Abstract
This study aimed to explore the relationship between female age and pregnancy outcomes in patients undergoing their first elective single embryo transfer (eSET) of in vitro fertilization (IVF) cycles. The retrospective cohort study encompassed 7089 IVF/intracytoplasmic sperm injection (ICSI) patients of the Reproductive Medicine Center, Henan Provincial Peoples’ Hospital of China, from September 1, 2016, to May 31, 2022. Patients all received the first eSET in their IVF/ICSI cycles. A generalized additive model (GAM) was employed to examine the the dose–response correlation between age and pregnancy outcomes, namely the clinical pregnancy rate (CPR) and ongoing pregnancy rate (OPR). Logistic regression model was employed to ascertain the correlation between the CPR/OPR and age. The study cohort has an average age of 30.74; 3843 patients got clinical pregnancy rate of 61.40% and ongoing pregnancy rate of 54.21%. The multiple pregnancy rate of is 1.24%. For patients aged 34 and above, the CPR decreased by 10% for every 1-year increase in age (adjusted OR 0.90, 95% CI 0.84–0.96, p < 0.0001). Similarly, the OPR decreased by 16% for every 1-year increase in age (adjusted OR 0.84, 95% CI 0.81–0.88, p < 0.0001). Patients aged 35–37 years had an acceptable OPR of 52.4% after eSET, with a low multiple pregnancy rate (1.1%). Pregnancy outcomes were significantly better in blastocyst cycles compared to cleavage embryo cycles, and this trend was more pronounced in older patients. There was a non-linear relationship between female age and pregnancy outcomes in patients undergoing their first eSET cycles. The clinical pregnancy rate and ongoing pregnancy rate decreased significantly with age, especially in women older than 34 years. For patients under 37 years old, single embryo transfer should be prioritized. For patients over 38 years old with available blastocysts, eSET is also recommended.
Keywords: Elective single embryo transfer (eSET), Female age, In vitro fertilization (IVF), Clinical pregnancy, Ongoing pregnancy
Subject terms: Diseases, Medical research
Introduction
Assisted reproductive technology (ART) has become an increasingly important option for individuals and couples struggling with infertility worldwide. The global use of ART has been steadily rising, with an estimated 1.5–6 million ART cycles performed annually across the globe1. Despite the advances in ART, one of the persistent challenges has been the high rate of multiple gestations associated with these treatments. According to the Clinic Outcome Reporting System of the Chinese Society of Reproductive Medicine (CSRM), the multiple pregnancy rate of in vitro fertilization and embryo transfer (IVF-ET) in China was over 30.3% in 2016, and it slightly decreased to 24% in 2020 (http://59.110.12.46). Multiple gestations, such as twin or triplet pregnancies, are known to carry significantly higher risks for both the mother and the offspring, including increased rates of preterm birth, low birth weight, and greater neonatal morbidity and mortality2. To address this issue, the practice of elective single embryo transfer (eSET) has emerged as an important strategy to reduce the incidence of multiple pregnancies while optimizing the chances of a successful pregnancy. eSET involves the transfer of a single embryo during an IVF/ICSI cycle, as opposed to the transfer of multiple embryos. This approach has been shown to dramatically lower the risk of multiple gestations without compromising pregnancy rates3–6. As reported in the cochrane database of systematic reviews (2020)7, elective single embryo transfer followed by a subsequent single embryo transfer in a fresh or frozen cycle could achieve a comparable cumulative live birth rate to double embryo transfer (DET), while effectively preventing multiple pregnancies among women with a good prognosis.
However, the success of eSET can be influenced by various patient and treatment-related factors, including the female partner's age. There is a lack of relevant investigations on the impact of age on pregnancy outcomes in this specific patient population, excluding confounding factors. The decision on the number of embryos to be transferred should be jointly made by the clinician and the patient, in accordance with national laws and recommendations for embryo transfer limits3. Currently, there is insufficient data to guide the care of patients effectively. Therefore, this study aimed to explore the relationship between female age and pregnancy outcomes in patients undergoing their first eSET of IVF/ICSI cycles. Understanding this relationship is crucial for clinicians to make informed decisions and provide personalized recommendations to patients seeking ART treatment.
Materials and methods
Study cohort and data acquisition
Elective single embryo transfer (eSET) is a specific approach in IVF/ICSI, where a single, high-quality embryo is deliberately selected and transferred to the uterus, as opposed to the transfer of multiple embryos3. According to prior research, there is a dearth of agreement over the eSET definition. The American Society for Reproductive Medicine (ASRM) describes eSET as the presence of several high-quality embryos, with only one high-quality embryo transferred per cycle8, while other studies defined it as those single embryo transfers (SET) in which supernumerary embryos were frozen9–11. In clinical practice, patients who have a history of cesarean section, ectopic pregnancy, small stature, or OHSS tendency also prefer to choose ‘SET’, who may not have more than one high-quality embryo12–14. Thus, our study adopts the latter definition of eSET to explore the possibility of a wider range of SET population, which defines eSET as having supernumerary embryos frozen.
The patients in this study were from Reproductive Center of Henan Provincial People's Hospital. The raw data of this study were extracted from the artificial reproductive technology (ART) database of Henan Provincial People’s Hospital. We obtained the desensitization data of individuals who completed their first IVF/ICSI therapy between September 2016 and May 2022. Data were processed from medical records into standardized research datasets for further analysis.The research underwent evaluation and received approval from the Reproductive Medicine Ethics Committee of Henan Provincial People's Hospital (Grant No.: SYSZ-LL-2021091501).The need for individual consent was waived by the committees due to the retrospective character of the study. Data was desensitized to hide personal information before being processed.
