Abstract
Introduction
Lebrikizumab is a novel monoclonal antibody with established efficacy in patients with moderate-to-severe atopic dermatitis (AD) in multiple Phase 3 trials. One of the ultimate treatment goals for patients with moderate-to-severe AD is to achieve stable disease control without concern for planning future life events.
Methods
In ADvocate1 and ADvocate2, lebrikizumab-treated patients meeting the protocol-defined response criteria at Week 16 were re-randomized 2:2:1 to receive lebrikizumab every 2 weeks (Q2W), lebrikizumab every 4 weeks (Q4W), or placebo Q2W (lebrikizumab withdrawal) for 36 additional weeks. In this post hoc analysis, we evaluated the proportions of patients with no or minimal fluctuations of efficacy during the 36-week maintenance period and plotted individual patient trajectories. We defined no or minimal fluctuations as achieving and maintaining the defined endpoint (≥ 75% improvement in the Eczema Area and Severity Index [EASI 75], ≥ 90% improvement in EASI, Pruritus Numeric Rating Scale [NRS] ≥ 4-point improvement, or Pruritus NRS ≥ 3-point improvement) for ≥ 80% of the study visits. If patients used rescue medication, discontinued treatment, or transferred to the escape arm, data collected at or after the event were imputed as non-response.
Results
The proportions of lebrikizumab responders who maintained EASI 75 with no or minimal fluctuations were 70.8% (lebrikizumab Q2W), 71.2% (lebrikizumab Q4W), and 60.0% (lebrikizumab withdrawal). Of the patients with baseline Pruritus NRS ≥ 4 and who achieved ≥ 4-point improvement at Week 16, 66.1% (lebrikizumab Q2W), 62.7% (lebrikizumab Q4W), and 55.2% (lebrikizumab withdrawal) maintained ≥ 4-point Pruritus NRS improvement with no or minimal fluctuations.
Conclusions
Patients who met the response criteria at Week 16 and continued treatment with lebrikizumab Q2W or Q4W demonstrated a stable response with no or minimal fluctuations of efficacy in measures of skin and itch up to Week 52.
Clinical Trial Registration
NCT04146363 (ADvocate1) and NCT04178967 (ADvocate2).
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01226-9.
Keywords: Atopic dermatitis, Eczema, Fluctuations, Lebrikizumab, Response, Stability
Plain Language Summary
Atopic dermatitis, also known as atopic eczema (or just eczema), is a common skin disease that causes itchy, dry skin. Patients with eczema are often unsure of when disease flares will happen, even while receiving treatment. In two global studies, ADvocate1 and ADvocate2, lebrikizumab improved the signs and symptoms of moderate-to-severe eczema after 16 weeks of treatment. Most of these patients also saw improvement up to 52 weeks. We wanted to know if patients continued to feel better between Week 16 and Week 52. Patients who responded to lebrikizumab after 16 weeks were given lebrikizumab every 2 weeks, lebrikizumab every 4 weeks, or placebo every 2 weeks. We tested how many patients experienced stable response to therapy, which we said was maintaining the same level of improvement on skin signs and itch symptoms for at least 80% of study visits from Week 16 to Week 52. In patients treated with lebrikizumab every 2 weeks or every 4 weeks, we saw that about seven of every ten patients maintained a stable response in skin improvement and about six of every ten patients maintained stable response in itch symptoms. In patients who stopped lebrikizumab therapy, six out of every ten patients maintained a stable skin improvement and more than five of every ten patients maintained a stable improvement in itch symptoms. In ADvocate1 and ADvocate2, most lebrikizumab-treated patients showed a stable response over time on skin and itch with dosing every 2 weeks or every 4 weeks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01226-9.
Key Summary Points
| Why carry out this study?. |
| One of the ultimate treatment goals for patients with moderate-to-severe atopic dermatitis is to achieve stable disease control. |
| The purpose of this analysis is to illustrate the response trajectories of individual patients treated with lebrikizumab and report the proportions of patients who demonstrated a stable response with no or minimal fluctuations of efficacy from Week 16 to Week 52 of treatment. |
| What was learned from the study?. |
| In patients with moderate-to-severe atopic dermatitis, the major and clinically relevant response achieved on skin and itch with lebrikizumab at Week 16 is maintained with no or minimal fluctuations up to Week 52. |
| Stability of response is shown with lebrikizumab at both the every-2-week and every-4-week dose regimens. |
Digital Features
This article is published with digital features, including visual graphs to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.26124106.
