Abstract
Although significant strides have been made in non-pharmacologic management of atrial fibrillation (AF), these treatments remain a work in progress. While catheter ablation is often effective for management of paroxysmal AF, it is less successful in patients with persistent or longstanding persistent AF. This review was undertaken to clarify the risks, benefits, and alternatives to catheter ablation for non-pharmacologic AF management. In order to clarify the roles of surgical and hybrid ablation, this narrative review was undertaken by searching MEDLINE to identify peer-reviewed clinical trials, randomized controlled trials, meta-analyses, review articles, and other clinically relevant studies. The search was limited to English-language reports published between 1960 and 2023. Atrial fibrillation was searched using the terms surgical ablation, catheter ablation, hybrid ablation, stroke prevention, left atrial occlusion, and atrial excision. Google and Google Scholar, as well as bibliographies of identified articles, were also reviewed for additional references. The Cox-maze surgical approach is still the most efficacious non-pharmacological treatment for AF. Hybrid ablation, combining cardiac surgical and catheter ablation techniques, has become an attractive option for persistent or longstanding persistent AF.
Keywords: Atrial fibrillation, Surgical ablation, Hybrid ablation, Catheter ablation
Key Summary Points
| Atrial fibrillation is the most common sustained tachyarrhythmia and increases the risk of morbidity and mortality. |
| Catheter ablation is effective for paroxysmal atrial fibrillation but is less effective for persistent or longstanding persistent atrial fibrillation. |
| Increasing evidence suggests hybrid (surgical plus catheter) ablation is a useful approach for persistent or longstanding persistent atrial fibrillation. |
| Basic and clinical research should aim to clarify atrial fibrillation’s mechanisms and optimize its management. |
| Continued cooperation and teamwork between cardiac surgeons and cardiac electrophysiologists promises to result in greater results and fewer complications. |
Introduction
Development of atrial fibrillation (AF) increases the risk of morbidity and mortality. These sequelae include: (1) an irregularly irregular rhythm that may result in uncomfortable palpitations and emotional distress; (2) loss of atrioventricular synchronous contraction, which impairs cardiac hemodynamics and may cause or exacerbate congestive heart failure; and (3) static left atrial blood flow, resulting in enhanced vulnerability to thromboembolism [1]. While early initiation of pharmacologic therapy may provide arrhythmia suppression [2], and reduce the detrimental sequelae of AF, the benefit does not extend to all patients with AF.
Patient selection, indications, techniques (and technologies), and benefits of AF catheter ablation as well as complications associated with these procedures have been described in detail elsewhere [3–6]. Therefore, this treatise will focus on surgical and hybrid (surgical + catheter based) treatment of AF. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the author. Because evidence-based medicine forms the essence of medical practice, this review aims to provide a critical summary of the existing literature as well as the current state of scientific evidence on surgical and hybrid AF ablation. Key articles and research findings are summarized in order to describe current areas of agreement as well as controversies and debates, point out gaps in current knowledge, depict unanswered questions, and suggest directions for future research [7].
History of AF Surgery
The first surgical approach was ablation of the His bundle with requisite permanent ventricular pacing [8, 9]. This technique was supplanted by catheter ablation of the His bundle with direct current shocks and subsequently with use of radiofrequency (RF) energy delivered to the right and/or left atrioventricular junction [10, 11]. Surgical left atrial isolation was reported in a canine model in 1980. Although a normal cardiac output could be maintained, left atrial fibrillation could continue and the risk of thromboembolism was likely to be unchanged [1, 12] (see Table 1).
Table 1.
Creation of atrioventricular junction block for life-threatening or disabling drug refractory supraventricular tachyarrhythmias
| First author references (year) | Cases/participants | Age, years | Follow-up, years (unless otherwise indicated) | Study type | Key findings/messages |
|---|---|---|---|---|---|
| Sealy et al. [9] | 42 patients with life-threatening or disabling atrial arrhythmias | 49 (only reported for patients without accessory pathways) | Not available | Single-center surgical series |
Ablation successful (AV block) in 36/42 patients (86%) 4/6 failures using cryothermy 2/6 failures using surgical incisions |
| Scheinman et al. [10] | 5 patients (4 male) with drug refractory supraventricular tachycardia | 56 ± 15 | 4–12 months | Single-center series |
Direct current shocks delivered via catheter Complete AV block achieved in all patients; 1 patient died suddenly 6 weeks post ablation |
| Trohman et al. [11] | 61 patients with drug refractory supraventricular tachycardia | 62 ± 12.7 | 9.7 ± 4.7 months | Single-center series | The AV junction was successfully ablated using radiofrequency (RF) energy in 60/61 patients (98%). Right-sided ablation was unsuccessful in 7 patients. 6/7 underwent left-sided ablation which created complete AV block |
All patients with complete atrioventricular (AV) block required permanent pacing
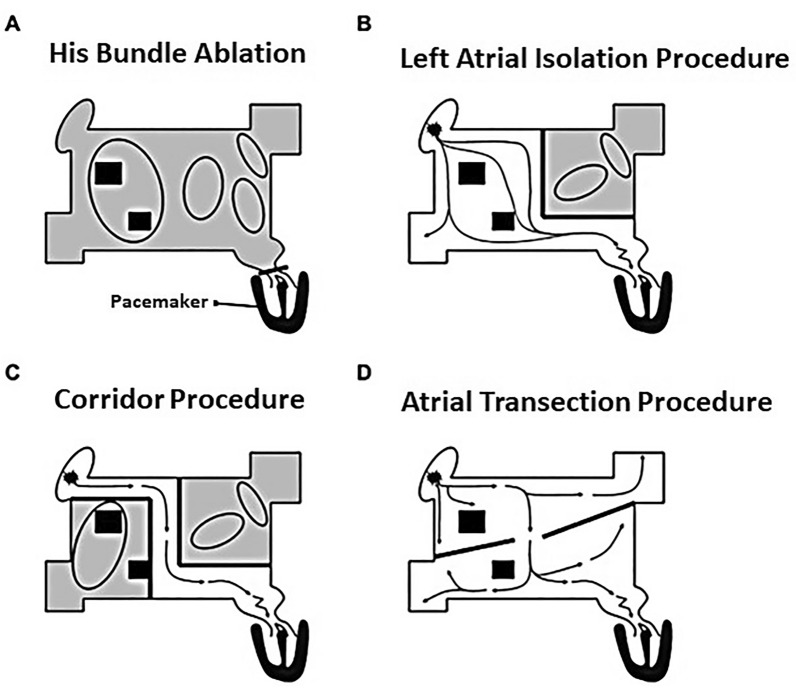
In 1985, Guiradon and colleagues [13, 14] reported an open-heart technique that isolated an atrial septal strip (containing both sinoatrial and atrioventricular [AV] nodes) from the remaining atrial myocardium. Although isolated from the septal corridor postoperatively, the atria continued to fibrillate and, likewise, were isolated from their respective ventricles, precluding atrioventricular synchrony [14–16]. In a long-term follow-up (21 ± 20 months), seven of nine patients remained in sinus rhythm. Two patients had recurrent AF (one patient was effectively controlled with a single antiarrhythmic drug). While effective in a canine model, the “atrial transection procedure” was only transiently effective in a patient with AF [1, 14, 16, 17]. Figure 1 provides schematic views of the procedures noted above [8]. Table 2 summarizes results of early surgical attempts to achieve sinus rhythm.
Fig. 1.
Early surgical management of atrial fibrillation (AF). Each of these early interventions did not successfully address the primary rhythm disturbance (AF) or the risk of thromboembolism. Additional details are available in the text and in Table 2.
Adapted from reference 8, with permission
Table 2.
Early surgical attempts to restore sinus rhythm
| First author references (year) | Cases/participants | Age, years | Follow-up, years (unless otherwise indicated) | Study type | Key findings/messages |
|---|---|---|---|---|---|
| Williams et al. [12] | Ten dogs | Adult | Postoperatively | Animal model |
Aimed to isolate the left atrium (LA) to treat refractory ectopic supraventricular tachycardia arising in the left atrium Dog’s left atrium was paced rapidly and no atrioventricular (AV) conduction was noted after LA isolation The loss of left atrial kick did not appear to adversely impact hemodynamics The risk of thromboembolism was unlikely to be unchanged |
|
Guiradon et al. [13] Leitch et al. [15]* |
9* | 48 ± 12* | 21 ± 20 months* |
Original description (13) Follow-up results |
Sinus rhythm maintained in 7/9* Four patients required permanent pacing for sinus node dysfunction* The risk of thromboembolism was unlikely to be significantly changed* |
| D’Agostino HJ Jr, et al. [16] | Animal model 1 patient | N/A | N/A | Surgical ablation of atrial fibrillation in canine model of chronic MR | Unsuccessful in one patient and abandoned |
*Data from Leitch et al. MR mitral regurgitation
The Cox-maze procedure (CMP) was initially performed in 1987 [1, 5, 18, 19]. Although this version was efficacious, two problematic issues resulted: (1) sinus rate responses to maximal exercise were frequently insufficient and (2) left atrial dysfunction occasionally occurred [5, 15, 19, 20].
The incision through the sinus node area was eliminated in the maze II procedure. The transverse atriotomy across the left atrial dome was shifted posteriorly to facilitate intra-atrial conduction. Unfortunately, complete transection of the superior vena cava (SVC) was then required to gain left atrial exposure [5, 14, 20].
In the Cox-maze III procedure, the septal incision was shifted posteriorly to the orifice of the SVC thus improving left atrial exposure. The Cox-maze III was technically less demanding and resulted in a higher incidence of postoperative sinus rhythm, improved long-term sinus node function, fewer pacemaker requirements, diminished arrhythmia recurrence, and long-term improvement in atrial transport function [5, 14, 20, 21].
Cox-maze III results were compared between patients who underwent “concomitant AF surgery” and patients with lone AF who underwent “stand-alone AF surgery”. Mitral valve repair, mitral valve replacement, and coronary artery bypass grafting were the most common concomitant surgeries. Paroxysmal AF was present in 72/112 (64%) patients and persistent AF was present in 40 (36%) patients in the lone AF group. Mean follow-up durations were 5.4 ± 2.7 in the concomitant and 5.4 ± 3.0 years in the lone AF groups. At the end of follow-up, 78 (79.6%) of stand-alone patients were not in AF while off antiarrhythmic drugs. Similarly, in the concomitant group, 58 (73.4%) patients were in sinus rhythm and free of antiarrhythmic medication. An additional 19 (24%) patients were AF free but were taking antiarrhythmic agents. Differences between the groups were not statistically significant [5, 22].
In 2002, minimally invasive approaches, plus use of cryothermal and RF procedures (to replace “cut and sew” techniques) were introduced. Alternate energy sources such as microwave, ultrasound, and laser were also employed. Generally speaking, alternate energy sources do not work as well as cryoablation or RF [5, 23]. Microwave ablation results have been unsatisfactory [5, 24, 25]. In surgical AF management, laser energy has been abandoned due to inconsistent creation of transmural lesions [23, 26]. It has been noted that cryoablation and unipolar RF energy delivery are less effective in creating transmural lesions and are associated with reconnection of the pulmonary veins (PVs) [27].
When new ablation technology, including bipolar RF energy and new cryoablation systems, is employed in the open chest and a complete biatrial Cox-maze lesion set performed, the procedure is deemed the Cox-maze IV procedure (CMP-IV) [5, 28]. In the Cox-maze IV procedure, the bilateral pulmonary veins were isolated, and a connecting lesion was added instead of the original box lesion around all of the PVs. Importantly, cross-clamp times were shorter with this procedure and procedural performance through a small, right inframammary incision was perfected. Two years later, the final CMP-IV version isolated the entire posterior left atrial wall with addition of a superior connecting line, recreating the box lesion-set [5, 28].
In 2007, Lall et al. [29] compared 242 patients who underwent Cox-maze III (154 patients) and Cox-maze IV (88 patients) surgical AF ablation. At 12 months, there was no significant difference in freedom from AF (96% for Cox-maze III and 93% for Cox-maze IV group) They concluded that bipolar RF ablation simplified the Cox-maze procedure, making surgical ablation a viable option for nearly all patients with AF undergoing concomitant cardiac surgery [5, 29].
