Abstract
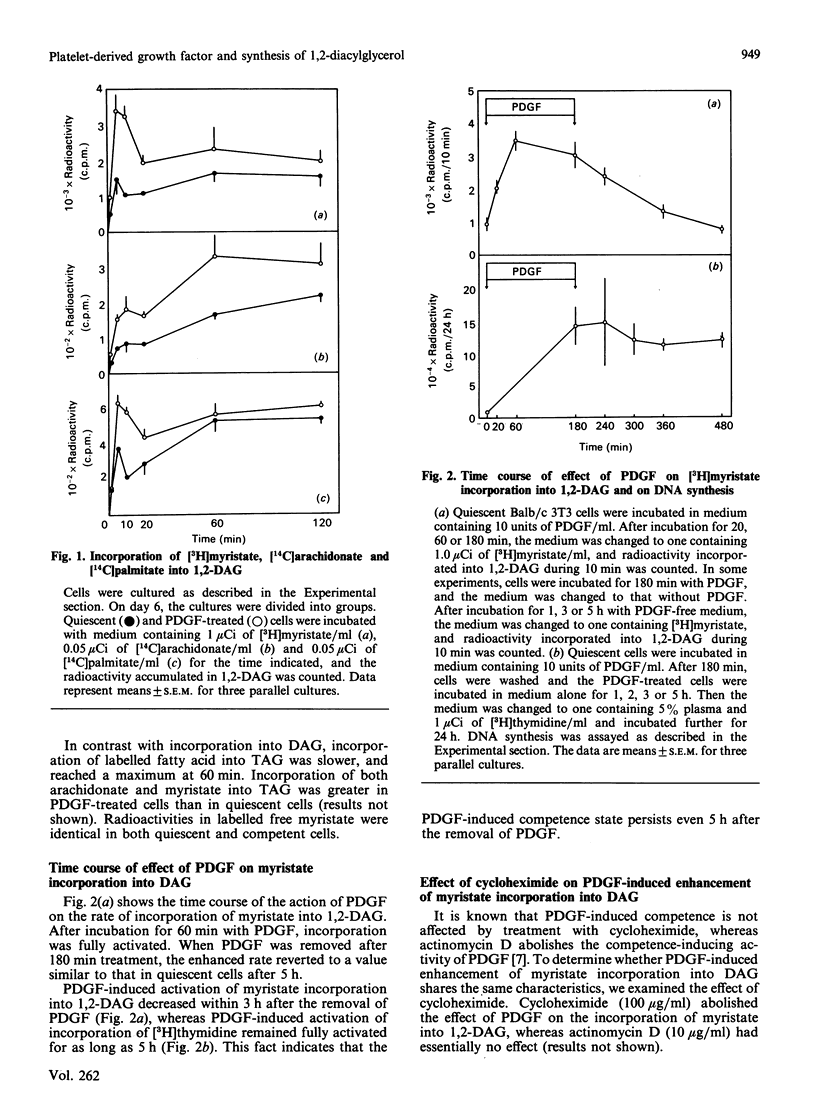
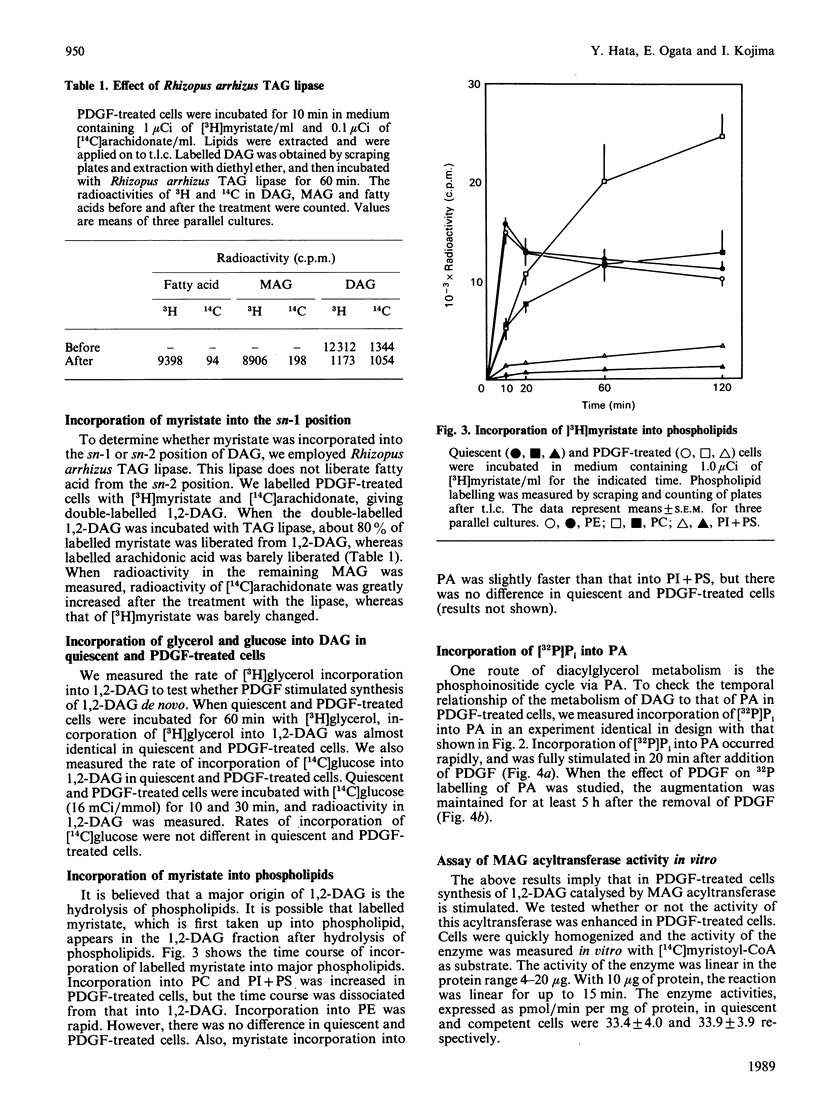
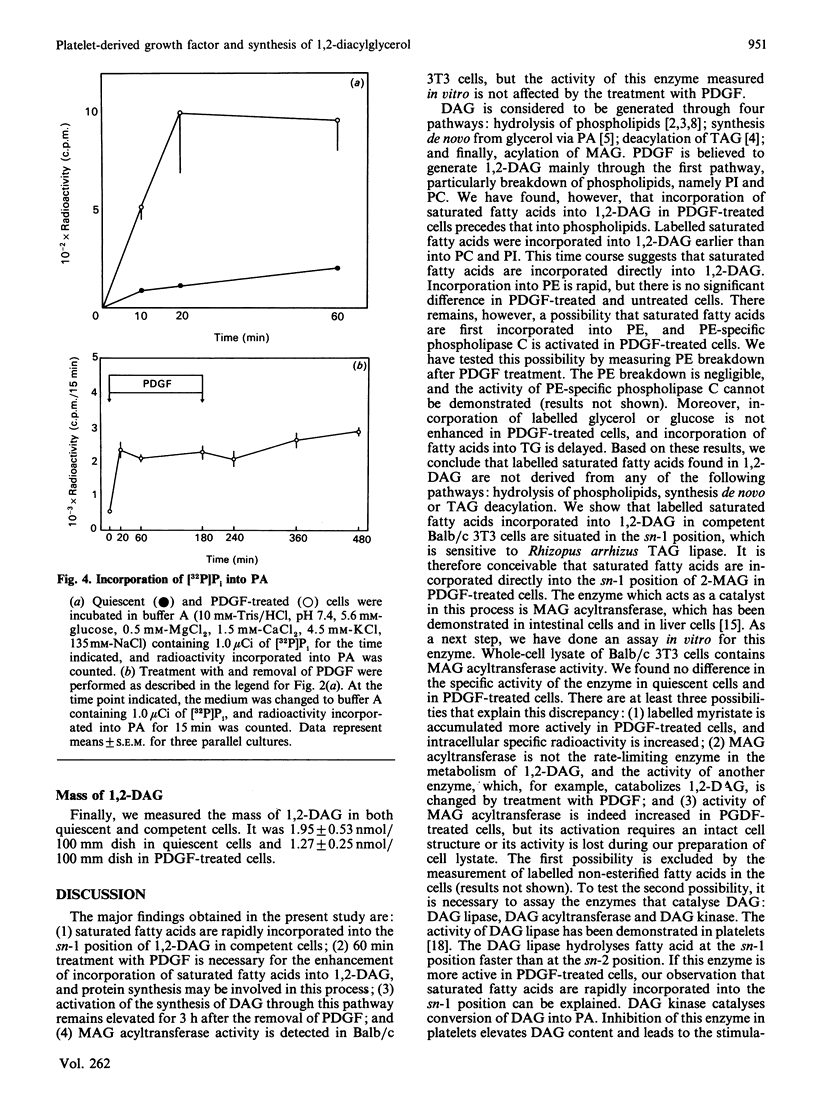
1,2-Diacylglycerol (1,2,-DAG) plays an important role in the protein kinase C-mediated signal-transduction system. Several reports have shown that 1,2-DAG is generated through various pathways other than classical phospholipid hydrolysis. We observed a rapid incorporation of [3H]myristate into 1,2-DAG in platelet-derived-growth-factor (PDGF)-treated Balb/c 3T3 cells. [14C]Palmitate was similarly incorporated into 1,2-DAG. The effect of PDGF, which was inhibited by cycloheximide, became maximal after 60 min treatment with PDGF, and disappeared 300 min after removal of PDGF. Treatment with triacylglycerol lipase revealed that labelled saturated fatty acid was incorporated into the sn-1 position. PDGF barely stimulated incorporation of [3H]glycerol or [14C]glucose into 1,2-DAG. Incorporation of [3H]myristate into 1,2-DAG preceded that into triacyglycerol and phospholipids. These results suggest that synthesis of 1,2-DAG from monoacylglycerol is enhanced in PDGF-treated cells. However, there is no significant difference in the activity of monoacylglycerol acyltransferase measured in vitro in quiescent and PDGF-treated cells. The reason for this discrepancy is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., O'Brien J. F. Use of (1-14C) aectic anhydride to quantitate diglycerides: a new analytical procedure. Anal Biochem. 