme. Mol Pharmacol. 1975 Jan;11(1):70–78. [PubMed] [Google Scholar]
- Yamamoto M., Kure S., Engel J. D., Hiraga K. Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J Biol Chem. 1988 Nov 5;263(31):15973–15979. [PubMed] [Google Scholar]