It is important to understand that AF is not a uniform entity. AF is classified based on the arrhythmia’s temporal characteristics. When AF is initially detected it is termed “recent-onset” (present for < 48–72 h) [4, 30, 31]. AF is deemed recurrent when patients develop ≥ 2 episodes. Episodes that terminate spontaneously (by consensus within 7 days) are paroxysmal or persistent if cardioversion (electrical or pharmacological) is required for AF termination. Successful termination of AF does not alter persistent AF classification. Longstanding persistent AF (≥ 1 year) not successfully terminated by cardioversion, or when cardioversion is not pursued, is deemed permanent [32].
Prospectively collected data (between 1992 and 2010) was assessed from 212 consecutive patients who underwent a stand-alone Cox-maze III (n = 112) or Cox-maze IV (n = 100) procedure. Their mean age, was 53.5 ± 10.4 years; 78% were male. AF was classified as paroxysmal in 48% and persistent or longstanding persistent in 52%. The preoperative median AF duration was six years. No intraoperative deaths occurred. Overall, 30-day mortality was 1.4% [28].
A strict follow-up regimen was implemented. All patients had ECGs or 24-h Holter monitoring at 3, 6, and 12 months and thereafter annually. At follow-up (mean 3.6 ± 3.1 years), 93% were AF-free (82% without antiarrhythmic drugs). At 10 years, 85% were free from symptomatic AF. Although 80% of patients were not receiving anticoagulation therapy, only one late stroke occurred. The less invasive CMP-IV resulted in shorter cross-clamp times (41 ± 13 vs. 92 ± 26 min; p < 0.001) while maintaining high success rates (at 2 years, 90% overall freedom from AF; 84% without antiarrhythmic drugs) [28].
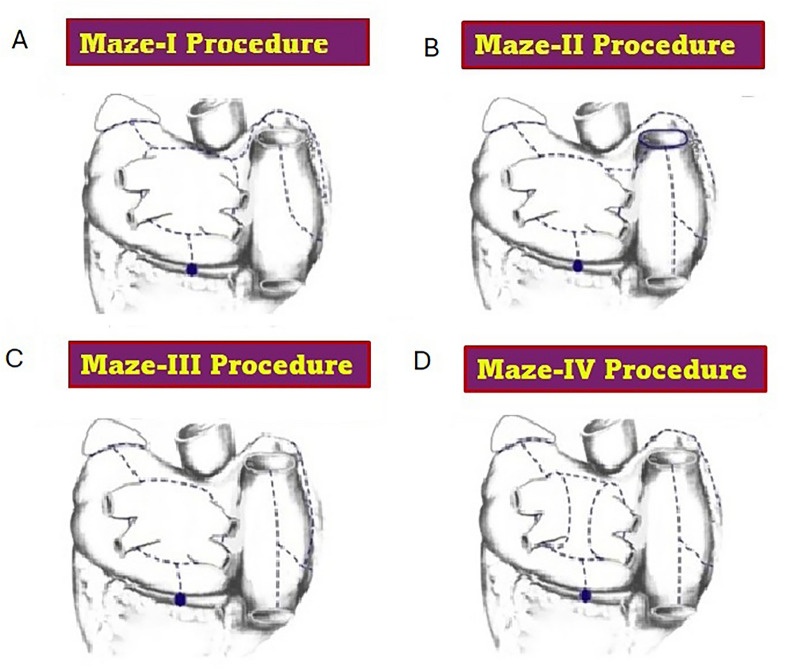
Figure 2 illustrates the evolving lesion sets from the four iterations of the Cox-maze procedure [8].
Fig. 2.
Evolution of the maze procedure from I to IV. The diagrams below illustrate the four iterations of the maze procedure, each of which is discussed extensively in the text. No procedure other than those diagrammed here should be called a “maze procedure”. A Maze I procedure. B Maze II procedure. C Maze III procedure. D Maze IV procedure.
Adapted from reference 8, with permission
More Recent Data in Larger Patient Cohorts
In 2015, Gillinov and associates [33] reported 260 patients with persistent or longstanding persistent AF who underwent mitral-valve surgery. These patients were randomly assigned to receive no ablation (control group) or to undergo concomitant surgical ablation. Ablation group patients were additionally randomized to pulmonary vein isolation or a biatrial maze procedure. Freedom from AF was not significantly different between the two ablative cohorts. More ablation group patients compared to the control group were AF free at both 6 and 12 months (63.2 vs. 29.4%, p < 0.001). Ablation led to more permanent pacemaker placement than occurred in the no ablation group (p = 0.01) [5, 33]. Responses to this study suggested the low biatrial maze procedure success rates indicated either inadequate lesion creation or incorrectly performed surgery. It was also pointed out that maze procedures include coronary sinus (CS) ablation, and that CS ablation was not mentioned [5, 34].
In terms of efficacy, although lesion sets were standardized, ablative energy sources were not [5, 27, 33]. Surgeons were permitted to use a combination of unipolar and bipolar RF as well as cryothermy [5, 33]. Pison et al. [27] pointed out that bipolar RF pulmonary vein isolation was performed in only 53 patients, compared to unipolar RF ablation or cryoablation in 110 patients. They noted that unipolar RF and cryoablation energies are less effective in creating transmural lesions and are associated with reconnection of the PVs [5, 27]. Therefore, they questioned whether more use of bipolar RF ablation would have resulted in greater success rates [5, 27]. Gillinov et al. [35] responded that bipolar RF was used in 43% of 67 patients who underwent PV isolation and greater AF freedom was not observed in this relatively small group.
The validity of results suggesting that the maze procedure caused more patients to require postoperative pacing was also questioned [34]. The authors [35] admitted their surprise that pacing requirements reached 17%. They raised the possibilities that this resulted from valve replacement, multi-valve surgical procedures (~ 50% of patients who underwent ablation had multivalve surgery) or that > 50% of ablation recipients were ≥ 70 years old, all factors that increase the risk of postoperative atrioventricular block [33]. In addition, they speculated that it could be due to the biatrial maze [35]. A previous trial noted that adding right atrial lesions to an extensive left atrial lesion set did not improve efficacy but did increase the rate of pacemaker placement to 16.5%. However, in that study’s cohort, sinus node dysfunction resulted in the increased pacing requirements [36].
In the Medicare-linked Society of Thoracic Surgeons database, 361,138 patients underwent coronary artery bypass grafting (CABG) between 2006 and 2013. Among these patients 34,600 (9.6%) had preoperative AF. Data from the 10,541 (30.5%) treated with surgical ablation (ablation group) were compared with 23,059 (69.5%) patients who were not (no ablation group) [37].
In order to overcome differences in potential confounders between the two study groups, a one-to-one optimal matching algorithm was employed to obtain a propensity score matched sample. Hence, 9771 patients were analyzed in each group [37].
Operative (3.2 vs. 2.5%; p < 0.006) and in hospital mortality (3.8 vs. 3.2%; p < 0.017) were higher in the ablation group. Patients in the ablation group had more new renal failure and prolonged ventilation compared to the no ablation group. However, mortality was lower in the ablation group among patients surviving past 2 years, (HR 0.89, 95% CI, 0.82, 0.97; log-rank p = 0.04). Likewise, incidence of stroke or systemic embolization was lower in the ablation group among patients surviving past 2 years, (HR 0.73, 95% CI 0.61, 95% 0.87; Gray’s test p = 0.0005) [37].
A smaller study from Poland used propensity matching to compare 918 cases of CABG alone to 306 cases of CABG + ablation. Surgical ablation was associated with a lower risk of 30-day mortality [risk ratio 0.37, 95% confidence interval (CI) 0.15, 0.91; p = 0.032] and multiorgan failure (risk ratio 0.29, 95% CI 0.10–0.94; p = 0.029). During a mean follow-up of 4.7 ± 3.5 years, surgical ablation was associated with a significant 33% improvement in overall survival rate: hazard ratio 0.67, 95% CI 0.49, 0.90; p = 0.008 [38].
These investigators also reported survival after AF surgical ablation accompanying mitral valve surgery (MVS). A cohort of 11,381 patients with baseline AF (mean age 65.6 ± 9.0 years) who underwent mitral valve surgery between 2006 and 2017 in Poland was analyzed. Within the group, 2449 (21.5%) underwent AF surgical ablation. Patients in the AF surgical ablation group were younger (63.8 ± 8.7 years vs. 66.1 ± 9.0 years; p < 0.001) and had lower (EuroSCORE, 2.86 vs. 3.69; p < 0.001) baseline surgical risk. There was significant survival benefit during the 12-year study period (hazard ratio, 0.71; 95% confidence interval, 0.63, 0.79; p < 0.001) for MVS + AF ablation compared to MVS alone [39].
Musharbash et al. [40] retrospectively reviewed patients (n = 10,859) who underwent cardiac surgery between 2001 and 2016. Patients were divided into 3 groups: those with an AF history receiving a concomitant Cox-maze IV procedure (n = 438), those with an AF history that was not surgically addressed (n = 1510), and those without AF (n = 8911). Propensity score matching was conducted between these three groups [40].
Performing concomitant Cox-maze IV procedures did not significantly increase postoperative morbidity or mortality and was associated with improved late survival compared with patients whose AF was not surgically treated and similar survival to the patients without an AF history. Kaplan–Meier analysis revealed greater survival with concomitant Cox-maze IV compared to AF that was untreated (p = 0.004). Ten-year survival was 62% compared to 42% for untreated AF. The adjusted hazard ratio was 0.47 (95% confidence interval, 0.26, 0.86, p = 0.014). Kaplan–Meier analysis revealed that there was no significant difference in survival between Cox-maze IV recipients and the group without AF with the (p = 0.847). Ten-year survival was 63% for Cox-maze IV recipients and 55% for the no AF group. The adjusted hazard ratio was 1.03 (95% confidence interval, 0.51, 2.11, p = 0.929) [40].
A retrospective analysis of 20,407 consecutive coronary artery bypass grafting (CABG) or valve procedures performed from 2008 to 2015 was conducted among seven centers reporting to a prospectively maintained clinical registry. Patients undergoing surgery with documented preoperative AF were included (n = 2740). Surgical ablation was performed in 23.1% of these patients. The rate of surgical ablation increased significantly over the study period (p < 0.001) [41].
Concomitant surgical ablation was performed in 16.2% of CABG, 30.6% of valve, and 24.3% of CABG plus valve procedures. Patients receiving surgical ablation were compared with those not receiving surgical ablation. All-cause mortality was the primary end point. Secondary end points included in-hospital mortality and morbidity. Despite longer bypass times (p < 0.001) with surgical ablation, there were no differences found in postoperative complications. Length of stay was significantly shorter (p < 0.001) in the surgical ablation group [41].
Substantial improvement was found in unadjusted survival among individuals undergoing surgical ablation (hazard ratio 0.54, 95% confidence interval: 0.42, 0.70). After risk adjustment, surgical ablation patients had improved 5-year survival (hazard ratio 0.69, 95% confidence interval: 0.51, 0.92). This benefit was observed across all operations [41].
Badhwar et al. [42] examined United States surgical ablative performance trends for six operative categories: (1) aortic valve replacement (AVR) ± coronary artery bypass graft surgery (CABG), (2) mitral valve repair or replacement (MVRR) ± CABG, (3) AVR with MVRR, 4) CABG alone, (5) stand-alone surgical ablation, and (6) surgical ablation plus other concomitant operations. Overall, 48.3% of patients (42,066 of 86,941) underwent surgical ablation and the frequency of ablation performance increased throughout the study period (July 2011 to June 2014). Mitral valve operations had the highest rate of surgical ablation (MVRR ± CABG 68.4% and MVRR + AVR 59.1%) and isolated CABG had the lowest rates (32.8%). Following propensity matching, surgical ablation was associated with a reduction in relative risk (RR) of stroke (RR 0.84, 95% CI 0.74, 0.94), as well as 30-day mortality (RR 0.92, 95% CI 0.85, 0.99), but had increased risk of renal failure (RR 1.12, 95% CI 1.03, 1.22) and permanent pacemaker placement (RR 1.33, 95% CI 1.24, 1.43) [42].