The inclusion criteria were: (1) Female patients who received their first IVF/ICSI (using GnRH agonist protocol or GnRH antagonist protocol)15; (2) patients were transferred with SET in a fresh cycle or in the first frozen-thawed cycle after whole embryo freezing with at least one frozen embryo. The research excluded patients with one or more of the following conditions: (1) chromosomal abnormalities of either side of the parents, (2) endocrine diseases, (3) recurrent abortion, and (4) operative sperm extraction cycle.
Treatment
The same medical team selects the appropriate ovulation promotion protocol according to the patient's medical conditions. Each patient who satisfied the specified criteria received controlled ovarian stimulation using either a GnRH agonist or a flexible GnRH antagonist regimen. The reproductive endocrinologists set both the stimulation procedure and the dosage of gonadotropin (Gn). The Gn dosage was selected in every instance to maximize the quantity of oocytes obtained while reducing the likelihood of ovarian hyper-stimulation (OHSS)15.
The administration of a long-acting GnRH agonist (Diphereline, Ipsen, Tianjin) involves a single injection of 3.75 mg. This injection is given on either the second or fourth day of the menstrual cycle, as part of the GnRH agonist protocol. Alternatively, a daily injection of a short-acting GnRH agonist called Decapeptyl, with a dosage of 0.1 mg per day, (Ferring in Germany) is given for a duration of 14 to 18 days. This treatment starts during the middle of the luteal phase of the preceding menstrual cycle. This protocol typically takes around 33 days to complete. The GnRH antagonist Protocol included administering Gn injections beginning from the 2nd or 3rd day of menstruation. The administration of a GnRH antagonist at a dosage of 0.25 mg per day was initiated when a dominant follicle reached an average diameter of 12 mm, when the estrogen level was equal to or greater than 200 ng/L, or when there was a noticeable upward trend in blood luteinizing hormone (LH) levels. The dosage was taken till the day of urine human chorionic gonadotropin (hCG) injection. When there were at least two follicles measuring 18 mm or larger or three follicles measuring 17 mm or larger, subcutaneous injection of 4000 to 10,000 IU hCG (Lizhu Pharmaceutical Trading, China) was given to initiate the process. Oocyte retrieval, guided by transvaginal ultrasonography, was conducted 35–37 h after the first procedure.
Procedure for transferring embryos and providing support during the luteal phase
This study employed a standardized approach for embryo selection and transfer. Embryos at the cleavage or blastocyst stage exhibiting superior quality were chosen for elective single embryo transfer (eSET), based on well-defined morphological criteria16,17. The ET procedure was performed under ultrasound guidance, 3 or 5 days after oocyte retrieval, with only a single high-quality embryo loaded into the transfer catheter. This eSET strategy was adopted based on patient characteristics and embryo quality, rather than due to a lack of additional embryos. Supernumerary embryos were cryopreserved for potential future use. For frozen ET cycles, the endometrial preparation was tailored to each patient, and the same embryo assessment approach was utilized. Pregnancy was confirmed by measuring serum hCG levels 14 days post-transfer, and clinical pregnancy was defined as the detection of at least one gestational sac. Luteal support was provided from the day of oocyte retrieval until the 8-10th week of pregnancy or a negative beta-hCG result.
Outcomes
The main results of this research were the CPR and OPR. Clinical pregnancy was stated as the detection of at least one gestational sac within the uterus during a period of 4–5 weeks following the embryo transfer18. An ongoing pregnancy is characterized as a living pregnancy inside the uterus that endures until the 12th week of gestation19. Clinical pregnancy rate is calculated as a percentage with the numerator is the number of women who achieved a clinical pregnancy after the IVF embryo transfer and the denominator is the total number of women who underwent embryo transfer. Ongoing pregnancy rate is calculated as a percentage with the numerator is the number of women who had an ongoing pregnancy and the denominator is the total number of women who underwent embryo transfer.
Statistical analysis
The R language was used for statistical analysis. Continuous variables were reported as mean ± SD or median (Q1-Q3), whereas categorical variables were reported as N (%). The variables were compared across groups employing the one-way ANOVA and Chi-square test for categorical variables. A generalized additive model (GAM) was used to examine the association between the CPR/OPR and age in terms of dose–response. A logistic regression model was employed to assess the correlation between the CPR/OPR and age. The findings are shown as odds ratios (ORs) together with their corresponding 95% confidence intervals (95% CIs). The analysis includes both crude regression results and estimates that have been adjusted for covariates. The confounders were chosen based on their distinct correlation with the results. Upon careful evaluation of the clinical importance and covariates examination, we made adjustments for the following covariates: duration of infertility, type of infertility, causes causing infertility, COS protocols, AMH levels, type of embryo transplanted, number of frozen embryos, and endometrial thickness.
Subsequently, we employed a two-piece-wise linear regression model to investigate the threshold impact of age on pregnancy rate (Table 4). The inflection point for female age was determined by an "exploratory" approach, in which the trial inflection point was adjusted within a predefined range, and the one with the greatest model likelihood was chosen. We conducted a comparison between the one-line linear regression model and the two-piece-wise linear model and then carried out a log-likelihood ratio test. The alpha level was chosen at 0.05, with a two-sided significance. The statistical analyses were conducted employing the EmpowerStats program (www.empowerstats.com, X&Y solutions, Inc. Boston MA) and R software version 3.6.1 (http://www.r-project.org).
Table 4.