Introduction
The high disease burden of moderate-to-severe atopic dermatitis (AD) and its impact on daily activities, social relationships, and quality of life in adults and adolescents are well established [1–7]. Although AD is clinically defined by the presence of pruritic and recurring eczema, the presentation of the disease is often far more complex, owing to the heterogeneous nature of the disease [8]. The location, type, and symptoms of AD lesions often differ among patients and may change with time in individual patients. Recurring, highly pruritic skin lesions in patients with moderate-to-severe AD are unpredictable, persistent, and represent a current unmet need [9]. Furthermore, conventional regulatory and clinical trial endpoints are cross-sectional in nature at a specific time point but do not address long-term control in this chronic, unstable disease.
Across chronic conditions, patients commonly report fears of illness or symptoms recurring or worsening, which can have a deleterious impact on quality of life [10]. Significant emotional and psychosocial burden can result from the uncertainty of when the next AD flare will occur which, by one study, averaged nine times per year with each flare lasting 15 days at a time [11]. In the same study, 75% of patients stated that being able to effectively control their AD would be the single most important improvement to their quality of life [11]. Given the association between inadequate disease control and disease burden [12] in patients with moderate-to-severe AD, a stable therapeutic response between injections is critical to improving patient quality of life. Although individual treatment goals vary, the unpredictability of AD suggests that one of the ultimate treatment goals for these patients is to achieve stable disease control without concern for planning future life events.
Lebrikizumab is a novel monoclonal antibody that binds with high affinity and slow off-rate to interleukin (IL)-13, thereby blocking the downstream effects of IL-13 with high potency. In patients who responded to lebrikizumab during the first 16 weeks of treatment [13], the majority showed a durable, robust response through Week 52 [14]. Here, we illustrate the response trajectories of individual patients and report the proportions of patients who demonstrated a stable response with no or minimal fluctuations of efficacy from Week 16 to Week 52 of treatment.
Methods
Study Design and Patient Population
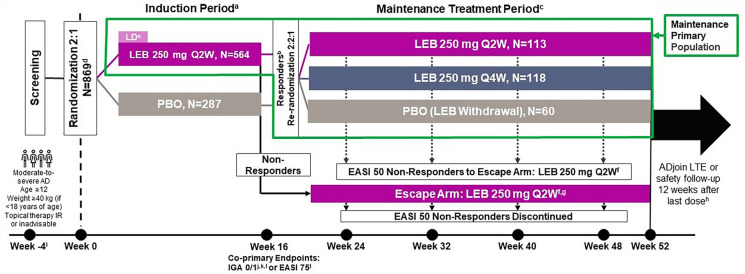
Full details and all pre-specified primary outcomes of the ADvocate1 (NCT04146363) and ADvocate2 (NCT04178967) trials were previously reported [13, 14]. Briefly, these were identically designed, randomized, double-blind, placebo-controlled, parallel-group, 52-week Phase 3 trials evaluating the efficacy and safety of lebrikizumab monotherapy in adults and adolescents with moderate-to-severe AD. A visual of the study design for ADvocate1 and ADvocate2 is included in Fig. 1. During the 16-week induction period, patients were randomly assigned in a 2:1 ratio to receive either lebrikizumab 250 mg or placebo every 2 weeks (Q2W) with a loading dose given at baseline and Week 2. After 16 weeks of treatment, patients were assessed for response, which was defined as achieving ≥ 75% improvement in the Eczema Area and Severity Index (EASI 75) or an Investigator’s Global Assessment score of 0 or 1 with a ≥ 2-point improvement (IGA 0/1) without the use of rescue medication. Patients who met this response criterion were re-randomized in a 2:2:1 ratio to receive lebrikizumab 250 mg Q2W, lebrikizumab 250 mg every 4 weeks (Q4W), or placebo Q2W (lebrikizumab withdrawal) for a period of 36 weeks. The analysis described in this manuscript includes lebrikizumab-treated patients who met the criteria for response at Week 16, were re-randomized, and continued the study for an additional 36 weeks.
Fig. 1.