Thus, surgical ablation of AF has beneficial effects on survival and reduces the risk of stroke. Bipolar application of RF energy appears to be more effective than other energy sources. These results are summarized in Table 3.
Table 3.
Data from recent trials in larger cohorts
| First author references (year) | Cases/participants | Age, years | Follow-up, years (unless otherwise indicated) | Study type | Key findings/messages |
|---|---|---|---|---|---|
| Gillinov et al. [33] |
260 persistent or longstanding persistent AF patients who were to undergo mitral valve surgery were randomized to maze surgery or PVI only versus no AF surgery |
69 ± 10 | 6-month assessment and 12-month assessment | Randomized multi-center study |
Significantly more patients in the ablation group were free of AF at 6 and 12 months (P < 0.001) More permanent pacemaker placement required in surgical group Ablative energy sources were not uniform and may have reduced success rates |
| Suwalski et al. [39] |
11,381 patients with AF underwent mitral valve surgery (MVS) 2449 (21.5%) also underwent AF surgical ablation |
65.6 ± 9.0 | Median follow-up 5 years | Retrospectively collected data from the KROK (Polish National Registry of Cardiac Surgery Procedures) registry |
AF surgical ablation group was younger (63.8 ± 8.7 years vs. 66.1 ± 9.0 years; p < 0.001) MVS + AF ablation resulted in significant survival benefit over MVS alone (p < 0.001) |
| Malaisrie et al. [37] | Data from the 9771 CABG patients treated with surgical ablation were compared with 9,771 CABG patients who were not surgically ablated | 74 | Median follow-up 4 years | A one-to-one optimal matching algorithm was employed to obtain propensity score matched samples |
Operative and in hospital mortality were significantly higher in the ablation group Among patients surviving past 2 years mortality and incidence of stroke or systemic embolization was lower in the ablation group |
| Suwalski et al. [38] | 306 cases of isolated CABG + ablation versus 918 of isolated CABG alone | Median 62 | 4.7 ± 3.5 years |
1:3 propensity matching Data collected retrospectively from the KROK (Polish National Registry of Cardiac Surgery Procedures) registry |
CABG + ablation was associated with a statistically lower risk of 30-day death (P = 0.032) Performing ablation at the time of isolated CABG was also associated with a significant long-term survival benefit (P = 0.008) |
| Musharbash et al. [40] |
10,859 total AF + Cox-maze IV 438 AF—Cox-maze IV 1510 No AF 8911 |
After matching: AF + Cox-maze IV = 68 ± 11 versus AF w/o Cox-maze IV = 68 ± 14 AF + Cox-maze IV = 66 ± 11 versus No AF = 66 ± 12 |
Mean follow-up between the AF + Cox-maze IV and AF w/o Cox-maze IV groups was 4.2 ± 3.4 years and 3.8 ± 3.8 years. Between the AF + Cox-maze IV and the No AF group was 4.0 ± 3.4 years and 3.9 ± 3.8 years | Retrospective review of patients undergoing cardiac surgery 2001 to 2016 with propensity matching |
Survival greater with concomitant Cox-maze IV compared to AF that was untreated No significant difference in 10-year survival between Cox-maze IV recipients and the group without AF |
| Iribarne et al. [41] | 2740 with documented pre-op AF |
Surgical ablation recipients 71.7 No ablation patients 71.9 |
Mean long-term survival follow-up was 2.6 years | Retrospective multicenter cohort of patients with AF who underwent CABG, valve, and valve plus CABG comparing surgical ablation versus no surgical ablation |
No differences in postoperative complications Length of stay shorter in ablation recipients (p < 0.001) Ablated patients had improved 5-year survival |
| Badhwar et al. [42] |
86,941 total 42,066 (48.3%) underwent surgical ablation 28,739 pairs were propensity matched |
Median (interquartile range)* Overall: 72 (65–79) No ablation: 73 (65–80) Ablation: 71 (64–78) |
47 months** | US surgical ablative propensity matched performance trends for six operative categories: 1) AVR ± CABG, 2) MVRR ± CABG, 3) AVR with MVRR, 4) CABG alone, 5) stand-alone surgical ablation, and 6) surgical ablation plus other concomitant operations | Risk-adjusted mortality, stroke, and prolonged ventilation were reduced in ablation recipients. However, renal failure and new pacemakers were increased after surgical ablation |
US United States, AF atrial fibrillation, AVR aortic valve replacement, CABG coronary artery bypass graft surgery, MVRR mitral valve repair or replacement, MVS mitral valve surgery, PVI pulmonary-vein isolation, w/o without. *Unadjusted values. **Ill-defined in original manuscript
Evolution of Hybrid Surgical Techniques
The first description of the autonomic nervous system’s importance in initiating AF was provided by Coumel in the 1990s [43–45]. In 2005, Wolf and colleagues [46] demonstrated the feasibility of minimally invasive video-assisted left atrial appendage exclusion and bilateral pulmonary vein isolation for AF. In 2010, Edgerton and associates [47] reported prospective, nonrandomized study results from consecutive patients with symptomatic paroxysmal AF who underwent video-assisted, minimally invasive surgical ablation. The procedure reported included pulmonary vein isolation with bipolar RF application, intraoperative confirmation of transmurality, mapping for and ablation of ganglionated plexi (GP), division of the ligament of Marshall and excision or exclusion of the left atrial appendage [47].
Between March 2005 and January 2008, the procedure was performed in 52 patients with paroxysmal AF. Freedom from symptoms attributed to atrial tachyarrhythmias was 78.0% at 6 months and 63.8% at 12 months. Long-term monitoring, by either a 24-h Holter monitor, 2–3-week event monitoring, or interrogation of an implanted pacemaker, obtained at 6 and 12 months revealed freedom from atrial tachyarrhythmias was 86.3% and 80.8% at 6 and 12 months respectively. Although the authors concluded that the use of clinical symptoms underestimated clinical success as compared with long-term monitoring, it seems equally possible that 24-h Holter monitor and 2–3-week event monitoring overestimated clinical success [47].
Autonomic ganglia are known to play an important role in AF initiation and maintenance [48]. As seen in Fig. 3, the five major left atrial ganglionic plexi (GPs) include the Marshall tract ganglionic plexus (GP), the superior left GP, the anterior right GP, the inferior left GP, and inferior right GP. The GP are located consistently in areas where highly fractionated atrial potentials (FAPs), also known as CFAEs (complex fractionated atrial electrograms). GP may be localized via high-frequency pacing (e.g., cycle length 50 ms, at 12–15 V, 10-ms pulse width) delivered through mapping or ablation catheters to left atrial locations with FAPs to identify sites that exhibit transient AV block during AF. Occasionally, different signs of GP activation (such as activation of ectopic excitation from a PV other than the one adjacent to the GP stimulated) may occur [48].
Fig. 3.
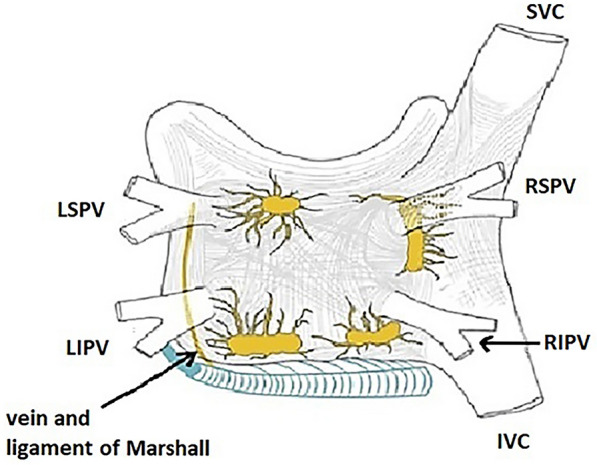
Major left atrial ganglionic plexi. The five major left atrial automatic ganglionic plexi (GP) and axons (superior left GP, inferior left GP, anterior right GP, inferior right GP, and ligament of Marshall) are shown in yellow. The coronary sinus, which is enveloped is muscular fibers that have connections to the atrium is shown in blue. The vein and ligament of Marshall, which travels from the coronary sinus to the region between the left superior PV and the left atrial appendage is also shown in blue. IVC inferior vena cava, LIPV left inferior pulmonary vein, LSPV left superior pulmonary vein, RIPV right inferior pulmonary vein, RSPV right superior pulmonary vein, SVC superior vena cava
Adapted from reference 48, with permission
Despite the role of autonomic ganglia in initiating and maintaining AF, surgical ablation of AF does not seem to be enhanced by targeting the autonomic nervous system. In 2012, Fragakis et al. [49] noted that ganglionated plexi ablation was becoming popular among groups adopting surgical ablation. However, they noted that randomized data did not exist to clarify its potential benefit [49]. In 2015, Gelsomino and colleagues [50] reported 519 subjects with persistent or longstanding persistent AF who underwent RF maze IV procedures during open heart surgery from January 2006 to July 2013 at three institutions without (Group 1) or with (Group 2) ganglionated plexi (GP) ablation. The primary outcome was AF recurrence off antiarrhythmic drugs. The percentage of patients in sinus rhythm off-antiarrhythmic drugs did not differ between groups. Thus, GP ablation was not demonstrated to be beneficial to maintain stable postoperative sinus rhythm [50]. In 2016, the AFACT Study (Atrial Fibrillation Ablation and Autonomic Modulation Via Thorascopic Surgery), GP ablation for advanced AF during thoracoscopic surgery had no detectable impact on AF recurrence but caused a greater number of major adverse events such as sinoatrial node dysfunction, pacemaker implantation and major bleeding [51]. The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of AF noted that ablation of autonomic ganglia as an initial or repeat ablation strategy for paroxysmal, persistent, and longstanding persistent AF was not well established [48].
In 2011, Krul and colleagues [52] described a “hybrid” AF ablation approach. The authors used extensive periprocedural electrophysiological testing during thoracoscopic GP ablation, and pulmonary vein antrum isolation. Additional left atrial ablation lines (ALAL) were created in patients with persistent AF and longstanding persistent AF [52].
GPs were located with high-output, high-frequency pacing (18 V, 1-ms pulse width, 1000 Hz) which was confirmed by pacing induced AV block or an increase in the R-R interval > 50%. Pulmonary vein antrum isolation was achieved with bipolar RF energy applied to clamps placed around the PV antrum. The PV antrum was considered isolated if either no bipolar potentials were recorded distal to the scar or if potentials of a slow automatic rhythm, dissociated from the atrial depolarizations were recorded. When ALAL were created, a superior line connecting the encircling lesions around the left and right PVs was added to prevent reentry around both scars. An additional line, similar to a mitral isthmus line, was created during ablation between the superior line and the left fibrous trigone. In selected patients an additional inferior line was created. If an inferior line was created, electrical isolation of the posterior wall box was demonstrated by entry and exit block. At the procedure’s conclusion, an endoscopic stapling and cutting device (Endo Gia stapler, Tyco Healthcare Group, North Haven, CT) was used to remove the left atrial appendage [52]. Thirty-one patients with AF (16 paroxysmal, 13 persistent, two longstanding persistent) were treated. Thirteen of 15 patients with nonparoxysmal AF received ALAL. After a year of follow up, 19 of 22 patients (86%) had no recurrent atrial tachyarrhythmias and were not using antiarrhythmic drugs. Three patients required a sternotomy because bleeding could not be controlled with thoracoscopic surgery. One patient developed a hemothorax due to bleeding from a thoracoscopic port entrance. There were three minor events; during admission, one patient developed a pneumothorax after the chest drains were removed. Two patients had pneumonia treated successfully with antibiotics. No deaths or thromboembolic events occurred [52].
