Abstract
Few population-based studies including younger adults have examined the potential of olfactory function tests to capture the degree of atrophy in memory-associated brain regions, which cannot be adequately explained by cognitive function tests screening for cognitive impairment. This population-based study investigated associations between high-resolution olfactory test data with few odours and grey matter volumes (GMVs) of the left and right hippocampi, amygdala, parahippocampi, and olfactory cortex, while accounting for differences in cognitive decline, in 1444 participants (aged 31–91 years). Regression analyses included intracranial volume (ICV)-normalised GMVs of eight memory-related regions as objective variables and age, sex, education duration, smoking history, olfaction test score, and the Montreal Cognitive Assessment-Japanese version (MoCA-J) score as explanatory variables. Significant relationships were found between olfactory test scores and ICV-normalised GMVs of the left and right hippocampi and left amygdala (p = 0.020, 0.024, and 0.028, respectively), adjusting for the MoCA-J score. The olfactory test score was significantly related to the right amygdalar GMV (p = 0.020) in older adults (age ≥ 65 years). These associations remained significant after applying Benjamini–Hochberg multiple testing correction (false discovery rate < 0.1). Therefore, olfactory and cognitive function tests may efficiently capture the degree of atrophy in the hippocampi and amygdala, especially in older adults.
Keywords: Brain atrophy, Olfactory function, Cognitive function, Grey matter volume, Cross-sectional studies, Multiple regression
Subject terms: Neurological disorders, Dementia
Introduction
In clinical settings, a comprehensive assessment for dementia includes history-taking, physical examination, activities of daily living evaluation, and cognitive function tests to screen for cognitive impairment before undertaking neuroimaging techniques, such as magnetic resonance imaging (MRI), to investigate brain atrophy1. Owing to cognitive reserve ability achieved through cognitive function enhancement via life experiences, some individuals with dementia may have less cognitive function decline than the degree indicated by brain atrophy2. Though newer treatments, such as lecanemab therapy, can effectively treat cognitive decline, treatment efficacy remains poor after cognitive impairment has progressed. Therefore, early detection of the risk of cognitive decline is important for the effective implementation of new treatments and preventive interventions. Olfactory function decline occurs more than 15 years before the onset of cognitive dysfunction3. If simple olfactory function tests can capture brain atrophy that cannot be detected by cognitive function tests to screen for cognitive impairment, conducting both olfactory and cognitive tests can facilitate the early detection of the risk of cognitive decline. However, it remains unclear whether olfactory tests with multiple odour intensity levels using a few odours can capture the degree of atrophy in brain regions associated with memory, which cannot be adequately explained by traditional cognitive function tests to screen for cognitive impairment.
The olfactory pathway overlaps with important memory-related brain regions, including the hippocampi, amygdala, and parahippocampal gyri4. Therefore, olfactory loss is closely related to cognitive decline, particularly memory. We hypothesised that olfactory and cognitive decline might be related to the atrophy of these regions observed on MRI.
In this study, we aimed to evaluate the association between olfactory test data with multiple odour intensity levels using a few odours and grey matter volumes (GMVs) of the hippocampi, amygdala, parahippocampal gyri, and olfactory cortex under conditions that eliminated the effect of differences in cognitive decline among adult participants aged 31–91 years. The results are expected to provide scientific evidence for establishing a new, early, and accurate method to estimate the degree of brain atrophy in memory-related regions, which is associated with dementia, using olfactory and cognitive function tests.
Results
Characteristics of the study participants
Table 1 presents the characteristics of 1444 participants across olfactory test score quartiles.Data are presented as mean ± standard deviation (SD) for continuous variables and as number and percentage for categorical variables. In the study cohort, the mean age of participants was 63.4 (SD, 9.8) years, and the proportion of men was 36.1%. The olfactory test score was 2.68 (SD, 1.75), whereas the Montreal Cognitive Assessment-Japanese version (MoCA-J) score was 25.6 (SD, 2.6), and 44.0% of the participants (635 of 1444 participants) were suspected to have mild cognitive impairment (MCI) or dementia according to the MoCA-J criteria. The intracranial volume (ICV)-normalised GMV of the entire brain was 0.4029 (SD, 0.0356).
Table 1.