Study design for ADvocate1 and ADvocate2. aUse of topical/systemic treatments for AD prohibited. bResponders were patients who achieved an IGA 0/1 with a ≥ 2-point improvement or EASI 75, without use of rescue medication. cUse of intermittent topical rescue medications for AD was permitted. Responders who received PBO during induction and who were re-randomized to LEB received a LD of either 500 mg given at Week 16 or 500 mg given at Week 16 and Week 18, based on their treatment assignment. dFour hundred twenty-four patients (ADvocate1) and 445 patients (ADvocate2) with moderate-to-severe AD. eFive hundred mg LD at Week 0 and Week 2. fMaintenance of response assessed by EASI 50 at Week 24, Week 32, Week 40, and Week 48, respectively. Patients receiving systemic rescue medication will be required to washout for five half-lives prior to initiating treatment in the escape arm. gParticipants who are eligible for the escape arm at Week 16 will receive blinded LD at Week 16 and Week 18, based on their prior treatment assignment. hPatients completing ADvocate 1 or ADvocate2 will be offered treatment in ADjoin. Otherwise, patients will participate in a safety follow-up 12 weeks after their last dose; i ≤ 30-day screening period. jIGA 0/1 with ≥ 2-point improvement from baseline. kFDA primary endpoint. lEMA co-primary endpoint. AD atopic dermatitis, EASI Eczema Area and Severity Index, EASI 50 ≥ 50% improvement in EASI, EASI 75 ≥ 75% improvement in EASI, EMA European Medicines Agency, FDA Food and Drug Administration, IGA 0/1 Investigator’s Global Assessment of 0 or 1, IR inadequate responder, LD loading dose, LEB lebrikizumab, LTE long-term extension, N number of patients in the analysis population, PBO placebo, Q2W every 2 weeks, Q4W every 4 weeks
Both trials were approved by the applicable ethics review boards at each of the sites in North America, Europe, and the Asia-Pacific region. The main, central ethics review board for both studies was WCG. A full listing of ethics review boards is in Tables S2 and Table S3. Both trials were performed in accordance with the Helsinki Declaration of 1964 and its later amendments, the Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent to participate in the trials.
Outcomes
We measured skin improvement using EASI 75 and ≥ 90% improvement in Eczema Area and Severity Index (EASI 90). We measured itch improvement using Pruritus Numeric Rating Scale (NRS) ≥ 4-point and ≥ 3-point improvement from baseline. EASI percent change from baseline and Pruritus NRS change from baseline were used to visualize data at the individual patient level. EASI is used to assess the severity and extent of skin involvement in AD. It is a composite index with scores ranging from 0 to 72, with higher values indicating more severe or extensive disease. The minimal clinically important difference (MCID) of EASI is 6.6 points [15]. The Pruritus NRS is a patient-reported, single-item, 11-point scale. The Pruritus NRS is used by participants to rate their worst itch severity over the past 24 h, with 0 indicating “No itch” and 10 indicating “Worst itch imaginable.” The MCID is 3 points [16], while a 4-point change is a more conservative assessment of clinical impact indicating itch relief. The Pruritus NRS was recorded daily by the patient in an electronic diary and is reported as a prorated weekly mean.
We defined stable skin response with no or minimal fluctuations as the proportion of lebrikizumab responders maintaining EASI 75 for at least 80% (minimal fluctuations) of the 10 maintenance period study visits (i.e., Week 16 to Week 52). We also report the proportion of lebrikizumab responders maintaining EASI 75 for all (no fluctuations) of the maintenance period study visits. Finally, we report the proportion of lebrikizumab responders to achieve and maintain the more stringent response of EASI 90 for at least 80% of the maintenance period study visits and separately for all of the maintenance period study visits. We defined stable itch response with no or minimal fluctuations by two separate definitions: the proportion of lebrikizumab responders who maintained a Pruritus NRS ≥ 4-point improvement for ≥ 80% of the visits from Week 16 to 52 and the proportion of patients who maintained Pruritus NRS ≥ 3-point improvement for ≥ 80% of the visits from Week 16 to Week 52.
Statistical Analysis
All analyses were performed post hoc. During the 36-week maintenance period, patients were permitted to use topical rescue medication intermittently without discontinuing from the studies, and the use of short-term systemic rescue medication was evaluated on a case-by-case basis. For patients who used any topical or systemic treatment for AD, discontinued treatment, or transferred to the escape arm, data collected at or after the event were imputed as non-response. EASI percent change from baseline and Pruritus NRS change from baseline values for individual patients were plotted using observed data collected up to these events.
All analyses were performed on the modified pooled population of ADvocate1 and ADvocate2 patients who met the protocol-defined criteria for response to lebrikizumab at Week 16 (EASI 75 or an IGA 0/1 with a ≥ 2-point improvement without the use of rescue medication). In ADvocate2, analyses were performed on a modified population, excluding 18 patients (from a single study site) who did not meet the eligibility criteria of having moderate-to-severe AD. Of these patients, 14 entered the maintenance primary population and were excluded from the maintenance efficacy analysis of the lebrikizumab Week 16 responders. In addition to meeting the protocol-defined criteria, the Pruritus NRS ≥ 4-point improvement outcome includes patients who presented with a baseline score of ≥ 4 and achieved a ≥ 4-point improvement at Week 16. Similarly, the Pruritus NRS ≥ 3-point improvement outcome includes only per protocol responders who presented with a baseline score of ≥ 3 and achieved a ≥ 3-point improvement at Week 16.