Threshold impact analysis of the female age and clinical pregnancy rate/ongoing pregnancy rate.
| Models | CPR | OPR | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Model I | ||||
| One line effect | 0.96 (0.95, 0.97) | < 0.0001 | 0.96 (0.94, 0.97) | < 0.0001 |
| Model II | ||||
| Turning point (K1, K2) | 34 | 34 | ||
| Female age < K1 | 0.99 (0.98, 1.01) | 0.516 | 1.00 (0.98, 1.01) | 0.739 |
| Female age ≥ K2 | 0.90 (0.84, 0.96) | < 0.0001 | 0.84 (0.81, 0.88) | < 0.0001 |
| P value for LRT test* | < 0.001 | < 0.001 | ||
Threshold effect analysis of the female age and pregnancy outcomes. Data were presented as OR (95% CI) P value; Model I, linear analysis; Model II, non-linear analysis. Adjust for infertility years, types of fertilization, infertility factors, AMH, COS protocols, frozen embryos, embryo type, and endometrial thickness. CI confidence interval, OR odds ratio, LRT logarithm likelihood ratio test. *P < 0.05 manifests that Model II is significantly different from Model I.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Reproductive Medicine Ethics Committee of Henan Provincial People's Hospital committees (No: SYSZ-LL-2021091501), China. The procedures followed were in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association. The Reproductive Medicine Ethics Committee of Henan Provincial People's Hospital committees waived the need for individual permission owing to the study's retrospective character.
Results
Study population
The reproductive center of Henan Provincial Hospital of China conducted a total of 46,656 IVF/ICSI cycles from September 2016 to May 2020, 8594 cycles of which were transferred by a single embryo with at least one frozen embryo at the same time for their first IVF/ICSI transfer, and 7089 cycles were eligible for the study. Figure 1 illustrates the precise procedure for including and excluding patients. The study cohort has an average age of 30.74, and 3843 patients had ongoing pregnancy (54.21%). The multiple pregnancy rate of all the patients is 1.22%, and the clinical pregnancy rate (CPR) is 61.40%.
Figure 1.
The flowchart for the screening process.
Baseline patients and cycle characteristics
Table 1 shows the baseline characteristics of the five groups of patients. Table 2 demonstrates that increasing age contributes a hindrance in the quantity of frozen embryos, as well as the rates of clinical pregnancy and continued pregnancy (P < 0.001). The CPRs showed a notable decline as age increased, declining from 64.69% in patients under 32 to 24.2% in patients over 40. Consequently, there was a considerable reduction in continuing pregnancy rates each cycle, ranging from 57.2 to 12.9%. Moreover, as age increased, there was a substantial decrease in the likelihood of GnRH-ant protocol COS and the number of oocytes obtained (P < 0.001). However, the multiple pregnancy rate remained relatively stable among groups (P > 0.05).
Table 1.
Comparison of demographic and clinical characteristics of the five groups.
| Age < 32 | 32 ≤ age ≤ 34 | 35 ≤ age ≤ 36 | 37 ≤ age ≤ 38 | 38 ≤ age ≤ 40 | age > 40 | P | |
|---|---|---|---|---|---|---|---|
| No. of cases | 4894 | 982 | 539 | 345 | 197 | 132 | |
| Male age (year) | 29.58 ± 3.52 | 34.02 ± 3.43 | 35.73 ± 3.84 | 37.95 ± 3.78 | 39.72 ± 4.24 | 42.49 ± 4.49 | < 0.001 |
| Male BMI | 25.20 ± 3.82 | 25.64 ± 3.68 | 25.73 ± 3.46 | 25.61 ± 3.23 | 25.81 ± 3.23 | 25.44 ± 3.39 | < 0.001 |
| BMI | 23.04 ± 3.74 | 23.06 ± 3.61 | 23.52 ± 3.41 | 23.21 ± 3.19 | 23.72 ± 2.88 | 23.82 ± 3.21 | 0.002 |
| Infertility year | 3.08 ± 2.09 | 3.94 ± 2.81 | 4.53 ± 3.67 | 4.43 ± 4.00 | 4.23 ± 3.70 | 4.98 ± 4.77 | < 0.001 |
| Infertility type (%) | < 0.001 | ||||||
| Primary infertility | 2305 (47.11%) | 660 (67.28%) | 405 (75.28%) | 287 (83.19%) | 177 (89.85%) | 124 (93.94%) | |
| Secondary infertility | 2588 (52.89%) | 321 (32.72%) | 133 (24.72%) | 58 (16.81%) | 20 (10.15%) | 8 (6.06%) | |
| Infertility factors (%) | < 0.001 | ||||||
| Pelvic or tubal factor | 51.2 | 63 | 64.7 | 59.7 | 60.9 | 54.5 | |
| Ovary factor | 3.5 | 3 | 2.8 | 1.7 | 4.1 | 2.3 | |
| Uterine or cervix factor | 33.5 | 24.3 | 22.8 | 27.5 | 25.9 | 28 | |
| Endometriosis | 0.8 | 0.3 | 0.6 | 0.9 | 0.5 | 0.8 | |
| Male factor | 8.2 | 7.3 | 6.3 | 6.4 | 7.1 | 6.8 | |
| Other factors | 2.7 | 2 | 2.8 | 3.8 | 1.5 | 7.6 | |
| AMH (ng/ml) | 5.29 ± 3.91 | 4.26 ± 3.14 | 3.83 ± 2.93 | 3.40 ± 2.83 | 2.76 ± 2.23 | 2.29 ± 2.22 | < 0.001 |
| Basal AFC | 16.53 ± 6.21 | 14.36 ± 6.02 | 13.22 ± 5.76 | 11.52 ± 5.68 | 10.12 ± 5.31 | 8.09 ± 5.53 | < 0.001 |
Male age and male BMI are defined as the age and BMI of male infertility patients.
Table 2.
