Abstract
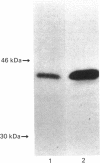
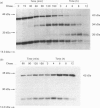
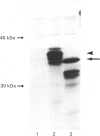
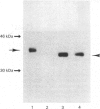
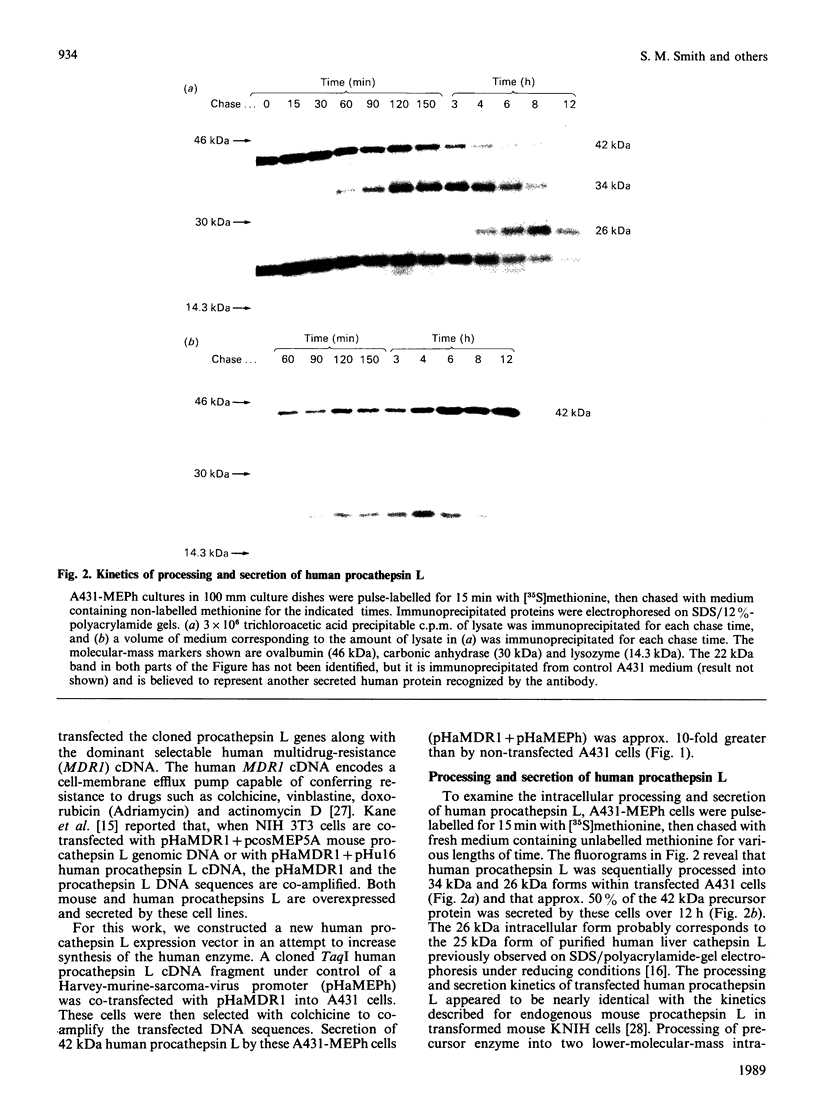
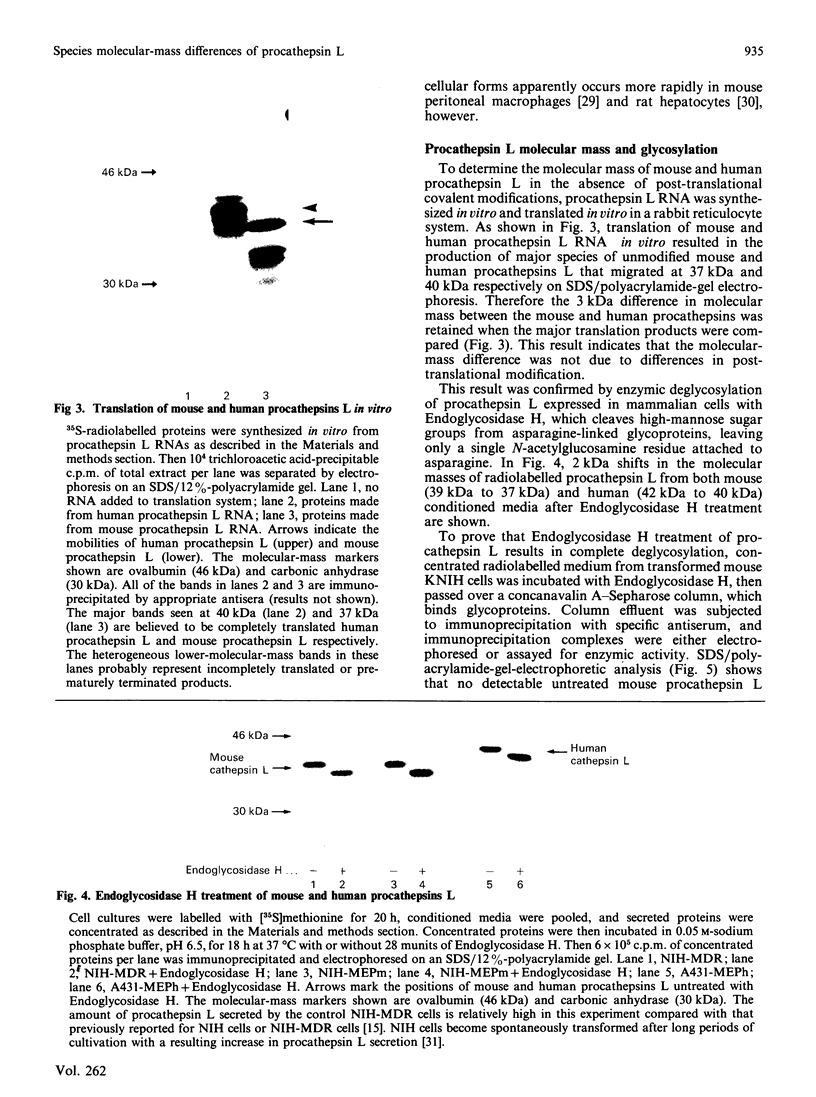
Cathepsin L is a major lysosomal cysteine proteinase in mouse and human cells. Despite similar predicted molecular masses, procathepsin L in these two species migrates on SDS/polyacrylamide gels with apparent molecular masses of 39 kDa and 42 kDa respectively. To determine if glycosylation differences account for this discrepancy, and to ascertain whether glycosylation is essential for enzymic activity, mouse and human procathepsins L were expressed at high concentrations in mouse NIH 3T3 cells or in human A431 cells after DNA-mediated transfection of cloned DNAs for these enzymes. In pulse-chase studies, human procathepsin L transfectants synthesized and secreted large amounts of enzymically active 42 kDa proenzyme and processed it into 34 kDa and 26 kDa intracellular peptides, a pattern of secretion and processing similar to that seen with endogenous or transfected mouse procathepsin L. Both translation of cloned procathepsin L cDNAs in vitro and Endoglycosidase H treatment of 39 kDa mouse and 42 kDa human procathepsin L resulted in non-glycosylated proteins 2 kDa lower in molecular mass than the untreated proteins for both species. This suggests that glycosylation differences are not responsible for the molecular-mass disparity between the two species. Moreover, Endoglycosidase H-treated mouse enzyme retained full proteolytic activity, indicating that glycosylation of cathepsin L is not essential for enzymic function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. The determination of similarities in amino acid composition among proteins separated by two-dimensional gel electrophoresis. Anal Biochem. 1978 Dec;91(2):548–556. doi: 10.1016/0003-2697(78)90542-0. [DOI] [PubMed] [Google Scholar]
- Cantz M., Gehler J. The mucopolysaccharidoses: inborn errors of glycosaminoglycan catabolism. Hum Genet. 1976 Jun 29;32(3):233–255. doi: 10.1007/BF00295816. [DOI] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. Isolation and sequence of a cDNA for human pro-(cathepsin L). Biochem J. 1988 Jul 1;253(1):303–306. doi: 10.1042/bj2530303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Cabral F. Purification and characterization of a transformation-dependent protein secreted by cultured murine fibroblasts. Biochemistry. 1981 Mar 17;20(6):1659–1665. doi: 10.1021/bi00509a039. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Troen B. R., Gal S., Ueda K., Pastan I., Gottesman M. M. Use of a cloned multidrug resistance gene for coamplification and overproduction of major excreted protein, a transformation-regulated secreted acid protease. Mol Cell Biol. 1988 Aug;8(8):3316–3321. doi: 10.1128/mcb.8.8.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang L., Reitman M., Tang J., Roberts R. M., Kornfeld S. Lysosomal enzyme phosphorylation. Recognition of a protein-dependent determinant allows specific phosphorylation of oligosaccharides present on lysosomal enzymes. J Biol Chem. 1984 Dec 10;259(23):14663–14671. [PubMed] [Google Scholar]