Participant characteristics.
| ALL | Q1 | Q2 | Q3 | Q4 | P-value | |
|---|---|---|---|---|---|---|
| Range of olfactory score | 0–6 | 0–1 | 2 | 3–4 | 5–6 | |
| Number of samples | 1444 | 428 | 245 | 469 | 302 | |
| Age, years | 63.4 ± 9.8 | 68.4 ± 8.6 | 64.5 ± 9.1 | 61.5 ± 9.4 | 58.2 ± 8.9 | < 0.001 |
| Men, n (%) | 521 (36.1) | 195 (45.6) | 111 (45.3) | 137 (29.2) | 78 (25.8) | < 0.001 |
| Education, years | 13.5 ± 1.8 | 13.3 ± 1.9 | 13.5 ± 1.8 | 13.6 ± 1.7 | 13.6 ± 1.7 | 0.010 |
| Smoking ever, n (%) | 542 (37.5) | 165 (38.6) | 105 (42.9) | 156 (33.3) | 116 (38.4) | 0.355 |
| MoCA-J score | 25.6 ± 2.6 | 24.7 ± 2.7 | 25.5 ± 2.5 | 26.1 ± 2.5 | 26.4 ± 2.2 | < 0.001 |
| ICV-normalised GMV for brain (× 10−3) | 402.9 ± 35.6 | 390.3 ± 34.9 | 399.6 ± 35.7 | 407.6 ± 34.2 | 416.0 ± 32.9 | < 0.001 |
Continuous data are presented as mean ± standard deviation. Categorical data are presented as number (percentage). The olfactory score is the total score for spearmint and stuffy socks. The MoCA-J is the Japanese version of the Montreal Cognitive Assessment. The ICV-normalised GMV represents the ratio of grey matter volume to intracranial volume. P-values were derived using the Jonckheere–Terpstra and Cochran–Armitage tests for trends in the distributions of continuous and categorical variables, respectively.
Participants who were younger, women, had more years of education, had higher MoCA-J scores, and had higher ICV-normalised GMV were more likely to retain olfactory function.
Association of the olfactory test score with the GMV
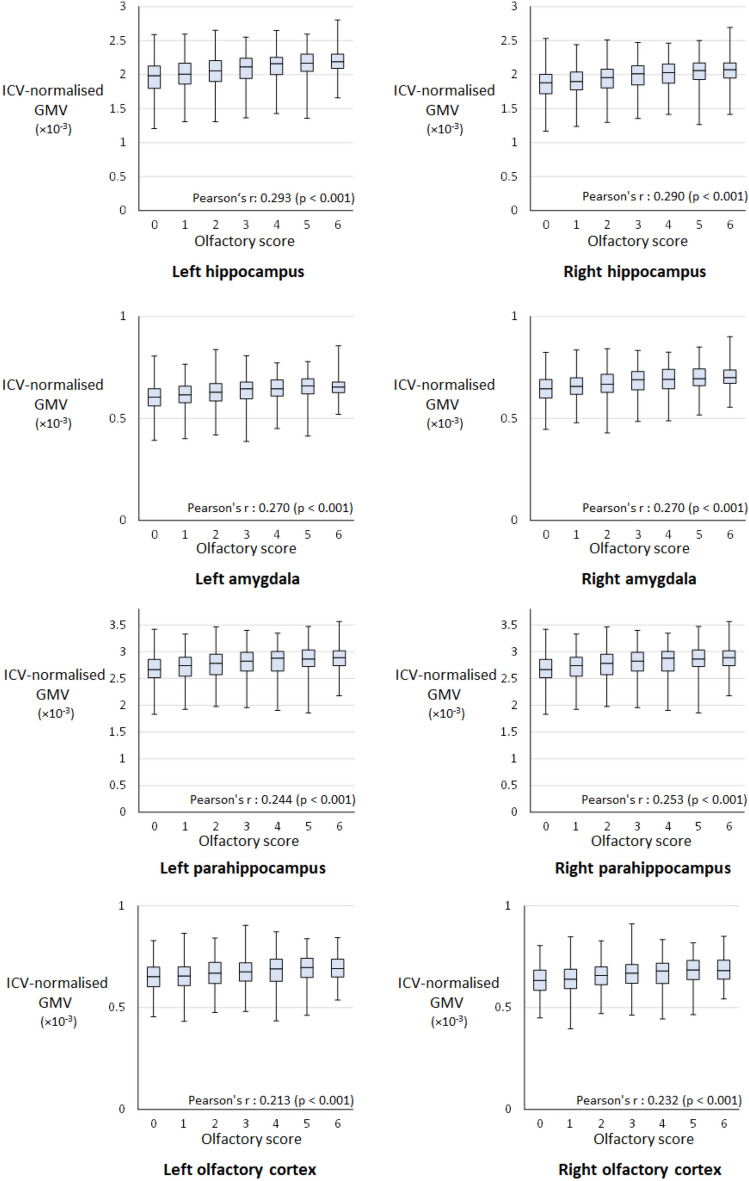
Figure 1 shows a box plot graph depicting the distribution of the ICV-normalised GMV of each region of interest (ROI) by olfactory scores and Pearson’s correlation coefficient between the olfactory test score and ICV-normalised GMV to indicate the approximate relationship between the olfactory test score and ICV-normalised GMV of each ROI. The higher the olfactory score, the larger the ICV-normalised GMV. The correlations between the olfactory score and ICV-normalised GMVs for the eight ROIs were statistically significant. The correlations were the strongest with the hippocampi, followed by the amygdala, parahippocampi, and olfactory cortex in that order.