Results
Baseline Characteristics
In ADvocate1 and ADvocate2, 291 lebrikizumab-treated patients met the criteria for response at Week 16 and were re-randomized to receive lebrikizumab Q2W (N = 113), lebrikizumab Q4W (N = 118), or placebo (lebrikizumab withdrawal; N = 60) from weeks 16 to 52. At baseline of the studies, patient demographics and disease characteristics for the Week 16 per protocol responders were balanced among treatment groups (Table 1). The mean (standard deviation [SD]) patient age was 35.5 (17.0) years, and the mean (SD) duration since the onset of AD was 21.8 (14.6) years. The proportions of patients who scored ≥ 4 on the Pruritus NRS at baseline were 97.3% (lebrikizumab Q2W), 92.2% (lebrikizumab Q4W), and 96.6% (lebrikizumab withdrawal).
Table 1.
Baseline demographics and disease characteristics in Week 16 lebrikizumab responders
| LEB 250 mg Q2W (N = 113) | LEB 250 mg Q4W (N = 118) | PBO (LEB Withdrawal) (N = 60) | |
|---|---|---|---|
| Age, years | 36.1 (17.0) | 35.8 (17.3) | 33.8 (16.6) |
| Adolescent (≥ 12 to < 18 years), n (%) | 13 (11.5) | 17 (14.4) | 8 (13.3) |
| Adult (≥ 18 years), n (%) | 100 (88.5) | 101 (85.6) | 52 (86.7) |
| Female, n (%) | 53 (46.9) | 69 (58.5) | 36 (60.0) |
| Region, n (%) | |||
| USA | 44 (38.9) | 51 (43.2) | 22 (36.7) |
| Europe | 40 (35.4) | 38 (32.2) | 18 (30.0) |
| Rest of the world | 29 (25.7) | 29 (24.6) | 20 (33.3) |
| Race, n (%) | |||
| White | 80 (70.8) | 86 (72.9) | 33 (55.0) |
| Asian | 19 (16.8) | 17 (14.4) | 15 (25.0) |
| Black | 9 (8.0) | 12 (10.2) | 8 (13.3) |
| BMI, kg/m2 | 26.3 (6.9) | 26.2 (5.9) | 25.3 (4.8) |
| Prior systemic treatment, n (%) | 51 (45.1) | 66 (55.9) | 30 (50.0) |
| Disease duration since AD onset, years | 21.7 (14.2) | 22.6 (14.8) | 20.4 (14.9) |
| IGA, n (%) | |||
| 3 (Moderate) | 70 (61.9) | 78 (66.1) | 37 (61.7) |
| 4 (Severe) | 43 (38.1) | 40 (33.9) | 23 (38.3) |
| EASI | 29.5 (10.8) | 28.8 (12.6) | 28.9 (11.2) |
| BSA % involvement | 45.3 (20.6) | 43.9 (23.2) | 42.9 (22.4) |
| SCORADa | 66.9 (11.5) | 64.7 (13.0) | 65.5 (12.0) |
| Pruritus NRSb | 7.2 (1.7) | 7.0 (2.1) | 7.5 (1.8) |
| < 4, n (%) | 3 (2.7) | 9 (7.8) | 2 (3.4) |
| ≥ 4, n (%) | 108 (97.3) | 107 (92.2) | 57 (96.6) |
Responders achieved EASI 75 or IGA 0/1 with ≥ 2-point improvement at Week 16 without rescue medication use
Data are mean (standard deviation) unless stated otherwise
AD atopic dermatitis, BMI body mass index, BSA body surface area, EASI Eczema Area and Severity Index, EASI 75 ≥ 75% improvement in EASI, IGA Investigator’s Global Assessment, LEB lebrikizumab, N number of patients in the analysis population, n number of patients in the specified category, NRS numeric rating scale, PBO placebo, Q2W every 2 weeks, Q4W every 4 weeks, SCORAD SCORing Atopic Dermatitis
aNumber of patients with non-missing data, used as denominator (n = 111 for LEB Q2W, n = 114 for LEB Q4W, and n = 59 for LEB withdrawal)
bNumber of patients with nonmissing data, used as denominator (n = 111 for LEB Q2W, n = 116 for LEB Q4W, and n = 59 for LEB withdrawal)
Skin Improvement
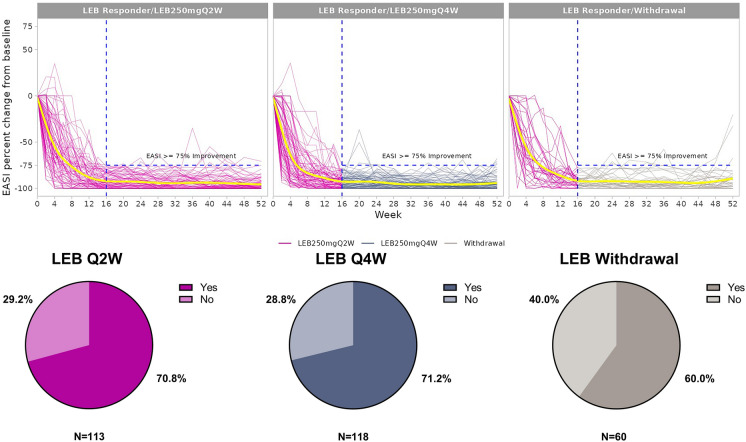