Ovarian stimulation characteristics and outcomes among the five group.
| Age ≤ 32 | 32 < age ≤ 34 | 34 < age ≤ 36 | 36 < age ≤ 38 | 38 < age ≤ 40 | age > 40 | P | |
|---|---|---|---|---|---|---|---|
| COS protocols (%) | < 0.001 | ||||||
| GnRH agonist protocol | 4122 (84.23%) | 811 (82.59%) | 422 (78.29%) | 248 (71.88%) | 119 (60.41%) | 57 (43.18%) | |
| GnRH antagonist protocol | 772 (15.77%) | 171 (17.41%) | 117 (21.71%) | 97 (28.12%) | 78 (39.59%) | 75 (56.82%) | |
| Gn dosage (IU) | 2181.21 ± 947.55 | 2355.91 ± 901.05 | 2434.18 ± 828.04 | 2540.49 ± 816.40 | 2622.72 ± 742.41 | 2567.50 ± 840.26 | < 0.001 |
| Gn duration (day) | 10.88 ± 2.79 | 10.92 ± 2.64 | 10.70 ± 2.47 | 10.62 ± 2.59 | 10.37 ± 2.30 | 9.82 ± 2.96 | 0.012 |
| Starting dosage of Gn (IU) | 161.28 ± 56.20 | 175.04 ± 60.36 | 192.50 ± 64.73 | 207.45 ± 62.40 | 222.72 ± 59.18 | 245.68 ± 52.94 | < 0.001 |
| No. of retrieved oocytes | 10.95 ± 4.63 | 10.61 ± 4.55 | 10.11 ± 4.66 | 10.09 ± 4.87 | 10.23 ± 4.88 | 8.22 ± 4.70 | < 0.001 |
| No. of frozen embryos | 5.20 ± 3.41 | 4.61 ± 3.01 | 4.22 ± 2.98 | 3.80 ± 2.66 | 3.43 ± 2.53 | 2.72 ± 2.16 | < 0.001 |
| Methods of ART (%) | 0.133 | ||||||
| IVF | 4064 (83.04%) | 841 (85.64%) | 467 (86.64%) | 295 (85.51%) | 170 (86.29%) | 114 (86.36%) | |
| ICSI | 669 (13.67%) | 108 (11.00%) | 64 (11.87%) | 41 (11.88%) | 23 (11.68%) | 16 (12.12%) | |
| Combined | 161 (3.29%) | 33 (3.36%) | 8 (1.48%) | 9 (2.61%) | 4 (2.03%) | 2 (1.52%) | |
| Endometrial thickness (mm) on transfer day | 10.49 ± 2.49 | 10.59 ± 2.60 | 10.56 ± 2.73 | 10.34 ± 2.71 | 10.43 ± 2.98 | 9.62 ± 2.13 | 0.003 |
| Transferred embryo type (%) | 0.004 | ||||||
| Embryos in the cleavage stage | 1358 (27.75%) | 313 (31.87%) | 176 (32.65%) | 113 (32.75%) | 52 (26.40%) | 53 (40.15%) | |
| Blastocyst | 3536 (72.25%) | 669 (68.13%) | 363 (67.35%) | 232 (67.25%) | 145 (73.60%) | 79 (59.85%) | |
| Transferred embryo quality (%) | < 0.001 | ||||||
| Not good | 530 (12.31%) | 138 (15.56%) | 80 (16.99%) | 58 (19.27%) | 42 (27.45%) | 18 (18.56%) | |
| Good | 3774 (87.69%) | 749 (84.44%) | 391 (83.01%) | 243 (80.73%) | 111 (72.55%) | 79 (81.44%) | |
| Cycle type (%) | < 0.001 | ||||||
| Fresh embryo transfer | 2466 (50.39%) | 578 (58.86%) | 318 (59.00%) | 199 (57.68%) | 110 (55.84%) | 50 (37.88%) | |
| FET | 2428 (49.61%) | 404 (41.14%) | 221 (41.00%) | 146 (42.32%) | 87 (44.16%) | 82 (62.12%) | |
| Clinical pregnancy rate (%) | 3166 (64.69%) | 605 (61.61%) | 289 (53.62%) | 181 (52.46%) | 80 (40.61%) | 32 (24.24%) | < 0.001 |
| Ongoing pregnancy rate (%) | 2832 (57.87%) | 531 (54.07%) | 245 (45.45%) | 155 (44.93%) | 63 (31.98%) | 17 (12.88%) | < 0.001 |
| Multiple pregnancy rate (%) | 43(1.36%) | 6(1.16%) | 3(1.10%) | 1(0.57%) | 0 | 0 | 0.332 |
Age and clinical outcome of eSET
Multivariate logistic regression analysis
The outcomes of the multivariate logistic regression analysis revealed a substantial connection between age and CPR, with an OR of 0.951 (95% CI 0.935–0.968). Similarly, age was also shown to be significantly linked with OPR, with an OR of 0.949 (95% CI 0.933–0.965). Infertility years, types of fertilization, AMH, COS protocols, frozen embryos, transferred embryo type and endometrial thickness were also significantly linked to CPR with OR (95% CI) of 0.973 (0.956–0.991), 1.169 (1.061–1.288), 1.044 (1.029–1.059), 0.617 (0.546–0.696), 1.119 (1.101–1.138), 2.048 (1.845–2.273), 1.038 (1.019–1.059) respectively (P < 0.001), and with OPR with OR (95% CI) of 0.968 (0.951–0.985), 1.165 (1.060–1.280), 1.037 (1.024–1.051), 0.651 (0.577–0.734), 1.096 (1.079–1.113), 1.856 (1.674–2.059), 1.050 (1.030–1.070) respectively (P < 0.001) (shown in affiliated table).