- Marx J. L. A new wave of enzymes for cleaving prohormones. Science. 1987 Jan 16;235(4786):285–286. doi: 10.1126/science.2879353. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Gal S., Gottesman M. M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem J. 1987 Dec 1;248(2):449–454. doi: 10.1042/bj2480449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Taylor M. A., Etherington D. J. The purification and properties of cathepsin L from rabbit liver. Biochem J. 1984 Jan 1;217(1):209–217. doi: 10.1042/bj2170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K., Gal S., Schwartz R. H., Gottesman M. M. An acid protease secreted by transformed cells interferes with antigen processing. J Cell Biol. 1988 Jun;106(6):1879–1884. doi: 10.1083/jcb.106.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset M., Capony F., Rochefort H. The 52-kDa estrogen-induced protein secreted by MCF7 cells is a lysosomal acidic protease. Biochem Biophys Res Commun. 1986 Jul 16;138(1):102–109. doi: 10.1016/0006-291x(86)90252-4. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Leduc M., Recklies A. D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981 Dec 15;662(2):173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Characterization of a thiol proteinase secreted by malignant human breast tumours. Biochim Biophys Acta. 1980 Jul 10;614(1):134–143. doi: 10.1016/0005-2744(80)90174-6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Furuno K., Kato K. Biosynthesis and processing of lysosomal cathepsin L in primary cultures of rat hepatocytes. Arch Biochem Biophys. 1988 May 15;263(1):107–116. doi: 10.1016/0003-9861(88)90618-2. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987 May 28;316(22):1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Sahagian G. G., Gottesman M. M. The predominant secreted protein of transformed murine fibroblasts carries the lysosomal mannose 6-phosphate recognition marker. J Biol Chem. 1982 Sep 25;257(18):11145–11150. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Troen B. R., Ascherman D., Atlas D., Gottesman M. M. Cloning and expression of the gene for the major excreted protein of transformed mouse fibroblasts. A secreted lysosomal protease regulated by transformation. J Biol Chem. 1988 Jan 5;263(1):254–261. [PubMed] [Google Scholar]
- Yamaguchi N., Kawai K. Acid protease secreted from human pancreatic carcinoma cell line HPC-YT into serum-free, chemically defined medium. Cancer Res. 1986 Oct;46(10):5353–5359. [PubMed] [Google Scholar]