The proportions of lebrikizumab responders who maintained EASI 75 for at least 80% of study visits in the maintenance period were 70.8% (lebrikizumab Q2W), 71.2% (lebrikizumab Q4W), and 60.0% (lebrikizumab withdrawal). Figure 2 visualizes the individual trajectories of the patients with no or minimal fluctuations in EASI 75. Animation 1 visualizes individual patient data over time for all re-randomized patients. The proportions of lebrikizumab responders who maintained EASI 75 at all study visits were 53.1% (lebrikizumab Q2W), 55.1% (lebrikizumab Q4W), and 38.3% (lebrikizumab withdrawal). For the more stringent response of EASI 90, the proportions of lebrikizumab responders to maintain (or achieve and maintain) the endpoint for at least 80% of study visits were 45.1% (lebrikizumab Q2W), 50.8% (lebrikizumab Q4W), and 35.0% (lebrikizumab withdrawal). The proportions of patients who maintained (or achieved and maintained) EASI 90 at all study visits were 29.2% (lebrikizumab Q2W), 26.3% (lebrikizumab Q4W), and 13.3% (lebrikizumab withdrawal). Complete results are given in Table S1.
Fig. 2.
Individual patient trajectories for EASI percent change from baseline through Week 52 and the proportions of patients maintaining EASI 75 for ≥ 80% of study visits. The individual patient trajectories include only patients with stable response for patients treated with LEB Q2W (n = 80), LEB Q4W (n = 84), and LEB withdrawal (n = 36). Data collected after rescue medication use (any topical or systemic treatment), treatment discontinuation, and moving to escape arm were imputed as non-response. The yellow lines are the smoothing lines fitted by locally estimated scatterplot smoothing (LOESS). EASI Eczema Area and Severity Index, EASI 75 ≥ 75% improvement in EASI, LEB lebrikizumab, Q2W every 2 weeks, Q4W every 4 weeks
Animations available on article HTML.
Animation 1 (MP4 17579 KB)
Itch Improvement
Of the patients with baseline Pruritus NRS ≥ 4 and achieving a ≥ 4-point improvement at Week 16, 158 met the protocol criteria for response at Week 16 and were re-randomized to receive lebrikizumab Q2W (N = 62), lebrikizumab Q4W (N = 67), or lebrikizumab withdrawal (N = 29). At the end of the maintenance period, 66.1% (lebrikizumab Q2W), 62.7% (lebrikizumab Q4W), and 55.2% (lebrikizumab withdrawal) of patients maintained a ≥ 4-point Pruritus NRS improvement for ≥ 80% of the maintenance period study visits (Fig. 3).
Fig. 3.
Proportions of patients maintaining Pruritus NRS ≥ 4-point improvement for ≥ 80% of study visits. Patients had baseline Pruritus NRS ≥ 4 and achieved a ≥ 4-point improvement at Week 16. Data collected after rescue medication use (any topical/systemic treatment), treatment discontinuation, and moving to escape arm were imputed as non-response. LEB lebrikizumab, NRS numeric rating scale, Q2W every 2 weeks, Q4W every 4 weeks
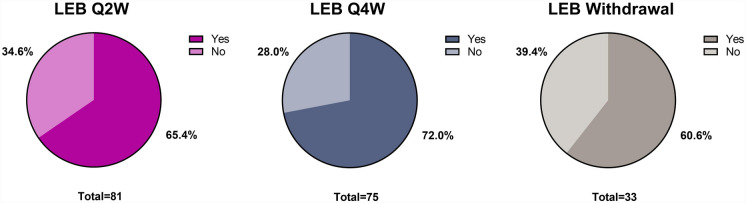
Of the patients with baseline Pruritus NRS ≥ 3 and a ≥ 3-point improvement at Week 16, 189 met the protocol criteria for response at Week 16 and were re-randomized to receive lebrikizumab Q2W (N = 81), lebrikizumab Q4W (N = 75), or lebrikizumab withdrawal (N = 33). At the end of the maintenance period, 65.4% (lebrikizumab Q2W), 72.0% (lebrikizumab Q4W), and 60.6% (lebrikizumab withdrawal) maintained a ≥ 3-point improvement for ≥ 80% of the maintenance period study visits (Fig. 4).