Adjust for infertility years, types of fertilization, infertility factors, AMH, COS protocols, frozen embryos, transferred embryo type, and endometrial thickness.
When female age was analyzed as a continuous variable, inverse associations were found with clinical pregnancy after eSET. After adjusting for confounding variables, including infertility years, types of fertilization, infertility factors, AMH, COS protocols, frozen embryos, transferred embryo type, and endometrial thickness, for every 1-year elevation in the age of infertile women, the likelihood of clinical pregnancy decreased by 4.9%. Contrasted with the reference group (≤ 32 years old), the CPR of the other five groups was significantly lower contrasted with the reference group (P < 0.01). Contrasted with the reference group, the clinical pregnancy probability in the 39–40 years group decreased by 54%. The information is detailed in Table 3.
Table 3.
Associations of female age with pregnancy outcomes following eSET in IVF/ICSI cycles using multivariable logistic regression analysis.
| Item | CPR | OPR | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-adjusted | Adjust | Non-adjusted | Adjust | |||||
| OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | P | |
| Age | 0.942 (0.931, 0.953)) | < 0.0001 | 0.951 (0.935, 0.968) | < 0.0001 | 0.940 (0.930, 0.951) | < 0.0001 | 0.949 (0.933, 0.965) | < 0.0001 |
| Age group | ||||||||
| ≤ 32 years | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 33–34 years | 0.88 (0.76, 1.01) | 0.0661 | 0.93 (0.80, 1.09) | 0.3766 | 0.86 (0.75, 0.98) | 0.0284 | 0.90 (0.77, 1.04) | 0.1539 |
| 35–36 years | 0.63 (0.53, 0.75) | < 0.0001 | 0.71 (0.58, 0.86) | 0.0006 | 0.61 (0.51, 0.73) | < 0.0001 | 0.67 (0.55, 0.82) | < 0.0001 |
| 37–38 years | 0.60 (0.48, 0.75) | < 0.0001 | 0.71 (0.55, 0.90) | 0.0048 | 0.59 (0.48, 0.74) | < 0.0001 | 0.68 (0.54, 0.87) | 0.0018 |
| 39–40 years | 0.37 (0.28, 0.50) | < 0.0001 | 0.46 (0.33, 0.63) | < 0.0001 | 0.34 (0.25, 0.46) | < 0.0001 | 0.41 (0.29, 0.57) | < 0.0001 |
| > 40 years | 0.17 (0.12, 0.26) | < 0.0001 | 0.27 (0.18, 0.42) | < 0.0001 | 0.11 (0.06, 0.18) | < 0.0001 | 0.16 (0.10, 0.27) | < 0.0001 |
Curve fitting and threshold effect analysis
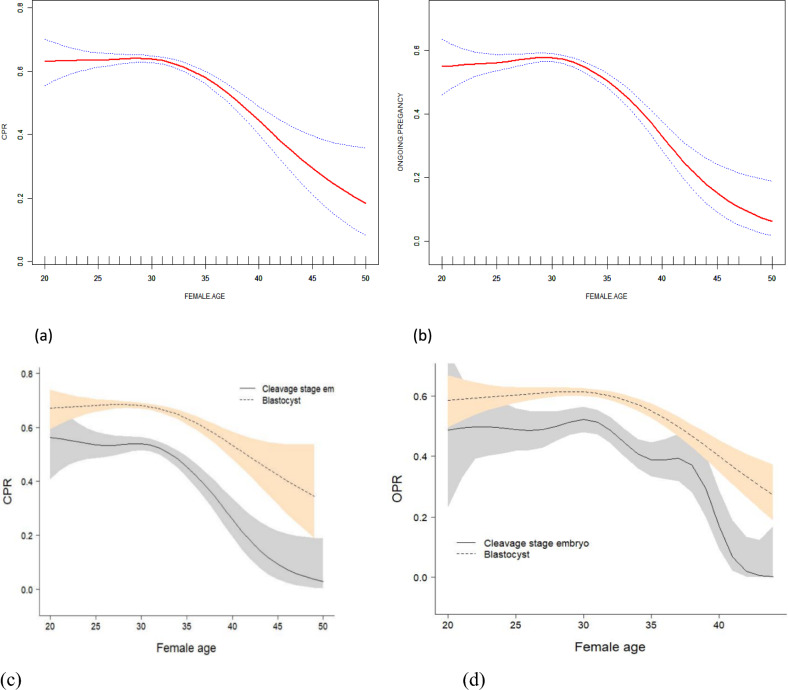
The outcomes of the curve fitting analysis, using the age of the woman as the x-axis and the ongoing pregnancy rate as the y-axis, revealed that the ongoing pregnancy rate of eSET remained consistent when the age was below 34 years old. However, it exhibited a significant decline as the age exceeded 35 years old (Fig. 2b). The OPR of people older than or equal to 38 years old was more than 50% lower compared with that of people less than 35 years old (Fig. 2a). 70.87% of cycles had blastocyst embryos transferred in our eSET population. A total of 5024 blastocyst eSET cycles were analyzed, of which 81.22% had been carried out with D5 blastocyst transferred. However, consistent with cycles, a comparable age correlated decrease in CPR and OPR was also seen in both blastocyst and cleavage eSET (P < 0.001). The detailed results are shown in Fig. 2c,d. Overall, CPR and OPR in blastocyst cycles (CPR: 66.44%; OPR: 58.67%) were significantly higher than in cleavage embryo cycles, respectively (CPR: 49.15%; OPR: 43.34%). This trend seemed more pronounced in older patients (more than 38). The detailed results are shown in Fig. 2 (See the affiliated table for specific values).