In the same year (and month), Mahapatra and associates [53] compared results from 15 patients who sequentially underwent surgical epicardial-catheter endocardial ablation for persistent or longstanding persistent AF versus 30 who were treated only with catheter ablation. All patients had failed prior catheter ablation. Sequential catheter ablation was performed 4.3 ± 1.3 days after their surgical ablation. After a mean follow-up of 20.7 ± 4.5 months, 86.7% (13/15) of sequential patients were atrial tachyarrhythmia free off antiarrhythmic drugs, compared to 53.3% (16/30) of patients that had repeat catheter ablation alone (p = 0.04) [5, 53].
In 2012, Pison et al. [54] reported a cohort of 26 consecutive patients with AF who underwent simultaneous hybrid thoracoscopic surgical plus transvenous catheter ablation and were followed for up to 1 year. The epicardial lesions were not transmural in 23% of the patients, and endocardial touch-up was needed. Ten had persistent AF, 15 had paroxysmal AF, and 1 had longstanding persistent AF [54, 55]. Endocardial catheter ablation allowed completion of lesion sets that could (or were) not be completed surgically, especially the mitral isthmus and cavotricuspid isthmus lines. Endocardial ablation, guided by epicardial mapping, was used to fill gaps in the lesions applied surgically [54, 55]. The authors deemed the techniques feasible and safe. The single-procedure success rate at 1 year was 83% [54, 55]. Thus, the current form of hybrid ablation was shown to be effective [5, 54, 55].
A 2015 systematic review [56] compared the safety and efficacy of the hybrid procedure to the Cox-maze procedure (with cardiopulmonary bypass support [CPB]) and beating-heart epicardial ablation. At 1 year, sinus rhythm restoration rates were 70, 93, and 80% and sinus restoration without anti-arrhythmic medication was 71, 87, and 72%, for, the hybrid procedures, the Cox-maze, and epicardial surgical ablation, respectively. The minimally invasive Cox-maze procedure with CPB had the lowest incidence of reoperation for major bleeding as well as important safety advantages in conversion to sternotomy [56]. Minimally invasive thoracoscopic surgery may exceed AF catheter ablation results, but limitations in creation of transmural floor and roof lines on the posterior left atrial wall can reduce efficacy compared to open CPB surgery [5].
Despite use of a variety of catheter-based strategies for persistent AF, success rates after a single procedure have ranged from ~ 20–60% [5, 57, 58]. For the combination of persistent and longstanding persistent AF, efficacy rates are ~ 30–40%. Efficacy seems somewhat dependent on the approach used. Longstanding persistent AF may be effectively treated with a composite of extensive index catheter ablation, repeat procedures, and/or pharmaceuticals [5, 57–59]. Rostock and colleagues reported that after 2.3 ± 0.6 ablation procedures in 395 patients, 312 (79%) were arrhythmia free (including concomitant antiarrhythmic drug treatment in 38%) at mean follow-up of 15 ± 9 months after their last procedure [59]. However, a 2017 report (not confined to persistent and longstanding persistent AF) found that individuals who underwent an additional ablation had $39,409 greater costs during the subsequent year [60]. These results, as well as the fact that thoracoscopic surgical and catheter AF ablation may result in incomplete isolation of the PVs and the posterior left atrial wall, spurred hybrid strategy development combining these approaches [5, 57–59, 61].
Currently, hybrid AF ablation uses subsets of the Cox-maze IV minimally invasive epicardial lesion followed by endocardial catheter ablation to close non-transmural gaps between lesions as well as to address additional reentrant atrial circuits. Unfortunately, like surgical approaches [5, 18, 29, 35, 62], lack of uniform procedural approaches remain including (but not limited to) thoracoscopic vs. pericardioscopic approaches, energy sources used, lesion sets applied, timing between the respective surgical and catheter components, left atrial appendage (LAA) management as well as medical management of these patients [5, 62].
Superior outcomes have been achieved with a bilateral thoracoscopic ablative approach compared to using a pericardioscopic approach which include lower morbidity and mortality rates. The bilateral thoracoscopic approach excludes (e.g., ligation, clipping, or excision) the left atrial appendage in patients with persistent or longstanding persistent AF. Left atrial appendage exclusion has the potential to reduce lifetime risk of stroke as well as electrically isolating/eliminating AF triggers originating from the appendage [62, 63].
In 2019, Al-Jazairi et al. [64] reported the results of single-stage hybrid ablation in 50 consecutive patients. The PVs and superior vena cava were isolated, and a posterior left atrial box was created via thoracoscopic epicardial ablation. Isolation was assessed endocardially, and additional endocardial ablation was performed as needed. The pulmonary veins were isolated via the epicardial approach. Additional endocardial ablation was required to complete box isolation in 21 patients [64].
Five (10%) patients had paroxysmal, 34 (68%) patients had persistent, and 11 patients (22%) had longstanding persistent AF. Twenty-five (50%) individuals had unsuccessful prior catheter ablation(s) [64].
At 1 year, 76% of patients maintained sinus rhythm off antiarrhythmic drugs. Patients with paroxysmal AF had the greatest success, and patients with longstanding persistent AF had the poorest results. The procedure was successful in 100% of patients with paroxysmal AF versus 79% in those with persistent AF versus 55% in those with longstanding persistent AF, (p = 0.039) [64].
Seven patients suffered procedure related complications. Two had bleeding requiring thoracotomy and recovered without sequelae. One required a dual chamber pacemaker after restoration of sinus rhythm. Another one developed pleural and pericardial effusions that required drainage. Unfortunately, two suffered permanent phrenic nerve injury [64].
The authors emphasized that the likelihood of success and potential gains in patient quality of life had to be weighed against the risks of complications. They concluded that individuals with paroxysmal AF after unsuccessful catheter ablation, or those with relatively shorter durations of persistent AF appeared to be the best candidates for hybrid ablation [64].
The “European multicentre experience of staged hybrid atrial fibrillation ablation for the treatment of persistent and longstanding persistent atrial fibrillation” was reported by Haywood and associates in January of 2020 [65]. Patients from four European cardiothoracic centers, underwent a 1st stage video-assisted thoracoscopic (VATS) epicardial surgical AF ablative procedure. After ≥ 8 weeks, 166 of 175 (95%) patients underwent endocardial RF catheter ablation. The majority of gaps found during the endocardial procedure were found around the right PVs (28%) and in the roof line (36%) [65].
At a median follow up of 18 months 93/166 (56%) remained free of atrial tachyarrhythmia recurrence without antiarrhythmic drugs. At last clinic follow-up, 110/166 (62.9%) were in sinus rhythm without antiarrhythmic drugs and 132/175 (75.4%) were in sinus rhythm ± antiarrhythmic drugs. Implantable loop recorders were inserted in 56 patients. At a median 14 months of follow-up, 60% had remained fully arrhythmia free and 80% had an AF burden < 5%. Among all patients who underwent at least the 1st stage procedure, outcomes in persistent and longstanding persistent AF did not differ significantly [65].
Complications of first stage surgical ablation were relatively frequent and occurred in 35 of 175 patients (20%). These included phrenic nerve injuries (20; only four after 3–12 months), hemothorax (6), liver abrasion (1), pleural effusion (3), pneumonia (3), pericarditis (1), pericardial effusion (1), pericardial adhesions (1), gastrointestinal bleeding (1), obstructive ileus requiring hemicolectomy (1), transient ischemic attack (1), stroke (5), temporary acute kidney injury (3), and left atrial appendage thrombus (1; on preoperative transesophageal echo). Complications of second stage catheter ablation occurred in four of 166 patients (2.4%) and included pericarditis (1), bradycardia requiring permanent pacing (2), and damage to a permanent right atrial pacing lead (one; revision not required, pacing not needed). The authors speculated that patients severely affected by AF symptoms who have unfavorable characteristics for catheter ablation alone, may accept the procedural risks in order to gain the increased level of efficacy represented by this approach [65].
A multicenter randomized controlled trial, CONVERGE (Convergence of Epicardial and Endocardial Ablation for the Treatment of Symptomatic Persistent AF), evaluated the safety of hybrid ablation for treatment of persistent and longstanding persistent AF and compared its efficacy to catheter ablation [61]. Endocardial and epicardial procedures were performed in one setting [66].
This industry (AtriCure, Mason, OH, USA) sponsored trial, randomized (in a 2:1 ratio) patients (ages 18–80 years) with symptomatic persistent AF refractory to or intolerant of ≥ 1 class I or class III antiarrhythmic drugs with left atrial diameters ≤ 6 cm to endocardial catheter or hybrid convergent ablation. Longstanding persistent AF was present in 42% of the study participants [66].
Epicardial ablation, in contrast to seemingly preferable techniques described previously, was achieved using pericardioscopic access with vacuum-assisted delivery of unipolar RF energy (EPi-Sense, AtriCure, Mason, OH, USA). Subsequently, endocardial ablation was performed (using an irrigated RF catheter) aiming to assure that PV isolation was complete, linear lesion gaps were filled, and cavotricuspid isthmus block was achieved [66]. If AF termination did not result, complex fractionated atrial electrograms could, at the operator’s discretion, be targeted with/without intent to alter the GPs [66].
The follow-up duration targeted was 1 year [5, 66]. Following a 3-month blanking period, the primary end point, was freedom from atrial tachyarrhythmias [AF/atrial flutter (AFL)/atrial tachycardia (AT)] without use of class I or class III antiarrhythmic agents (except previously intolerable or failed drugs without dosage increase). Secondary endpoints included an AF burden reduction of 90% as well as freedom from AF (only) absent dose increases or new class I or class III antiarrhythmic agents [5, 65].
Hybrid convergent ablation was superior to endocardial catheter ablation for persistent and longstanding persistent AF. Freedom from atrial arrhythmias in the absence of new or increased doses of previously failed (class I/III) antiarrhythmic drugs was 67.7 vs. 50.0% (p = 0.036). Without antiarrhythmic drugs success rates were 53.5 vs. 32.0% (p = 0.0128). Follow-up (via 7-day Holter monitor) at 18 months revealed that ≥ 90% AF burden reduction was achieved in 74% of hybrid convergent patients versus just 55% among those who only underwent endocardial catheter ablation. Major adverse events were more common in the hybrid convergent group (8/102, 7.8% vs. 0/51, 0%, p = 0.0525), however, the difference was not quite statistically significant [5, 67].
In a 2022 report from Bhatia and colleagues [68], 81 consecutive patients underwent stage 1 epicardial (VATs) surgical ablation. During the surgical procedure, acute isolation of the PVs and PW was achieved in all patients. At the end of this stage, the left atrial appendage (LAA) was excluded (AtriClip, AtriCure, Mason, Ohio) [67].
Sixty-four patients underwent endocardial catheter mapping and ablation (stage 2). Endocardial ablation was not performed after surgical ablation in 17 patients. Twelve had stage 1 complications including two intracranial hemorrhages, two instances of cardiac thrombosis, two phrenic nerve injuries, and one instance each of gastrointestinal bleeding, heparin-induced thrombocytopenia, decompensated heart failure, perioperative stroke, pulmonary embolism, and constrictive pericarditis [68].
Stage 2 included mapping of the pulmonary veins and/or posterior wall (PW) seeking reconnections, as well as selective examination of low-voltage zones and searches for potential localized AF drivers. Modes of AF recurrence after surgical ablation were identified by systematic high-density mapping looking for pulmonary vein and posterior wall reconnection as well as other mechanisms during the endocardial portion of hybrid ablation. Gaps in PV isolation or PW isolating box lesion sets were recorded, ablated, and related to post-surgical AF recurrence. Completion of the endocardial lesion set was defined by isolation of the PVs and the PW [68].
Patient-tailored additional lesions were also delivered. Left atrial voltage maps were used to identify low-voltage zones (bipolar voltage < 0.45 mV in sinus rhythm or < 0.31 mV during AF) and were selectively ablated. Low-voltage-zone ablation was accomplished in six patients by creating a line of block to the nearest non-conducting structure. In 21 patients with sustained AF a 64-pole basket was advanced to the left and right atria to identify (a) focal impulses, with centrifugal activation, or (b) rotational activation (reentrant drivers), in localized regions for > 50% of the duration of mapped AF [63, 64]. Ablation was abandoned at sites that increased esophageal temperatures or overlayed regions of phrenic nerve capture [68, 69].