Figure 1.
Box plot depicting the distribution of the ICV-normalised GMV of each ROI by the olfactory score. Pearson's r Pearson’s correlation coefficient, ICV intracranial volume, GMV grey matter volume, ROI region of interest.
Next, we performed multiple regression analyses wherein the interaction terms between olfactory test scores and each adjustment variable were included as explanatory variables to assess the appropriateness of stratification. Table 2 indicates the estimated values of the coefficients, confidence intervals, and p-values for the interaction terms with olfactory test scores in the multiple regression analyses of the ICV-normalised GMVs to assess the appropriateness of stratification by age group (< 65 or ≥ 65 years), sex, education duration (≤ 12 and > 12 years), and smoking history. For age group, regression analyses were performed using age, sex, education duration, smoking history, olfactory test data, and the interaction term between age group and the olfactory test score as explanatory variables and the ICV-normalised GMV of ROIs as objective variables, with the explanatory variables being centred. For sex, education duration, and smoking history, we conducted the assessment similarly to that for age group. The interaction term between age group and the olfactory test score was significant in the regressions for the right amygdala and left parahippocampus (standardised coefficient [95% confidence interval, 95% CI] of the interaction term: right amygdala: 0.050 [0.008–0.093], p = 0.019 and left parahippocampus: 0.047 [0.003–0.090], p = 0.034). The interaction term between sex and the olfactory test score was significant in the regressions for the left olfactory cortex (standardised coefficient [95% CI] of the interaction term: − 0.045 [− 0.089 to − 0.002], p = 0.040). The interaction term between education duration and the olfactory test score was not significant in the regressions for any of the eight ROIs. The interaction term between smoking history and the olfactory test score was not significant in the regressions for any of the eight ROIs. Based on these results, we present the results of the analysis without stratification for the left and right hippocampi, left amygdala, right parahippocampus, and right olfactory cortex but stratified by age group for the right amygdala and left parahippocampus and stratified by sex for the left olfactory cortex.
Table 2.
Coefficients of the interaction terms with olfactory test scores in multiple regression analysis for ICV-normalised GMVs.
| Interaction terms | ROI | Regression coefficient of the interaction term | ||
|---|---|---|---|---|
| Estimate | [95% CI] | P-value | ||
| Age group × olfactory score | Left hippocampus | 0.030 | [− 0.010, 0.069] | 0.140 |
| Right hippocampus | 0.031 | [− 0.009, 0.070] | 0.125 | |
| Left amygdala | 0.025 | [− 0.017, 0.067] | 0.241 | |
| Right amygdala | 0.050 | [0.008, 0.093] | 0.019 | |
| Left parahippocampus | 0.047 | [0.003, 0.090] | 0.034 | |
| Right parahippocampus | 0.028 | [− 0.014, 0.071] | 0.190 | |
| Left olfactory cortex | − 0.007 | [− 0.050, 0.036] | 0.754 | |
| Right olfactory cortex | 0.009 | [− 0.034, 0.053] | 0.680 | |
| Sex × olfactory score | Left hippocampus | − 0.004 | [− 0.043, 0.036] | 0.856 |
| Right hippocampus | − 0.017 | [− 0.057, 0.022] | 0.390 | |
| Left amygdala | − 0.013 | [− 0.056, 0.029] | 0.538 | |
| Right amygdala | − 0.034 | [− 0.076, 0.009] | 0.119 | |
| Left parahippocampus | − 0.011 | [− 0.055, 0.033] | 0.621 | |