Fig. 4.
Proportions of patients maintaining Pruritus NRS ≥ 3-point improvement for ≥ 80% of study visits. Patients had baseline Pruritus NRS ≥ 3 and achieved a ≥ 3-point improvement at Week 16. Data collected after rescue medication use (any topical/systemic treatment), treatment discontinuation, and moving to escape arm were imputed as non-response. LEB lebrikizumab, NRS numeric rating scale, Q2W every 2 weeks, Q4W every 4 weeks
Figure S1a, b shows the individual trajectories of the patients with no or minimal fluctuations in maintaining a Pruritus NRS ≥ 4-point improvement and a ≥ 3-point improvement, respectively, through Week 52. Animation 2 and Animation 3 use individual patient data to visualize the stability of a Pruritus NRS ≥ 4-point improvement and a Pruritus NRS ≥ 3-point improvement, respectively, over time for all re-randomized patients.
Animations available on article HTML.
Animation 2 (MP4 18620 KB)
Animation 3 (MP4 19000 KB)
Discussion
In this analysis, we have shown that over 70% of Week 16 per protocol responders met the skin endpoint common to AD clinical trials (EASI 75) with no or minimal fluctuations in efficacy over the 36-week treatment period with lebrikizumab. Furthermore, over half of these patients maintained EASI 75 for 100% of study visits with approximately 28% of patients achieving and maintaining EASI 90 for 100% of study visits. The more stringent endpoint of EASI 90 usually correlates well with an IGA 0/1 and thus may more closely represent clear or almost clear skin. More than 60% of patients also maintained a ≥ 4-point improvement in Pruritus NRS with no or minimal fluctuations. These results further support the population-based analysis on the maintenance of response [14] and also indicate that, at the individual level, in most of the patients, lebrikizumab might be modifying the course of moderate-to-severe AD by successfully disrupting the natural waxing and waning features of the disease.
Importantly, similar improvements were observed in patients treated with both lebrikizumab Q2W and Q4W during maintenance treatment, indicating that a stable skin and itch response is possible with a once-monthly injection. Patients maintaining a high level of disease control while receiving medication only once per month may spend less time thinking about their disease and have a higher level of confidence in planning future life events. Similarly, providers can have confidence knowing their patient is under control and requires fewer follow-up visits. The concept of fluctuations in efficacy described in this analysis is distinct from the concept of disease flare. Data on prevention of disease flares were published separately for tacrolimus ointment in adults and children [17, 18], as well as more recently for dupilumab [19] and abrocitinib [20]. This analysis on stability of efficacy demonstrates minimal to no fluctuations in efficacy with Q4W dosing, a unique aspect of treatment with lebrikizumab for moderate-to-severe AD among the currently published data.
The visualization of these stability data in the available animations help to translate the population-based results to the experience of the individual patients treated with lebrikizumab Q2W and Q4W. Interestingly, many patients who were withdrawn from lebrikizumab after achieving the per protocol response at Week 16 continued to demonstrate stable efficacy up to Week 52. The stable response in the 36-week lebrikizumab withdrawal arm could be suggestive of disease modification [21]. This is an important consideration when patients and providers discuss biological treatment choices for AD.
Although EASI is the most frequently used endpoint by clinicians for signs of AD, itch is the most burdensome symptom and usually the first indication of a new disease flare. The results of this analysis confirm lebrikizumab’s achievement of itch improvement and skin clearance, with many patients maintaining more stringent endpoints (i.e., EASI 90 and Pruritus NRS ≥ 4-point improvement) up to 1 year. This denotes that many patients will maintain a response to lebrikizumab that is not only stable but also robust, deep, and sustained in both clinician-reported signs and the patient-reported symptom of itch.
This analysis has some limitations. First, it measures only the response during the maintenance period for patients who met a strict and standard clinical trial definition of response at Week 16, which was anchored to EASI 75 and IGA 0/1. This could be a limitation because, in the real world, more patients (such as patients achieving ≥ 50% improvement in EASI [EASI 50]) may benefit from the long-term response provided by lebrikizumab. Similarly, this analysis may underestimate the real-world efficacy of lebrikizumab by assigning patients to the escape arm who did not maintain EASI 50 at pre-specified time points during the maintenance period. A separate, similar analysis using observed data demonstrated a greater proportion of patients maintaining a stable response for up to 2 years [22]. Finally, this analysis was performed post hoc and was not pre-specified in the statistical analysis plan.
Conclusion
Treatment with lebrikizumab allows most patients to maintain a robust and stable response with no or minimal fluctuations in skin and itch relief for up to 1 year of treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing and process support were provided by Tyler Albright, PharmD of Eli Lilly and Company. Funding for medical writing and process support was provided by Eli Lilly and Company.