Figure 2.
Associations between the female age and pregnancy outcomes in patients who received their first eSET. A threshold, nonlinear association between the female age and CPR (a) and OPR (b) was found in a generalized additive model (GAM). The solid radial line depicts the continuous curve that best fits the relationship between the variables. The blue bars depict the 95% confidence interval derived from the fit. Adjust for infertility years, types of fertilization, infertility factors, AMH, COS protocols, frozen embryos, embryo type, and endometrial thickness. The two arcs represent a smooth curve fit between age and CPR (c) and OPR (d), stratified by whether the transplanted embryos are cleavage stage embryos or blastocysts. The colored bands represent 95% confidence intervals for the fit. Adjust for infertility years, types of fertilization, infertility factors, AMH, COS protocols, frozen embryos, embryo type, and endometrial thickness.
Table 4 manifests the threshold implications analysis of age on the continued pregnancy rate. After adjusting the confounding factors, age is an essential factor affecting ongoing pregnancy in patients with eSET. The results of the threshold effect analysis showed that the age threshold was 34 years old. The GAM identified a non-linear relationship between female age and CPR/OPR, as shown in Table 4. A comparison was made between the linear regression model and a two-piece-wise linear regression model, and the P value of the log-likelihood ratio test was found to be less than 0.001. The result suggests that the appropriate approach to fit the model is to use a two-piece-wise linear regression model. The CPR rate declined by 10% for every year above the age of 34 (OR = 0.90, 95% CI 0.84–0.96, P < 0.0001). Similarly, the OPR manifested a hindrance of 16% with every further year of age (OR = 0.84, 95% CI 0.81–0.88, P < 0.0001).
Discussion
To our knowledge, this is the first investigation focused on the dose–response relationship between female age and pregnancy outcomes following the first elective single embryo transfer (eSET) cycle. Our results showed an overall ongoing pregnancy rate of 54.21% after the initial eSET, highlighting the continued challenge in providing individualized treatment recommendations. Despite the overall satisfactory pregnancy rate, clinicians must carefully evaluate each patient's unique circumstances before determining the optimal embryo transfer strategy through shared decision-making, adhering to national guidelines. Continuous analysis of patient data and outcomes is crucial to refine the elective single embryo transfer strategy and ensure evidence-based, patient-centered care. According to ASRM, women below 35 years should be advised eSET, while those between 35 and 37 years should consider it20. A previous cost-effectiveness study supported elective single embryo transfer followed by a second frozen-thawed SET in women under 3221. However, the existing data is insufficient to adequately support these age-based recommendations, as most current articles only compare double and single embryo transfer outcomes without analyzing theelective single embryo transfer population separately22,23.
Our study established two models to characterize the relationship between female age and eSET outcomes. The linear model showed a 4% decrease in both clinical pregnancy and ongoing pregnancy rates per 1-year increase in age, regardless of the age group. In contrast, the non-linear model revealed a threshold effect, where age did not have a significant impact on eSET outcomes for women aged 34 years or younger, including those under 32 years old. This finding challenges the traditional approach of making elective single embryo transfer recommendations based solely on broad age categories. Our non-linear model provides a more nuanced understanding, suggesting that age may not be a crucial factor in elective single embryo transfer success up to 34 years of age. By incorporating both linear and non-linear perspectives, our study offers a more comprehensive assessment of the age-related dynamics in eSET. This information can guide clinicians in making individualized recommendations, going beyond simplistic age-based stratification. Further research is needed to integrate these novel insights with the existing literature and explore their broader implications for optimizing eSET outcomes in clinical practice.
Our investigation revealed that age played a crucial independent role in determining the outcomes of pregnancy after elective single embryo transfer. Consistent with previous research, female age has a significant function in determining the success rates of IVF procedures5. The primary factor is the age-related decline in oocyte number and quality, leading to a drop in female fertility24. Furthermore, there is data indicating that as individuals age, there is a higher occurrence of meiotic non-disjunction, resulting in chromosomal aneuploidy and early pregnancy loss25. Consequently, the majority of embryos from women over the age of 40 exhibit chromosomal abnormalities and never progress beyond the early stages of development25. The endometrial receptivity also decreases with age26. Our finding that younger age is linked to improved pregnancy outcomes after eSET aligns with the evidence that the number of follicles and oocyte quality decrease significantly beyond the age of 37–3824. We also found that patients between 35 and 37 can achieve an acceptable ongoing pregnancy rate (52.4%) after elective single embryo transfer, with a low multiple pregnancy rate (1.10%) compared to the multiple birth rate of about 20% reported after DET in this age group27. This supports the guidelines' recommendations to consider SET for individuals aged between 35 and 37 years.
Women over 38 had lower ongoing pregnancy rates after elective single embryo transfer, but blastocyst transfers resulted in significantly better outcomes compared to cleavage-stage transfers28. The non-linear age-related trend was consistent, but blastocyst transfers showed improved results, particularly in older patients, likely due to increased euploidy29,30. Thus, eSET with blastocyst transfer should be recommended for women over 38 to balance pregnancy success and minimize multiple births, given the greater risk of multiple births for older women and their offspring.