During stage 2, reconnection of the PVs was noted in 18/64 patients (28.1%). Nevertheless, no relationship was found between PV reconnection and the presence or absence of recurrent AF. Acutely, after (stage 2) endocardial ablation, all patients had complete PV isolation. PW isolation was achieved in 57/64 (89.1%) but was not able to be completed in seven patients due to rising esophageal temperatures [68].
Inefficacy events were defined as AF > 30 s in duration. Safety endpoints included atrioesophageal fistula, phrenic nerve paralysis, unsuccessful appendage ligation, stroke or transient ischemic attack, and all-cause mortality [68].
Recurrence of AT/AF after stages 1 and 2 was more prevalent in patients when PW isolation could not be achieved, compared to those with a completed lesion set (p = 0.042). Freedom from recurrence of AT/AF was not influenced by AF termination during ablation or the present/absence of low-voltage zones outside the epicardial lesion set. Nevertheless, low-voltage zones were associated with post-surgical stage 1 AF recurrences [68]. Similarly, Masuda et al. [70] have noted that patients with low-voltage areas demonstrated poor long-term rhythm outcomes irrespective of low-voltage area ablation.
Among the 64 patients who underwent Stages I and II, one patient died from cancer at 9 months. Another patient who only underwent surgical ablation suffered a respiratory arrest and died several months later [68].
The “Pivotal Study Of A Dual Epicardial and Endocardial Procedure (DEEP) Approach for Treatment of Subjects With Persistent or Long Standing Persistent Atrial Fibrillation With Radiofrequency Ablation” aimed to demonstrate safety and efficacy of combining epicardial with endocardial procedures for patients with persistent AF or longstanding persistent AF [5, 71]. Results from the DEEP treatment approach for persistent or longstanding persistent AF with RF energy application were summarized in a 2023 abstract [72].
Ninety patients were enrolled from 2015 to 2020. Inclusion criteria were failed class I/III antiarrhythmic drugs, a left atrial diameter (LAD) ≤ 5.5 cm, and ≤ 2 prior unsuccessful endocardial catheter ablations. Persistent AF was present in 83.3% and 16.7% had longstanding persistent AF [72].
Nearly half (48%) had failed endocardial catheter ablation. Their median AF duration was 3.8 years. Mean left atrial diameter was 4.5 cm. Epicardial surgical ablation consisted of PV isolation, creation of floor and roof lines (to form a posterior LA box), left superior PV to left atrial appendage ablation, ablation of ganglionic plexi plus the ligament of Marshall as well as left atrial appendage exclusion. Catheter ablation was performed 91–121 days after the epicardial procedure [72].
Primary effectiveness (defined as freedom from > 30 s of atrial tachyarrhythmia without addition of new or increased dosing of previously failed antiarrhythmic drugs) through 1 year, was 70.6% (60/85; 95% CI 60.9%, 80.3%, p = 0.0232). Through 12 months, freedom from AF was 78.8% (p = 0.0002). The rate of adverse events was 6.7% [72]. The authors concluded hybrid ablation was effective for persistent AF or longstanding persistent AF when the risk–benefit ratio is deemed favorable, given the limited treatment alternatives [72].
Due to its electroanatomic properties Bachmann’s bundle is the preferential pathway of interatrial conduction. It travels between the superior vena cava and the ascending aorta from the right atrium to the left atrium [73, 74]. Changes in Bachmann’s bundle structure may result in longitudinal dissociation in conduction via adjacent muscle fibers, facilitating re-entry and precipitation of AF [74].
In 2000, Kumagai et al. [75] demonstrated that RF application to Bachmann’s bundle could terminate and/or prevent induction/reinduction of AF episodes in six dogs with sterile pericarditis. In 2022, De Martino and colleagues reported results of a two-arm non-randomized study investigating the safety and efficacy of Bachmann’s bundle (BB) ablation in patients with longstanding persistent AF undergoing hybrid ablation [76].
Sixty consecutive patients underwent epicardial isolation of the pulmonary veins plus the left atrial posterior wall (box lesion) half with and half without additional BB ablation. Within 6 weeks post-surgery, each patient underwent an endocardial procedure to look for potential lesion gaps and assess the need for additional atrial substrate modification. Freedom from AF through 12 months of follow-up was the primary endpoint [76].
At completion of the procedure, 30 (100%) and 17 (56%) patients spontaneously converted to sinus rhythm within the BB ablation arm vs. conventional/non-BB ablation group, respectively (p < 0.001). Freedom from atrial tachyarrhythmia recurrence at 12 months was 96.6% within the BB ablation arm compared to 76.6% among non-BB ablation patients (p = 0.025). Despite these findings, no significant differences in complication rates or quality of life were noted. The authors concluded that adding BB ablation to hybrid procedures targeting longstanding persistent AF is both feasible and effective at increasing sinus rhythm maintenance without increasing procedural complications [76].
A recent trial compared hybrid ablation to repeated catheter ablation for individuals with long standing persistent AF. Forty-one ablation-naive patients with long standing persistent AF were randomized to catheter ablation (n = 22) or hybrid ablation (n = 19) and received pulmonary vein isolation, posterior left atrial wall isolation and, if needed, ablation of the cavotricuspid isthmus. Repeat catheter ablation, until 6 months after the initial catheter ablation procedure, did not count as a failure, whereas any re-intervention in the hybrid arm after the 3-month blanking period counted as a failure [77].
Surgical ablation was performed using the video-assisted thoracoscopic approach and bipolar RF applications. Isolation of the left PVs was performed, followed by creating roof and inferior lines to create a box lesion connecting the superior and inferior pulmonary veins with each other. Thereafter, right sided PV isolation was performed. Upon completion of the surgical ablation, endocardial validation was performed. If needed, touch-up endocardial RF ablative lesions were applied. In the event of a previously documented right- or left-sided atrial flutter or tachycardia, these arrhythmias were also ablated [77].
The minimal lesion set performed during endocardial catheter ablation was bilateral PV isolation and a box lesion excluding the posterior left atrium. Prior to ending the procedure, the box lesion and bilateral PVs were checked for entrance and exit block in sinus rhythm. Touch-up RF applications were delivered as needed [77].
Most patients in the two arms (90 and 91%) had persistent rather than long standing persistent AF. Severe left atrial dilatation and CHA2DS2-VASc scores > 3 were more frequent in the hybrid ablation group. A greater number of patients with chronic obstructive lung disease and obstructive sleep apnea were randomized to the hybrid ablations arm. More congestive heart failure patients were randomized to the catheter ablation arm [77].
Despite procedure times being longer in the hybrid arm (p < 0.001), radiation dose and total radiation exposure times were significantly increased in the catheter ablation group (p = 0.004 for dose; p < 0.001 for time). Hospital length of stay was shorter in catheter ablation recipients (p < 0.001) [77].
Freedom from supraventricular tachyarrhythmias with a duration > 5 min off antiarrhythmic drugs at 1 year was the primary efficacy endpoint. This endpoint was significantly greater in the hybrid group (89 vs. 41%, p = 0.002). Likewise, freedom from supraventricular tachyarrhythmias lasting > 30 s off antiarrhythmic drugs at 1 year (the secondary effectiveness outcome), was also significantly greater within the hybrid arm (89 vs. 36%, p < 0.001) [77].
Allowing antiarrhythmic drug use, freedom from documented atrial tachycardias was 95% within 12 months in the hybrid ablation arm versus 41% in the catheter ablation group (p < 0.001). Redo ablations were not performed within 6 months in catheter ablation group [77].
Among primary safety outcomes one patient in the hybrid arm had pericarditis requiring pericardiocentesis and one catheter ablation group patient had femoral arterial bleeding requiring surgical intervention. Among secondary safety outcomes a femoral arteriovenous fistula occurred in one hybrid group patient and one patient in the catheter ablation arm developed hematuria after urinary bladder catheter insertion and had their ablation postponed [77]. Table 4 summarizes the evolution of hybrid ablation.
Table 4.
Evolution of hybrid ablation
| First author, references (year) | Cases/participants | Age, years | Follow-up, years (unless otherwise indicated) | Study type | Key findings/messages |
|---|---|---|---|---|---|
| Wolf et al. [46] | 27 | 57.2 ± 14.9 | 173.6 days |
Single center: A new, minimally invasive, video-assisted thoracoscopic surgical (VATS) technique for AF ablation described |
Perioperative mortality: 0% Hospital stay: 3.3 ± 1.0 days Late mortality: 0% Reintervention: 0% AF-free at 3 months: (21/23) 91.3% AF-free at 3 months off AADs: 65.3% |
| Edgerton et al. [47] | 52 | 60.3 |
6 and 12 months via 24-h Holter monitor, 14-day event recorder or pacemaker interrogation |
Single center: Thoracoscopic RF pulmonary vein isolation, ablation of GPs, and excision or stapling of LAA in 88% |
Maintenance of sinus rhythm: 86.3% at 6 months and 80.8% at 12 months 81.4% (35/43) were not receiving AADs at 6 months and 89.2% (33/37) at 12 months |
| Krul et al. [52] |
31 Paroxysmal AF = 16 Persistent AF = 13 Longstanding persistent AF = 2 |
57 ± 7 years | 1 year |
Single center: Thoracoscopic PVI in all patients ALAL for persistent and longstanding persistent AF Extensive periprocedural electrophysiological testing during procedure |
At 1 year, 19 of 22 patients (86%) had no recurrent atrial tachyarrhythmias off AADs Three patients required a sternotomy because of uncontrolled bleeding There was 1 hemothorax, 1 pneumothorax, and 2 developed pneumonia treated with antibiotics |
| Mahapatra et al. [53] |
15 patients with persistent or longstanding AF underwent sequential hybrid ablation 30 patients with persistent or longstanding AF underwent catheter ablation |
Sequential:59.5 ± 2.4 Catheter only: 59.2 ± 1.5 |
Mean 20.7 ± 4.5 months |
Comparison between patients that underwent sequential surgical epicardial-catheter endocardial ablation for persistent or longstanding persistent AF versus 30 who were treated only with catheter ablation All failed at least 1 prior catheter ablation |
13/15 (86.7%) sequential patients versus 16/30 (53.3%) catheter-alone patients, were free of any atrial arrhythmia and off of AAD (p = 0.04). On AAD, 14/15 (93.3%) sequential patients were free of any atrial arrhythmia recurrence, compared to 17/30 (56.7%) catheter-alone patients (p = 0.01) |
| Pison et al. [54] |
26 total 15 paroxysmal AF 10 persistent AF 1 longstanding persistent AF |
56.8 ± 8.4 | 470 ± 154 days |
Single center simultaneous combined thoracoscopic surgical and transvenous CA of paroxysmal and persistent AF |
Over 1/3 of all patients had 1 or more previous catheter ablations Overall single-procedure success rate of 83% at 1 year |
| Je et al. [56] |
1877 total patients Minimally invasive endocardial Cox-maze 145 Minimally invasive epicardial surgical ablation 1382 Minimally invasive hybrid surgical ablation 350 |
59.1 |
15.1 ± 10.3 Range 6–55 months |
Systematic Review of 37 studies |
At 1 year, rates of sinus rhythm restoration were 93, 80, and 70%, and sinus restoration without anti-arrhythmic medications were 87, 72, and 71%, for Cox-maze, epicardial and hybrid procedures, respectively The minimally invasive Cox-maze procedure with CPB support also had important safety advantages in conversion to sternotomy and major bleeding |
| Al-Jazairi et al. [64] |
50 total 5 paroxysmal, 34 persistent 11 longstanding persistent |
57 ± 9 | Efficacy assessed with 12-lead EKG and 72-h Holter monitoring after 3, 6, and 12 months | Single center, single-stage hybrid (thoracoscopic surgical and transvenous CA) AF ablation |
At 1-year follow-up, 76% of patients maintained sinus rhythm off antiarrhythmic drugs Patients with paroxysmal AF had the greatest success, longstanding persistent AF patients had the poorest (significantly lower) results Complications occurred in 7 patients: Bleeding requiring thoracotomy (2); Permanent phrenic nerve injury (2); Pericardial and pleural effusions requiring drainage (1); Pleural effusion requiring drainage (1); Permanent pacing needed due to sinus node dysfunction (1) |
| Haywood et al. [65] |
175 total 9 did not undergo endocardial ablation 166 (95%) underwent staged hybrid ablation |
62.2 ± 8.5 | Median 18 months | Multicenter experience (four European centers) with staged hybrid ablation for persistent and longstanding persistent AF |
Endocardial procedure performed ≥ 8 weeks post-surgery After (median) 18 months follow-up 56% were AF-free of AADs At last clinic follow-up, 132/175 (75.4%) were in sinus rhythm ± AADs Complications of the surgical stage of ablation occurred in 35/175 (20%). Complications of the second stage occurred in 4/166 (2.4%)* |
| DeLurgio et al. [67] |
153 total randomized 2:1 to hybrid or endocardial catheter ablation Results from 12 months available in 149/153 (97.4%) |
Hybrid: 63.7 ± 9.6 Endocardial catheter ablation: 65.1 ± 6.7 |
Twenty-four–hour Holter monitoring at 6 and 12 months 7-day monitor at 18 months Phone follow-up at years 2–5 |
Multicenter, randomized controlled trial comparing hybrid ablation to catheter ablation in patients with a left atrial size ≤ 6.0 cm and drug-resistant persistent and longstanding (42%) persistent AF |
Hybrid convergent ablation had superior efficacy: AF freedom, absent new or increased dosage of previously failed class I/III AADs of 67.7% versus 50.0% (P = 0.036); AF freedom, off AADs, of 53.5% versus 32.0% (P = 0.0128) Major adverse events in the hybrid arm within 7 days: stroke (1), excessive bleeding (1), and excessive bleeding with late pericardial effusion (1) Major adverse events in the hybrid arm within 8–30 days: pericardial effusions (3), one phrenic nerve injury (1), and transient ischemic attack (1) Major adverse events were not reported in the catheter ablation arm |
| Ellenbogen et al. [72] |
90 total 48% had failed catheter ablation Persistent AF present in 83.3% Longstanding persistent AF present in 16.7% |
63.4 ± 7.74 |
1 year 24-h rhythm monitoring and ECG performed 6 and 12 months post catheter ablation |
Multicenter (17 sites) trial of staged hybrid ablation |
Catheter ablation was performed 91–121 days after epicardial procedure Primary effectiveness (freedom from atrial tachyarrhythmias [> 30 s] absent new/increased dose of previously failed AAD) was 70.6% (p = 0.0232); freedom from AF was 78.8% (p = 0.0002) Adverse events occurred in 6 patients and were equally distributed between the 2 procedural arms |
*Specific complications are enumerated in the text
AADs antiarrhythmic drugs; AF atrial fibrillation; ALAL additional left atrial lines; BPs ganglionic plexi; CA catheter ablation; CM centimeters; ECG electrocardiogram; LAA left atrial appendage; VATS video-assisted Thoracoscopic
Discussion: Assessing Risk–Benefit Among Surgical, Hybrid, and Catheter Ablation of AF
The most efficacious non-pharmacological treatment for AF is still the Cox-maze surgical approach [78]. A systematic review and meta-analysis, revealed freedom from AF at 12-months was superior among surgical vs. catheter ablation cohorts (78.4 vs. 53%; RR, 1.54; 95% CI 1.50, 2.14; I2 = 0%; p < 0.0001) and was similar for final pooled follow-up of all studies included (78.2 vs. 55.4%; RR, 1.39;95% CI 1.15, 1.68; I2 = 66%; p = 0.0006) [79].