1974 Jun;59(2):617–627. doi: 10.1016/0003-2697(74)90315-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Coleman R. A., Haynes E. B. Hepatic monoacylglycerol acyltransferase. Characterization of an activity associated with the suckling period in rats. J Biol Chem. 1984 Jul 25;259(14):8934–8938. [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Wykle R. L. A novel mechanism of diglyceride formation. 12-O-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986 Jul 15;261(20):9128–9132. [PubMed] [Google Scholar]
- Denning G. M., Figard P. H., Kaduce T. L., Spector A. A. Role of triglycerides in endothelial cell arachidonic acid metabolism. J Lipid Res. 1983 Aug;24(8):993–1001. [PubMed] [Google Scholar]
- Farese R. V., Konda T. S., Davis J. S., Standaert M. L., Pollet R. J., Cooper D. R. Insulin rapidly increases diacylglycerol by activating de novo phosphatidic acid synthesis. Science. 1987 May 1;236(4801):586–589. doi: 10.1126/science.3107122. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Goerig M., Gronwald R., Grulich J., Loth U., Schettler G. Cell cycle-dependent changes in arachidonic acid and glycerol metabolism in Swiss 3T3 cells stimulated by platelet-derived growth factor. J Biol Chem. 1985 Feb 10;260(3):1370–1373. [PubMed] [Google Scholar]
- McNeil P. L., McKenna M. P., Taylor D. L. A transient rise in cytosolic calcium follows stimulation of quiescent cells with growth factors and is inhibitable with phorbol myristate acetate. J Cell Biol. 1985 Aug;101(2):372–379. doi: 10.1083/jcb.101.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I., Hata Y., Ogata E., Kojima I. Insulin-like growth factor II stimulates calcium influx in competent BALB/c 3T3 cells primed with epidermal growth factor. Characteristics of calcium influx and involvement of GTP-binding protein. J Biol Chem. 1987 Sep 5;262(25):12120–12126. [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Shier W. T. Serum stimulation of phospholipase A2 and prostaglandin release in 3T3 cells is associated with platelet-derived growth-promoting activity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):137–141. doi: 10.1073/pnas.77.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kikkawa U., Kaibuchi K., Nishizuka Y. Membrane phospholipid metabolism and signal transduction for protein phosphorylation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:119–158. [PubMed] [Google Scholar]