It is important to recognize that pregnancy success is multifactorial, influenced by a range of patient and treatment-related characteristics besides age. Our analysis identified several other important variables that may act as confounding factors, including infertility history, type of fertilization, underlying infertility diagnoses, AMH, controlled ovarian stimulation (COS) protocols, cryopreserved embryo utilization, transferred embryo quality, and endometrial receptivity. The duration of infertility, for instance, has been linked to reduced implantation and pregnancy rates, potentially due to the cumulative impact of subfertility on uterine and embryonic health31,32. Similarly, the type of fertilization and specific infertility etiologies could introduce variability in embryo competence and uterine receptivity33. Ovarian reserve, as reflected by AMH levels, can also affect oocyte and embryo quality, thereby influencing eSET outcomes34. Furthermore, the choice of COS protocol, utilization of frozen embryos, and endometrial development may interact with maternal age to impact implantation and pregnancy maintenance35,36. Incorporating a broader perspective on potential confounding factors can enable a more comprehensive assessment of complexities in eSET and guide personalized decisions.
This single-center study's findings may have limited generalizability due to differences in patient demographics, treatment protocols, and IVF practices compared to other settings. For example, the study cohort included 674 women over 37 years out of a total of 7089 patients, which may limit the representativeness of the older age group. Validation through diverse multi-center studies is needed to confirm the identified age thresholds and non-linear relationships, strengthening the external validity to guide personalized elective single embryo transfer implementation across broader contexts. Despite these limitations, the study provides valuable insights to inform clinical decision-making within the constraints of the local patient population and healthcare system serve (Supplementary Table 1).
In conclusion, this study reveals a curvilinear relationship between female age and eSET outcomes. Pregnancy rates declined significantly after age 34, with a 16% decrease per additional year. Patients aged 35–37 achieved acceptable ongoing pregnancy rates with elective single embryo transfer while maintaining low multiple birth risks, so eSET should be prioritized for women under 37 to optimize pregnancy success and minimize twin births. For women over 38 with available blastocysts, elective single embryo transfer is also recommended, as this selective approach can reduce high twin rates in older ART patients without compromising overall pregnancy outcomes. By balancing reduced multiple births and acceptable pregnancy rates, this personalized eSET strategy can improve short- and long-term outcomes for infertile women undergoing ART. Future studies will focus on clinical and perinatal outcomes in women over 37, through multi-center randomized trials.
Supplementary Information
Author contributions
C-LZ supervised the whole research, including the processes, conception, design, and completion. She also contributed to the analysis of the study results and made amendments to the publication. XW made significant contributions to the analysis of the data and also wrote the first version of the paper. P-ZT conducted data collecting and authored portions of the text. JL、Y-JZ and C-YD was tasked with the acquisition of data. Each author made contributions to the paper and endorsed the submitted version. The authors affirm that they have no conflicts of interest.
Funding
This research was fully sponsored by the National Natural Science Foundation of China (No. U2004130 http://www.nsfc.gov.cn/).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xue Wang and Pei-zhe Tian.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70249-1.
References
- 1.de Mouzon, J. et al. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted reproductive technology 2012†. Hum. Reprod.35(8), 1900–1913. 10.1093/humrep/deaa090 (2020). 10.1093/humrep/deaa090 [DOI] [PubMed] [Google Scholar]
- 2.Wen, J. Y. et al. Artificial intelligence model to predict pregnancy and multiple pregnancy risk following in vitro fertilization-embryo transfer (IVF-ET). Taiwan J. Obstet. Gynecol.61(5), 837–846. 10.1016/j.tjog.2021.11.038 (2022). 10.1016/j.tjog.2021.11.038 [DOI] [PubMed] [Google Scholar]
- 3.Sun, Y. et al. The consensus of Chinese experts on the number of embryo transfer. J. Reprod. Med.27(10), 940–945 (2018). [Google Scholar]
- 4.ESHRE. ESHRE ART fact sheet. http://www.eshre.eu/ESHRE/English/Guidelines-Legal/ART-fact-sheet/page.aspx/1061 (Accessed 20 May 2012).
- 5.De Geyter, C. Single embryo transfer in all infertile couples treated with assisted reproduction produces excellent results and avoids multiple births. Swiss Med. Wkly.151, w20499 (2021). 10.4414/smw.2021.20499 [DOI] [PubMed] [Google Scholar]
- 6.Reimundo, P. et al. Single-embryo transfer: A key strategy to reduce the risk for multiple pregnancy in assisted human reproduction. Adv. Lab. Med.2, 179–198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath, M. S. et al. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst. Rev.8, CD003416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: An American Society for Reproductive Medicine Practice Committee opinion. Fertil. Steril.97, 825–34 (2012). 10.1016/j.fertnstert.2011.11.048 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro, B. S. et al. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil. Steril.99, 389–92 (2013). 10.1016/j.fertnstert.2012.09.044 [DOI] [PubMed] [Google Scholar]
- 10.Niinimäki, M. et al. Elective single-embryo transfer in women aged 40–44 years. Hum. Reprod.28, 331–5 (2013). 10.1093/humrep/des399 [DOI] [PubMed] [Google Scholar]