The major concern associated with thoracoscopic surgical ablation is the risk of adverse events. Major complications, including pulmonary vein (PV) stenosis, pericardial effusion, cardiac tamponade, pneumothorax, hemothorax, pneumonia, major bleeding, conversion to complete thoracotomy, TIA, stroke, myocardial infarction and death, were significantly higher in the surgical ablation cohort (28.2 vs. 7.8%; RR, 3.30; 95% CI 1.73, 6.29; I2 = 13%; p = 0.0003). However, when assessing complications individually between groups, only pneumothorax (6.2 vs. 0%) and pleural effusions (6.2 vs. 0%) were significantly different [79].
While catheter ablation of AF has been effective for paroxysmal AF, it is frequently insufficient for persistent and longstanding persistent AF. The evidence presented above demonstrates that hybrid ablation is a better option for persistent and longstanding persistent AF. Despite hybrid ablation having a slightly higher complication rate compared to catheter ablation, morbidity and mortality are, overall, low when performed by experienced operators [77].
Nevertheless, catheter ablation remains a common choice as first-line and second-line (after antiarrhythmic drug failure) therapy in the United States (US). A recent multicenter trial from nine US centers demonstrated that more than half of the patients with persistent AF underwent ablation prior to initiation of class I or III antiarrhythmic drug therapy. Arrhythmia-free survival was similar between those undergoing first-line ablation compared to those receiving second-line ablation. Not surprisingly, those undergoing second line ablation were more likely to continue receiving antiarrhythmic drugs [80]. Likewise, in the SARA study (Study of Ablation Versus antiaR-rhythmic Drugs in Persistent Atrial Fibrillation) the freedom atrial tachyarrhythmias was significantly higher in the ablation group (70.4 vs. 43.7%; P = 0.002) [81]. In a substudy of the CABANA trial [82], AF burden was reduced significantly in catheter ablation recipients, regardless of whether AF was paroxysmal or persistent [83].
The 2017 ACC/AHA/ACCP/HRS indications for hybrid and surgical ablation focused extensively on surgical ablation [48]. The 2023 indications for hybrid and surgical ablation are summarized in Table 5 [3]. Although far more succinct, the 2023 position on hybrid ablation fits the subsequently accumulated data more accurately. The 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation provides both a procedural perspective (see Table 6) as well as future directions to follow to improve management of AF (see below) [84].
Table 5.
Recommendations for surgical ablation
| COR | LOE | Recommendations |
|---|---|---|
 |
 |
1. For patients with AF who are undergoing cardiac surgery, concomitant surgical ablation is beneficial to reduce the risk of recurrent AF |
 |
 |
2. In patients undergoing surgical ablation, anticoagulation is reasonable for at least 3 months after the procedure to reduce thromboembolic risk |
 |
 |
3. For patients with symptomatic persistent AF refractory to antiarrhythmic drug therapy, hybrid epicardial and endocardial ablation might be reasonable to reduce the risk of recurrent atrial tachyarrhythmia |
COR Class (Strength) of recommendation
LOE Level (Quality) of evidence
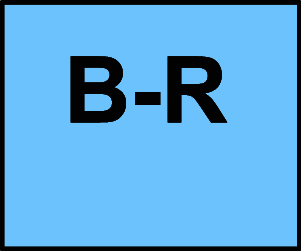
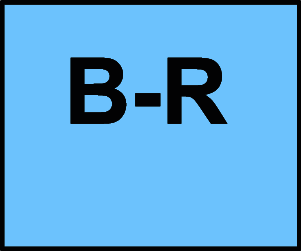
B-R Moderate-quality evidence from 1 or more randomized controlled trials (RCTs); Meta-analyses of moderate-quality RCTs
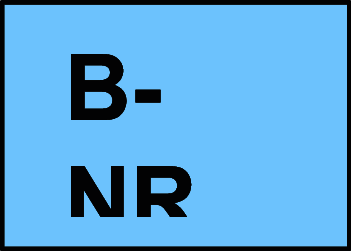
B-NR Data derived from nonrandomized trials
AF atrial fibrillation
Adapted from reference 3, with permission
Table 6.
2024 Guidelines for surgical and hybrid AF ablation
| Clinical scenarios | Advice/Recommendations |
|---|---|
| Concomitant surgical AF ablation is beneficial in patients with paroxysmal or persistent AF undergoing left atrial open cardiac surgery regardless of prior antiarrhythmic drug failure or intolerance | TO DO |
| Concomitant surgical AF ablation is beneficial in patients with paroxysmal or persistent AF intolerant or refractory to previous antiarrhythmic drug therapy, undergoing closed (non-left atrial open) cardiac surgery | TO DO |
| Biatrial Cox-maze procedure or a minimum of PVI plus left atrial posterior wall isolation is beneficial in patients undergoing surgical AF ablation concomitant to left atrial open cardiac surgery | TO DO |
| Documentation of exit and/or entrance block across pulmonary veins and completeness of deployed lines is beneficial during surgical AF ablation | TO DO |
| Concomitant surgical AF ablation is reasonable in patients with paroxysmal or persistent AF prior to initiation of Class I or III antiarrhythmic therapy, undergoing closed (non-left atrial open) cardiac surgery | May be appropriate TO DO |
| Stand-alone surgical or hybrid ablation is reasonable in symptomatic patients with persistent AF with prior unsuccessful catheter ablation as well as in those who are intolerant or refractory to antiarrhythmic drug therapy and prefer a surgical/hybrid approach, after careful consideration of relative safety and efficacy of treatment options | May be appropriate TO DO |
| Stand-alone surgical or hybrid ablation may be reasonable in symptomatic patients with paroxysmal AF with prior unsuccessful catheter ablations who prefer a surgical/hybrid approach, after careful consideration of relative safety and efficacy of treatment options | Area of uncertainty |
AF atrial fibrillation; PVI pulmonary vein isolation
Adapted from reference 84 with permission
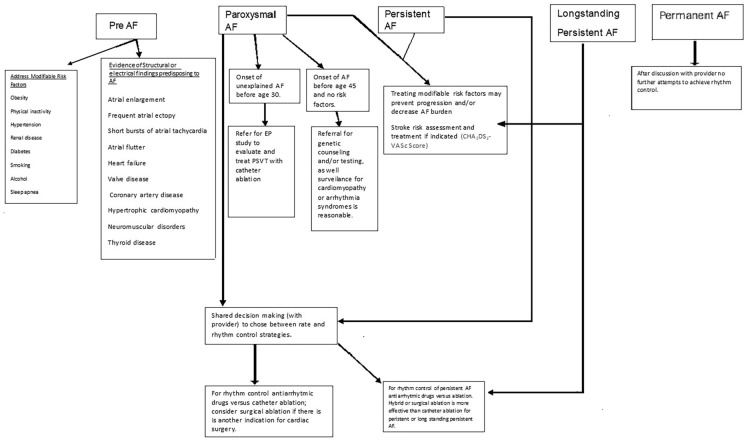
Although many treatment options exist, management of atrial fibrillation remains imperfect. Therapeutic options include risk factor management and awareness of structural/electrical abnormalities that may precipitate AF. Pharmacotherapy is available but may be limited by lack of efficacy and side effects. While catheter ablation is effective for paroxysmal AF, it is less so in persistent and longstanding persistent AF. Surgical ablation, despite its efficacy, seems most appealing when concomitant cardiac surgery is indicated. Hybrid ablation includes features of catheter and surgical ablation that are complimentary (see Table 7). Ultimately, the course of therapy should result from shared decision making between the patient and his/her physician(s) as outlined in Fig. 4.
Table 7.