Thanking Patient Participants
Eli Lilly and Company and Almirall S.A. would like to thank the clinical trial participants and their caregivers, without whom this work would not be possible.
Author Contributions
All authors contributed to the interpretation of data and reviewed the manuscript critically for intellectually important content. Jonathan I. Silverberg, Gaia Gallo, and Marta Casillas made substantial contributions to the conception or design of the work. Formal analysis of the data was performed by Yuxin Ding and Zhenhui Xu. The first draft of the manuscript was written by Tyler Albright. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
ADvocate1 and ADvocate2 were funded by Dermira, a wholly-owned subsidiary of Eli Lilly and Company. Eli Lilly and Company is responsible for funding the journal’s Rapid Service Fee. Almirall S.A. has licensed the rights to develop and commercialize lebrikizumab for the treatment of dermatology indications including atopic dermatitis in Europe. Lilly has exclusive rights for development and commercialization of lebrikizumab in the United States and the rest of the world outside of Europe.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Declarations
Conflict of Interest
Jonathan I Silverberg has received grants and/or personal fees from AbbVie, AFYX Therapeutics, Arena Pharmaceuticals, Asana BioSciences, Bluefin, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, GSK, Incyte, Kiniksa, LEO Pharma, Luna Pharma, Menlo Therapeutics, Novartis, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi. Andreas Wollenberg has served as an advisor and/or paid speaker for and/or participated in clinical trials (with honorarium paid to the institution) sponsored by AbbVie, Aileens, Alentis, Almirall S.A., Amgen, Beiersdorf, Bioderma, Bioproject, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, DKSH, Eli Lilly, Galapagos, Galderma, Glenmark, GSK, Hans Karrer, Hexal, Janssen-Cilag, Kyowa Kirin, Leo Pharma, L’Oreal, Maruho, MedImmune, MSD, Mylan, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Sandoz, Santen, Sanofi-Aventis, and UCB. Linda Stein Gold has served as an investigator/consultant and/or speaker for Amgen, AbbVie, LEO Pharma, Arcutis, Incyte, Dermavant, Sanofi, Regeneron, Eli Lilly and Company, Bristol Myers Squibb, UCB, Janssen, Ortho Derm, and Galderma. James Del Rosso has received grants as an investigator, honoraria for lecturing, and/or consulting fees from AbbVie, Amgen (Celgene), AOBiome, Aslan, Arbonne, Arcutis, Bausch Health (Ortho Derm), Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, Exeltis, Ferndale, Galderma, Incyte, IntraDerm, Johnson & Johnson, La Roche-Posay/L’Oréal, LEO Pharma, Menlo Therapeutics, Nektar, Pfizer, Pierre Fabre,, Regeneron/Sanofi Genzyme, Sun Pharma, Theraplex, UCB Pharma, Unilever, and Verrica Pharmaceuticals. Gil Yosipovitch has conducted clinical trials or received honoraria for serving as a member of the Scientific Advisory Board of AbbVie, Eli Lilly, GSK, Novartis, Regeneron, Sanofi, Galderma, Pfizer, Kiniksa, Escient, Arcutis, and LEO Pharma and received research funds from Pfizer, Novartis, Eli Lilly, and Sanofi Regeneron. Peter Lio has received grants as an investigator, honoraria for lecturing, and/or consulting fees from AbbVie, AOBiome, Arbonne, Burt’s Bees, Dermavant, Dermira, Eli Lilly and Company, Exeltis, Franklin Bioscience/Altus Labs, Incyte, IntraDerm, Johnson & Johnson, Kiniksa, La Roche-Posay/L’Oréal, LEO Pharma, Menlo Therapeutics, The National Eczema Association, Pfizer, Pierre Fabre, Realm Therapeutics, Regeneron/Sanofi Genzyme, Theraplex, TopMD, UCB Pharma, Unilever, and Verrica Pharmaceuticals. Jose-Manuel Carrascosa has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall S.A., Amgen, Boehringer Ingelheim, Galderma, Janssen-Cilag, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sanofi, Sandoz, and Bristol Myers Squibb. Gaia Gallo, Yuxin Ding, Marta Casillas, and Evangeline Pierce are employees and shareholders of Eli Lilly and Company. Zhenhui Xu is a full-time employee of CIMS Global LLC and a paid contractor for Eli Lilly and Company. Helena Agell is an employee of Almirall S.A. Sonja Ständer has served as an investigator for Celldex, Galderma, GSK, Incyte, Kiniksa Pharmaceuticals, and Trevi Therapeutics and has acted as a consultant, speaker, and/or served on advisory boards for AbbVie, Almirall S.A., Beiersdorf, Clexio Biosciences, Eli Lilly, Galderma, Incyte, IntegrityCE, Kiniksa Pharmaceuticals, Klirna, Pfizer, Professor Paul Gerson Unna Academy, Sanofi, TouchIME, Vifor Pharma, and WebMD.