- 11.Mancuso, A. C. et al. Elective single embryo transfer in women less than age 38 years reduces multiple birth rates, but not live birth rates, in United States fertility clinics. Fertil. Steril.106, 1107–1114 (2016). 10.1016/j.fertnstert.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang, L. et al. Ovarian hyperstimulation syndrome in a frozen-thawed embryo transfer pregnancy: A rare case report. BMC Pregnancy Childbirth20(1), 313. 10.1186/s12884-020-03014-7 (2020). 10.1186/s12884-020-03014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, T. et al. Reproductive outcomes of single embryo transfer in women with previous cesarean section. Reprod. Sci.28(4), 1049–1059. 10.1007/s43032-020-00345-w (2020). 10.1007/s43032-020-00345-w [DOI] [PubMed] [Google Scholar]
- 14.Trindade, V. D. et al. Tubal factor, cleavage stage and more than one embryo transferred were risk factors associated with ectopic pregnancy after assisted reproductive treatment. JBRA Assist. Reprod.26(2), 321–328. 10.5935/1518-0557.20210074 (2022). 10.5935/1518-0557.20210074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Practice Committee of the American Society for Reproductive Medicine. Prevention of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil. Steril.121(2), 230–245. 10.1016/j.fertnstert.2023.11.013 (2023). 10.1016/j.fertnstert.2023.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod.26(6), 1270–1283. 10.1093/humrep/der037 (2011). 10.1093/humrep/der037 [DOI] [PubMed] [Google Scholar]
- 17.Gardner, D. K., Pool, T. B. & Lane, M. Embryo nutrition and energy metabolism and its relationship to embryo growth, differentiation, and viability. Semin. Reprod. Med.18(2), 205–218. 10.1055/s-2000-12559 (2000). 10.1055/s-2000-12559 [DOI] [PubMed] [Google Scholar]
- 18.Zhang, J. et al. Effect of endometrium thickness on clinical outcomes in luteal phase short-acting GnRH-a long protocol and GnRH-ant protocol. Front. Endocrinol. (Lausanne)12, 578783. 10.3389/fendo.2021.578783 (2021). 10.3389/fendo.2021.578783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braakhekke, M. et al. Ongoing pregnancy qualifies best as the primary outcome measure of choice in trials in reproductive medicine: An opinion paper. Fertil Steril.101(5), 1203–1204. 10.1016/j.fertnstert.2014.03.047 (2014). 10.1016/j.fertnstert.2014.03.047 [DOI] [PubMed] [Google Scholar]
- 20.Practice Committee of American Society for Reproductive Medicine, Practice Committee of Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: A committee opinion. Fertil. Steril.99, 44–46 (2013). 10.1016/j.fertnstert.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 21.van Heesch, M. M. J. et al. Cost-effectiveness of embryo transfer strategies: A decision analytic model using long-term costs and consequences of singletons and multiples born as a consequence of IVF. Hum. Reprod.31, 2527–2540 (2016). 10.1093/humrep/dew229 [DOI] [PubMed] [Google Scholar]
- 22.Wu, Y. et al. Single embryo transfer improve the perinatal outcome in singleton pregnancy. J. Matern. Fetal Neonatal Med.33(19), 3266–3271. 10.1080/14767058.2019.1571029 (2019). 10.1080/14767058.2019.1571029 [DOI] [PubMed] [Google Scholar]
- 23.Wong, K. Y. et al. Outcomes and cost analysis of single-embryo transfer versus double-embryo transfer. Womens Health19, 17455057231206312. 10.1177/17455057231206312 (2023). 10.1177/17455057231206312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, M. et al. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol.28, 101327 (2020). 10.1016/j.redox.2019.101327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, C., Cavazza, T. & Schuh, M. Aneuploidy in human eggs: Contributions of the meiotic spindle. Biochem. Soc. Trans.49(1), 107–118 (2021). 10.1042/BST20200043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaling, Wu. et al. Unveiling uterine aging: Much more to learn. Ageing Res. Rev.86, 101879 (2023). 10.1016/j.arr.2023.101879 [DOI] [PubMed] [Google Scholar]
- 27.van Loendersloot, L. L. et al. Cost-effectiveness of single versus double embryo transfer in IVF in relation to female age. Eur. J. Obstet. Gynecol. Reprod. Biol.214, 25–30 (2017). 10.1016/j.ejogrb.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 28.Steinberg, M. L. et al. Elective single embryo transfer trends and predictors of a good perinatal outcome–United States, 1999 to 2010. Fertil. Steril.99, 1937–43 (2013). 10.1016/j.fertnstert.2013.01.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fragouli, E., Alfarawati, S., Spath, K. & Wells, D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol. Hum. Reprod.20(2), 117–126 (2014). 10.1093/molehr/gat073 [DOI] [PubMed] [Google Scholar]
- 30.Kroener, L. et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil. Steril.98, 876–880 (2012). 10.1016/j.fertnstert.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 31.Allahbadia, G. N. Intrauterine insemination: Fundamentals revisited. J. Obstet. Gynecol. India67(6), 385–392. 10.1007/s13224-017-1060-x (2017). 10.1007/s13224-017-1060-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, C. et al. The relationship between duration of infertility and clinical outcomes of intrauterine insemination for younger women: A retrospective clinical study. BMC Pregnancy Childbirth24(1), 199. 10.1186/s12884-024-06398-y (2024). 10.1186/s12884-024-06398-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamayou, S. et al. More blastocysts are produced from fewer oocytes in ICSI compared to IVF—Results from a sibling oocytes study and definition of a new key performance indicator. Reprod. Biol. Endocrinol.19(1), 116. 10.1186/s12958-021-00804-2 (2021). 10.1186/s12958-021-00804-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciepiela, P. et al. Oocyte matched follicular fluid anti-Müllerian hormone is an excellent predictor of live birth after fresh single embryo transfer. Hum. Reprod.34(11), 2244–2253. 10.1093/humrep/dez186 (2019). 10.1093/humrep/dez186 [DOI] [PubMed] [Google Scholar]
- 35.Guo, Y. et al. Efficacy of three COS protocols and predictability of AMH and AFC in women with discordant ovarian reserve markers: A retrospective study on 19,239 patients. J. Ovarian Res.14(1), 111. 10.1186/s13048-021-00863-4 (2021). 10.1186/s13048-021-00863-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallos, I. D. et al. Optimal endometrial thickness to maximize live births and minimize pregnancy losses: Analysis of 25,767 fresh embryo transfers. Reprod. Biomed. Online37(5), 542–548. 10.1016/j.rbmo.2018.08.025 (2018). 10.1016/j.rbmo.2018.08.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.