Rationale of hybrid ablation of atrial fibrillation
| Creation of completed lines |
|---|
| 1. Surgical approach may be more complete in making transmural ablation lines |
| (a) Ablation tools are designed for making lines |
| (b) Smooth epicardial surface ideal for surgical tools |
| (c) Visual imaging reveals the atrial surface, ablation lines, and gaps in lesions |
| 2. Catheter ablation may be most effective in targeting specific lesions |
| (a) Catheter ablation is designed to create point lesions |
| (b) Catheter ablation can slip off endocardial ridges, or trabeculations, thus breaking up lines |
| (c) Even with ultrasound imaging, assessing continuing of endocardial lesions may be difficult |
| Complementary nature of epicardial and endocardial ablation |
| 1. Epicardial ablation |
| (a) Heat sink of the circulating blood in the atrial chamber limits depth |
| (b) Epicardial lesions may be limited by fat |
| (c) Depth of ablation lesions may be insufficient |
| (d) May fail to penetrate the endocardium |
| 2. Endocardial ablation |
| (a) Creating transmural lesions may be difficult |
| (b) Endocardial ablation may result in collateral damage to anatomic structures |
| Together these techniques complement each other! |
| Role of mapping |
| 1. Epicardial mapping may be limited |
| (a) Constrained by pericardial reflections |
| (b) Absence of sophisticated tools and mapping systems designed for epicardial use |
| (c) Epicardial fat may limit mapping |
| 2. Endocardial mapping |
| (a) Extensive experience in mapping |
| (b) Large range of tools and technology |
| (c) Formally trained |
| (d) Mature enabling technology |
| Together these techniques complement each other! |
| 2. Unique targets |
| (a) Surgical epicardial ablation |
| (i) Full division of ligament of Marshall |
| (ii) LAA removal division |
| (iii) Targeted ganglionic plexi ablation |
| (iv) Safer superior vena cava isolation |
| (b) Transcatheter endocardial ablation |
| (i) More effective cavotricuspid isthmus line |
| (ii) Atrial flutter and atrial tachycardia ablation |
| (iii) Coronary sinus ablation |
| (iv) Map for flutter |
| (v) Mapping techniques, such as FIRM and CFAE |
| Together these techniques complement each other! |
CFAE indicates complex fractionated atrial electrograms; FIRM focal impulse and rotor modulation; and LAA left atrial appendage
Reproduced from reference (61) with permission
Fig. 4.
Flow chart for management of AF. AF atrial fibrillation, CHA2DS2-VASc Congestive heart failure/LV dysfunction (1 point); hypertension (1 point); aged ≥ 75 years (2 points); diabetes mellitus (1 point); stroke/transient ischemic attack/thromboembolism (1 point); vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque] (1 point); aged 65–74 years (1 point); female gender (1 point)
Adapted from reference 3, with permission
Future Perspectives
As the population ages, the worldwide prevalence of AF is increasing. It has been projected that the AF burden may increase more than 60% by 2050 [4]. In 2018, The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand reported an increasing prevalence of AF in older people as well as in Aboriginal and Torres Strait Islander peoples [85]. While we have identified risk factors associated with AF development, management of them is often difficult. The impact of risk factor modification on AF recurrence is not completely understood. Four main pathophysiological mechanisms contribute to AF development including electrical remodeling, structural remodeling, autonomic nervous system changes, and Ca++ handling abnormalities. AF is self-perpetuating because AF-induced remodeling enhances cardiac vulnerability to AF induction and maintenance [4]. However, our knowledge of AF’s mechanisms remains incomplete [4, 84]. Hopefully, basic science investigation will cast additional light on AF mechanisms. Genetic studies may provide new insights that contribute to prevention and therapeutic efficacy [84]. While pharmacotherapy for AF has been disappointing, the safety and efficacy of AF catheter ablation has improved. Nevertheless, many challenges remain. Optimization of cryoablation and radiofrequency energy delivery is a worthy target. While pulsed field (PFA) ablation is exciting, we need to learn the optimal settings for AF ablation. Clarifying whether PFA will improve the safety and efficacy of additional substrate modification or will be associated with unrecognized safety concerns will be pivotal [84]. Although catheter ablation is effective for paroxysmal AF, surgical and hybrid ablation are more effective than catheter ablation for treatment of persistent AF. Unfortunately, the risks of surgical complications exceed that of catheter ablation alone. Cooperative efforts to reduce the risk of hybrid ablation will be essential. The evolution of treatment strategies is certain to continue. Time will reveal which therapeutic ablation approach will evolve fastest and yield the best results.
Conclusions
AF is the most common sustained cardiac tachyarrhythmia, and its prevalence and incidence are increasing. Pharmacotherapy has significant limitations including limited efficacy, numerous side effects, and the potential for proarrhythmia. Surgical treatment of atrial fibrillation has potential to reduce mortality and prevent stroke. New ablative energy sources (such as pulsed field ablation and other evolving techniques) are promising but are far from a panacea. Hybrid ablation has emerged as an option for persistent and longstanding persistent AF. Continued cooperation and teamwork between cardiac surgeons and cardiac electrophysiologists promises to result in greater results and fewer complications.
Author Contributions
Richard G. Trohman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Richard G. Trohman Acquisition of data: Richard G. Trohman Analysis and interpretation of data: Richard G. Trohman. Drafting of the manuscript: Richard G. Trohman Critical revision of the manuscript for important intellectual content: Richard G. Trohman Administrative, technical, or material support: Richard G. Trohman.
Funding
No funding or sponsorship was received for this study or publication of this article.
Declarations
Conflict of Interest
Richard G. Trohman reported serving as an advisor to Boston Scientific/Guidant; receiving research grants from Boston Scientific/Guidant, Medtronic Inc, St Jude Medical (Abbott), Vitatron, and Wyeth- Ayerst/Wyeth Pharmaceuticals; serving as a consultant for Biosense Webster, Alta Thera Pharmaceuticals and Newron Pharmaceuticals P.s.A.; and receiving speakers fees or honoraria Boston Scientific/Guidant CRM, Medtronic Inc, Daiichi Sankyo, Alta Thera Pharmaceuticals and St Jude Medical (Abbott). Richard G. Trohman is an Editorial Board member of Cardiology and Therapy. Richard G. Trohman was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Richard G. Trohman attests that these potential conflicts of interest did not influence the contents of this manuscript.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Cox JL, Schuessler RB, Boineau JP. The development of the maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:2–14. 10.1016/S1043-0679(00)70010-4 [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–16. 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 3.Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2023;149:e1–156. [Google Scholar]
- 4.Trohman RG, Huang HD, Sharma PS. Atrial fibrillation: primary prevention, secondary prevention, and prevention of thromboembolic complications: part 1. Front Cardiovasc Med. 2023;10:1060030. 10.3389/fcvm.2023.1060030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trohman RG, Huang HD, Sharma PS. Atrial fibrillation: Primary prevention, secondary prevention, and prevention of thromboembolic complications Part 2. Front Cardiovasc Med. 2023;9:1060096. 10.3389/fcvm.2022.1060096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023;389:1660–71. 10.1056/NEJMoa2307291 [DOI] [PubMed] [Google Scholar]
- 7.Erol A. Basics of writing review articles. Noro Psikiyatr Ars. 2022;59(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox JL, Churyla A, Malaisrie SC, Kruse J, Pham DT, Kislitsina ON, et al. When is a maze procedure a maze procedure ? Can J Cardiol. 2018;34:1482–91. 10.1016/j.cjca.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 9.Sealy WC, Gallagher JJ, Kasell J. His bundle interruption for control of inappropriate ventricular responses to atrial arrhythmias. Ann Thorac Surg. 1981;32(5):429–38. 10.1016/S0003-4975(10)61774-2 [DOI] [PubMed] [Google Scholar]
- 10.Scheinman MM, Morady F, Hess DS, Gonzalez R. Catheter induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982;248:851–5. 10.1001/jama.1982.03330070039027 [DOI] [PubMed] [Google Scholar]
- 11.Trohman RG, Simmons TW, Moore SL, Firstenberg MS, Williams D, Maloney JD. Catheter ablation of the atrioventricular junction using radiofrequency energy and a bilateral cardiac approach. Am J Cardiol. 1992;70:1438–43. 10.1016/0002-9149(92)90296-B [DOI] [PubMed] [Google Scholar]
- 12.Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80:373–80. 10.1016/S0022-5223(19)37762-1 [DOI] [PubMed] [Google Scholar]
- 13.Guiraudon GM, Campbell CS, Jones DL, McLellan DG, MacDonald JL. Combined sinoatrial node atrio-ventricular isolation: a surgical alternative to His bundle ablation in patients with atrial fibrillation. Circulation. 1985;72:III–220. [Google Scholar]
- 14.Edgerton ZJ, Edgerton JR. History of surgery for atrial fibrillation. Heart Rhythm. 2009;6:S1–4. 10.1016/j.hrthm.2009.07.047 [DOI] [PubMed] [Google Scholar]
- 15.Leitch JW, Klein G, Yee R, Guiraudon G. Sinus node-atrioventricular node isolation: long-term results with the “corridor” operation for atrial fibrillation. J Am Coll Cardiol. 1991;17(4):970–5. 10.1016/0735-1097(91)90881-9 [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino HJ Jr, Harada A, Eisenberg SB, et al. Surgical ablation of atrial fibrillation in a canine model of chronic mitral regurgitation. Surg Forum XXXVII. 1986;302:303. [Google Scholar]
- 17.Cox JL. A brief overview of surgery for atrial fibrillation. Ann Cardiothorac Surg. 2014;3(1):80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox JL. The surgical treatment of atrial fibrillation IV Surgical technique. J Thorac Cardiovasc Surg. 1991;101:584–92. 10.1016/S0022-5223(19)36685-1 [DOI] [PubMed] [Google Scholar]
- 19.Stulak JM. Surgical treatment of atrial fibrillation-what have we learned? J Thorac Disease. 2015;7:E237–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox JL, Boineau JP, Schuessler RB, Jaquiss RD, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation I. rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110:473–84. 10.1016/S0022-5223(95)70244-X [DOI] [PubMed] [Google Scholar]
- 21.Stone CM, Chang BC, Tweddell JS, Sato S, Schuessler RB, Barzilai B, Boineau JP, Cox JL. Ablation of atrial fibrillation by the maze procedure. Surg Forum Am Coll Surg. 1989;40:213–5. [Google Scholar]
- 22.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. 10.1016/S0022-5223(03)01287-X [DOI] [PubMed] [Google Scholar]
- 23.Edgerton JR. Surgical therapy for atrial fibrillation. J Cardiovasc Med (Hagerstown Md). 2012;13:125–30. 10.2459/JCM.0b013e32834f2321 [DOI] [PubMed] [Google Scholar]
- 24.Pruitt JC, Lazzara RR, Ebra G. Minimally invasive surgical ablation of atrial fibrillation: the thoracoscopic box lesion approach. J Interv Card Electrophysiol. 2007;20:83–7. 10.1007/s10840-007-9172-3 [DOI] [PubMed] [Google Scholar]
- 25.Vicol C, Kellerer D, Petrakopoulou P, Kaczmarek I, Lamm P, Reichart B. Long-term results after ablation for long-standing atrial fibrillation concomitant to surgery for organic heart disease: is microwave energy reliable? J Thorac Cardiovasc Surg. 2008;136:1156–9. 10.1016/j.jtcvs.2008.05.041 [DOI] [PubMed] [Google Scholar]
- 26.Sandoval E, Castella M, Pomar JL. Current state of the surgical treatment of atrial fibrillation ? Cardiol Res Pract. 2011;2011:746054. 10.4061/2011/746054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pison L, Vroomen M, Crijns HJGM. Surgical ablation for atrial fibrillation. N Engl J Med. 2015;373:483. 10.1056/NEJMc1506893 [DOI] [PubMed] [Google Scholar]
- 28.Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, et al. The Cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades Circulation. Arrhythm Electrophysiol. 2012;5:8–14. 10.1161/CIRCEP.111.963819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–96. 10.1016/j.jtcvs.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 30.Recent-Onset Atrial Fibrillation. Available at: https://coreem.net/core/recent-onset-atrial-fibrillation/ (Accessed: 30 December 2023).