Ethical Approval
Both trials were approved by the applicable ethics review boards at each of the sites in North America, Europe, and the Asia–Pacific region. The main, central ethics review board was WCG IRB. A full listing of ethics review boards is in the Supplementary Appendix. Both trials were performed in accordance with the Helsinki Declaration of 1964 and its later amendments, the Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent to participate in the trials.
Footnotes
Prior Presentation Preliminary results of these analyses were presented at the April 2023 Revolutionizing Atopic Dermatitis Congress in Washington, DC, USA, and the October 2023 Fall Clinical Dermatology Congress in Las Vegas, NV, USA.
The original online version of this article was revised: Second part of figure 2 updated.
Change history
10/18/2024
A Correction to this paper has been published: 10.1007/s13555-024-01269-y
References
- 1.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7. [DOI] [PubMed] [Google Scholar]
- 2.Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–9. [DOI] [PubMed] [Google Scholar]
- 3.Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548-52.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci G, Bellini F, Dondi A, Patrizi A, Pession A. Atopic dermatitis in adolescence. Dermatol Reports. 2011;4(1):e1-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slattery MJ, Essex MJ, Paletz EM, Vanness ER, Infante M, Rogers GM, Gern JE. Depression, anxiety, and dermatologic quality of life in adolescents with atopic dermatitis. J Allergy Clin Immunol. 2011;128(3):668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US Adult Population. J Invest Dermatol. 2019;139(3):583–90. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Health utility scores of atopic dermatitis in US Adults. J Allergy Clin Immunol Pract. 2019;7(4):1246-52.e1. [DOI] [PubMed] [Google Scholar]
- 8.Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21(2):172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: implications for clinical practice. Am J Clin Dermatol. 2022;23(4):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebel S, Mutsaers B, Tomei C, Leclair CS, Jones G, Petricone-Westwood D, et al. Health anxiety and illness-related fears across diverse chronic illnesses: a systematic review on conceptualization, measurement, prevalence, course, and correlates. PLoS ONE. 2020;15(7): e0234124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuberbier T, Orlow SJ, Paller AS, Taïeb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226–32. [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Anderson P, Gadkari A, Blackburn S, Moon R, Piercy J, et al. Extent and consequences of inadequate disease control among adults with a history of moderate to severe atopic dermatitis. J Dermatol. 2018;45(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg JI, Guttman-Yassky E, Thaçi D, Irvine AD, Stein Gold L, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–91. [DOI] [PubMed] [Google Scholar]
- 14.Blauvelt A, Thyssen JP, Guttman-Yassky E, Bieber T, Serra-Baldrich E, Simpson E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188(6):740–8. [DOI] [PubMed] [Google Scholar]
- 15.Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106. [DOI] [PubMed] [Google Scholar]
- 16.Yosipovitch G, Rams A, Baldasaro J, Bunod L, Delbecque L, Strzok S, et al. Content validity and assessment of the psychometric properties and score interpretation of a pruritus numeric rating scale in atopic dermatitis. In: Revolutionizing Atopic Dermatitis, 11–13 December 2021. 2022;186(4):e135-e73.
- 17.Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63(7):742–50. [PubMed] [Google Scholar]
- 18.Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159(6):1348–56. [DOI] [PubMed] [Google Scholar]
- 19.Merola JF, Sidbury R, Wollenberg A, Chen Z, Zhang A, Shumel B, Rossi AB. Dupilumab prevents flares in adults with moderate to severe atopic dermatitis in a 52-week randomized controlled phase 3 trial. J Am Acad Dermatol. 2021;84(2):495–7. [DOI] [PubMed] [Google Scholar]
- 20.Flohr C, Cork MJ, Ardern-Jones MR, Eichenfield LF, Barbarot S, Feeney C, et al. Efficacy and safety of abrocitinib monotherapy in adolescents and adults: a post hoc analysis of the phase 3 JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN clinical trial. J Dermatol Treat. 2023;34(1):2200866. [DOI] [PubMed] [Google Scholar]
- 21.Bieber T. Disease modification in inflammatory skin disorders: opportunities and challenges. Nat Rev Drug Discov. 2023;22(8):662–80. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg JI, Wollenberg A, Lio P, Carrascosa JM, Casillas M, Gallo G, et al editors. Lebrikizumab provides stable skin response with no or minimal fluctuations for up to 2 years in patients with atopic dermatitis. American Academy of Dermatology: San Diego; 2024. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.