- 31.Lip GYH, Watson T. Atrial fibrillation (acute onset). BMJ Clin Evid. 2008;2008:0210. [PMC free article] [PubMed] [Google Scholar]
- 32.Dewar RI, Lip GYH. Guidelines Development Group for the NICE clinical guideline for the management of atrial fibrillation. Identification, diagnosis and assessment of atrial fibrillation. Heart (British Cardiac Society). 2007;93:25–8. 10.1136/hrt.2006.099861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399–409. 10.1056/NEJMoa1500528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox JL. Surgical ablation for atrial fibrillation. N Engl J Med. 2015;373:483. 10.1056/NEJMc1506893 [DOI] [PubMed] [Google Scholar]
- 35.Gillinov M, Moskowitz AJ, Argenziano M. Surgical ablation for atrial fibrillation. N Engl J Med. 2015;373:484. [DOI] [PubMed] [Google Scholar]
- 36.Soni LK, Cedola SR, Cogan J, Jiang J, Yang J, Takayama H, et al. Right atrial lesions do not improve the efficacy of a complete left atrial lesion set in the surgical treatment of atrial fibrillation, but they do increase procedural morbidity. J Thorac Cardiovasc Surg. 2013;145:356–61. 10.1016/j.jtcvs.2012.09.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malaisrie SC, McCarthy PM, Kruse J, Matsouaka RA, Churyla A, Grau-Sepulveda MV, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: Late outcomes in a Medicare population. J Thorac Cardiovasc Surg. 2021;161:1251-1261.e1. 10.1016/j.jtcvs.2019.10.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur J Cardio-Thoracic Surg. 2020;57:691–700. [DOI] [PubMed] [Google Scholar]
- 39.Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Thorac Cardiovasc Surg. 2019;157:1007–10184. 10.1016/j.jtcvs.2018.07.099 [DOI] [PubMed] [Google Scholar]
- 40.Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:159–70. 10.1016/j.jtcvs.2017.09.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iribarne A, DiScipio AW, McCullough JN, Quinn R, Leavitt BJ, Westbrook BM, et al. Surgical atrial fibrillation ablation improves long-term survival: a multicenter analysis. Ann Thorac Surg. 2019;107:135–42. 10.1016/j.athoracsur.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 42.Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical ablation of atrial fibrillation in the United States trends and propensity matched outcomes. Ann Thorac Surg. 2017;104:493–500. 10.1016/j.athoracsur.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 43.Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol. 1993;4:338–55. 10.1111/j.1540-8167.1993.tb01235.x [DOI] [PubMed] [Google Scholar]
- 44.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. 10.1093/eurheartj/15.suppl_A.9 [DOI] [PubMed] [Google Scholar]
- 45.Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. 10.1111/j.1540-8167.1996.tb00474.x [DOI] [PubMed] [Google Scholar]
- 46.Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB Jr, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:797–802. 10.1016/j.jtcvs.2005.03.041 [DOI] [PubMed] [Google Scholar]
- 47.Edgerton JR, Brinkman WT, Weaver T, Prince SL, Culica D, Herbert MA, et al. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg. 2010;140:823–8. 10.1016/j.jtcvs.2009.11.065 [DOI] [PubMed] [Google Scholar]
- 48.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fragakis N, Pantos I, Younis J, Hadjipavlou M, Katritsis DG. Surgical ablation for atrial fibrillation. Europace: European pacing arrhythmias, and cardiac electrophysiology. Europace. 2012;14:1545–52. 10.1093/europace/eus081 [DOI] [PubMed] [Google Scholar]
- 50.Gelsomino S, Lozekoot P, La Meir M, Lorusso R, Lucà F, Rostagno C, et al. Is ganglionated plexi ablation during maze IV procedure beneficial for postoperative long-term stable sinus rhythm? Int J Cardiol. 2015;192:40–8. 10.1016/j.ijcard.2015.04.259 [DOI] [PubMed] [Google Scholar]
- 51.Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, et al. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol. 2016;68:1155–65. 10.1016/j.jacc.2016.06.036 [DOI] [PubMed] [Google Scholar]
- 52.Krul SPJ, Driessen AHG, van Boven WJ, Linnenbank AC, Geuzebroek GSC, Jackman WM, et al. Thoracoscopic videoassisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–70. 10.1161/CIRCEP.111.961862 [DOI] [PubMed] [Google Scholar]
- 53.Mahapatra S, LaPar DJ, Kamath S, Payne J, Bilchick KC, Mangrum JM, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and longstanding persistent atrial fibrillation with long-term follow-up. AnnThorac Surg. 2011;91:1890–8. 10.1016/j.athoracsur.2011.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. 10.1016/j.jacc.2011.12.055 [DOI] [PubMed] [Google Scholar]
- 55.Calkins H. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation: is this the answer we are searching for? J Am Coll Cardiol. 2012;60:62–3. 10.1016/j.jacc.2012.01.068 [DOI] [PubMed] [Google Scholar]
- 56.Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-maze procedure, beating-heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardio-thorac Surg. 2015;48:531–40. 10.1093/ejcts/ezu536 [DOI] [PubMed] [Google Scholar]
- 57.Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–46. 10.1016/j.hrthm.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 58.Pak HN. Catheter ablation of long-standing persistent atrial fibrillation: a reckless challenge or a way to real cure. Korean Circ J. 2019;49:134–45. 10.4070/kcj.2018.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K, et al. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011;8:1391–7. 10.1016/j.hrthm.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 60.Mansour M, Karst E, Heist EK, Dalal N, Wasfy JH, Packer DL, et al. The impact of first procedure success rate on the economics of atrial fibrillation ablation. JACC: Clin Electrophysiol. 2017;3:129–38. [DOI] [PubMed] [Google Scholar]
- 61.Khoynezhad A, Ellenbogen KA, Al-Atassi T, Wang PJ, Kasirajan V, Wang X, et al. Hybrid atrial fibrillation ablation: current status and a look ahead. Circ Arrhythm Electrophysiol. 2017;10:e005263. 10.1161/CIRCEP.117.005263 [DOI] [PubMed] [Google Scholar]
- 62.Khoynezhad A, Warrier N, Worthington T, Shandling A. A narrative review of hybrid ablation for persistent and longstanding persistent atrial fibrillation. Ann Transl Med. 2021;9:947. 10.21037/atm-21-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. 10.1161/CIRCULATIONAHA.109.928903 [DOI] [PubMed] [Google Scholar]
- 64.Al-Jazairi MIH, Rienstra M, Klinkenberg TJ, Mariani MA, Van Gelder IC, Blaauw Y. Hybrid atrial fibrillation ablation in patients with persistent atrial fibrillation or failed catheter ablation. Neth Heart J. 2019;27:142–51. 10.1007/s12471-019-1228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haywood GA, Varini R, Osmancik P, Cireddu M, Caldwell J, Chaudhry MA, et al. European multicentre experience of staged hybrid atrial fibrillation ablation for the treatment of persistent and longstanding persistent atrial fibrillation. Int J Cardiol Heart Vasc. 2020;26:100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeLurgio DB, Ferguson E, Gill J, Blauth C, Oza S, Mostovych M, et al. Convergence of epicardial and endocardial RF ablation for the treatment of symptomatic persistent AF (CONVERGE Trial): rationale and design. Am Heart J. 2020;224:182–91. 10.1016/j.ahj.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 67.DeLurgio DB, Crossen KJ, Gill J, Blauth C, Oza SR, Magnano AR, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial circulation. Arrhythmia Electrophysiol. 2020;13:e009288. 10.1161/CIRCEP.120.009288 [DOI] [PubMed] [Google Scholar]
- 68.Bhatia NK, Shah RL, Deb B, Pong T, Kapoor R, Rogers AJ, et al. Mapping atrial fibrillation after surgical therapy to guide endocardial ablation Circulation. Arrhythmia Electrophysiol. 2022;15:e010502. 10.1161/CIRCEP.121.010502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. 2014;63:1761–8. 10.1016/j.jacc.2014.02.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, et al. Low-voltage-area ablation in paroxysmal atrial fibrillation -extended follow-up results of the VOLCANO trial. Circ J. 2022;86:245–52. 10.1253/circj.CJ-21-0476 [DOI] [PubMed] [Google Scholar]
- 71.Pivotal Study of a Dual Epicardial and Endocardial Procedure (DEEP) Approach (DEEP Pivotal). 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02393885 (Accessed: 21 Sept 2022)
- 72.Ellenbogen K, Khoynezhad A, de Asmundis C, Kasirajan V, Koneru J, Johnkoski J, et al. Effectiveness and safety of the dual epicardial & endocardial Procedure (DEEP) approach for treatment of subjects with persistent or long standing persistent atrial fibrillation (PersAF/LSPAF) with radiofrequency ablation. Circulation. 2023;148:A15606. 10.1161/circ.148.suppl_1.15606 [DOI] [Google Scholar]
- 73.James TN. The connecting pathways between the sinus node and A-V node and between the right and the left atrium in the human heart. Am Heart J. 1963;66:498–508. 10.1016/0002-8703(63)90382-X [DOI] [PubMed] [Google Scholar]
- 74.van Campenhout MJH, Yaksh A, Kik C, de Jaegere PP, Ho SY, Allessie MA, et al. Bachmann’s bundle: a key player in the development of atrial fibrillation? Circ: Arrhythmia Electrophysiol. 2013;6:1041–6. [DOI] [PubMed] [Google Scholar]
- 75.Kumagai K, Uno K, Khrestian C, Waldo AL. Single site radiofrequency catheter ablation of atrial fibrillation: studies guided by simultaneous multisite mapping in the canine sterile pericarditis model. J Am Coll Cardiol. 2000;36:917–23. 10.1016/S0735-1097(00)00803-2 [DOI] [PubMed] [Google Scholar]
- 76.De Martino G, Nasso G, Gasperetti A, Moscarelli M, Mancusi C, Della Ratta G, et al. Targeting Bachmann’s bundle in hybrid ablation for long-standing persistent atrial fibrillation: a proof of concept study. J Interv Cardiac Electrophysiol. 2022;64:273–80. 10.1007/s10840-021-00971-7 [DOI] [PubMed] [Google Scholar]
- 77.van der Heijden CAJ, Weberndörfer V, Vroomen M, Luermans JG, Chaldoupi SM, Bidar E, et al. Hybrid ablation versus repeated catheter ablation in persistent atrial fibrillation: a randomized controlled trial JACC. Clin Electrophysiol. 2023;9:1013–23. 10.1016/j.jacep.2022.12.011 [DOI] [PubMed] [Google Scholar]
- 78.Badhwar N, Tschopp DR, Lee RJ. Surgical and concomitant epicardial-endocardial (hybrid) ablation of persistent and longstanding persistent atrial fibrillation. Curr Probl Cardiol. 2015;40:245–67. 10.1016/j.cpcardiol.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 79.Phan K, Phan S, Thiagalingam A, Medi C, Yan TD. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardio-thorac Surg. 2016;49:1044–51. 10.1093/ejcts/ezv180 [DOI] [PubMed] [Google Scholar]
- 80.Barkagan M, Milman A, Zahavi G, Younis A, Dhakal B, Dixit S, Wong CX, Gerstenfeld EP, Narayan SM, Bunch JT, Cerbin L, Tzou WS, Metzl M, Khanani A, Siddiqui UR, Mohanty S, Natale A, Medina A, Anter E. Catheter ablation as first-line therapy in persistent atrial fibrillation patient characteristics and clinical outcomes. JACC Clin Electrophysiol. 2024;12:2405–500. 10.1016/j.jacep.2024.02.035. (Epub ahead of print. PMID: 38703164). 10.1016/j.jacep.2024.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mont L, Bisbal F, Hernandez-Madrid A, Pérez-Castellano N, Viñolas X, Arenal A, Arribas F, Fernández-Lozano I, Bodegas A, Cobos A, Matía R, Pérez-Villacastín J, Guerra JM, Ávila P, López-Gil M, Victor Castro V, Arana JI, Brugada J, On behalf of SARA investigators. Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35(8):501–7. 10.1093/eurheartj/eht457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL, CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261–74. 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, Al-Khalidi HR, Rosenberg Y, Mark DB, Lee KL, Packer DL, CABANA Investigators and ECG Rhythm Core Lab. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA Trial. J Am Coll Cardiol. 2020;75(25):3105–18. 10.1016/j.jacc.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tzeis S, Gerstenfeld EP, Kalman J, et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. J Interv Card Electrophysiol. 2024. 10.1016/j.hrthm.2024.03.017. 10.1016/j.hrthm.2024.03.017 [DOI] [PubMed] [Google Scholar]
- 85.Brieger D, Amerena J, Attia JR, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani HM, Hendriks J, Hespe CM, Hung J, Kalman JM, Sanders P, Worthington J, Yan T, Zwar NA. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Med J Aust. 2018;209(8):356–62. 10.5694/mja18.00646 [DOI] [PubMed] [Google Scholar]