| Right parahippocampus | − 0.037 | [− 0.080, 0.005] | 0.086 | |
| Left olfactory cortex | − 0.045 | [− 0.089, − 0.002] | 0.040 | |
| Right olfactory cortex | − 0.034 | [− 0.078, 0.010] | 0.125 | |
| Education duration group × olfactory score | Left hippocampus | − 0.015 | [− 0.055, 0.024] | 0.450 |
| Right hippocampus | − 0.015 | [–0.055, 0.024] | 0.453 | |
| Left amygdala | –0.022 | [− 0.064, 0.021] | 0.316 | |
| Right amygdala | − 0.011 | [− 0.053, 0.032] | 0.622 | |
| Left parahippocampus | − 0.011 | [− 0.054, 0.033] | 0.632 | |
| Right parahippocampus | − 0.003 | [− 0.045, 0.040] | 0.894 | |
| Left olfactory cortex | − 0.014 | [− 0.057, 0.029] | 0.521 | |
| Right olfactory cortex | 0.003 | [–0.041, 0.046] | 0.906 | |
| Smoking history × olfactory score | Left hippocampus | − 0.016 | [− 0.055, 0.024] | 0.431 |
| Right hippocampus | 0.001 | [− 0.039, 0.040] | 0.976 | |
| Left amygdala | − 0.012 | [− 0.054, 0.030] | 0.584 | |
| Right amygdala | 0.024 | [–0.018, 0.067] | 0.262 | |
| Left parahippocampus | − 0.00048 | [− 0.044, 0.043] | 0.983 | |
| Right parahippocampus | 0.023 | [− 0.019, 0.066] | 0.282 | |
| Left olfactory cortex | 0.00007 | [− 0.043, 0.043] | 0.997 | |
| Right olfactory cortex | − 0.001 | [− 0.044, 0.043] | 0.981 | |
CI confidence interval, ICV intracranial volume, GMV grey matter volume. Regression analyses were performed using olfactory score, age, sex, education duration, smoking history, and interaction terms as explanatory variables and ICV-normalised GMVs as objective variable.
According to the stratification policy mentioned above, we analysed the relationship between olfactory test scores and ICV-normalised GMVs. Table 3 describes the standardised partial regression coefficients for the olfactory test scores in the multiple regression analyses of the ICV-normalised GMVs. The results showed that the olfactory test score was significantly related to the left hippocampus, right hippocampus, and left amygdala (standardised coefficient [95% CI]: 0.056 [0.013–0.099], p = 0.011; 0.054 [0.011–0.097], p = 0.014; and 0.057 [0.011–0.103], p = 0.015, respectively) and also significantly related to the right amygdala in individuals aged ≥ 65 years (standardised coefficient [95% CI]: 0.089 [0.023–0.156], p = 0.009). Those relationships remained significant after applying the Benjamini–Hochberg (B–H) procedure5 for multiple testing correction (false discovery rate < 0.1) (Table 3).
Table 3.
Standardised partial regression coefficients for the olfactory test scores in the multiple regression analyses of ICV-normalised GMVs (No adjustment by MoCA-J score).
| ROI | Standardised partial regression coefficient | ||||
|---|---|---|---|---|---|
| Estimate | [95% CI] | P-value | P-valueBH | ||
| No stratification | Left hippocampus | 0.056 | [0.013, 0.099] | 0.011 | 0.061 |
| Right hippocampus | 0.054 | [0.011, 0.097] | 0.014 | 0.050 | |
| Left amygdala | 0.057 | [0.011, 0.103] | 0.015 | 0.040 | |
| Right parahippocampus | 0.044 | [− 0.003, 0.090] | 0.064 | 0.118 | |
| Right olfactory cortex | 0.031 | [− 0.016, 0.079] | 0.195 | 0.307 | |
| Stratification by age groups | |||||
| Age < 65 years | Right amygdala | 0.015 | [− 0.048, 0.078] | 0.639 | 0.703 |
| Left parahippocampus | 0.006 | [− 0.055, 0.067] | 0.859 | 0.859 | |
| Age ≥ 65 years | Right amygdala | 0.089 | [0.023, 0.156] | 0.009 | 0.096 |
| Left parahippocampus | 0.072 | [0.001, 0.143] | 0.048 | 0.106 | |
| Stratification by sex | |||||
| Men | Left olfactory cortex | − 0.032 | [− 0.115, 0.050] | 0.441 | 0.607 |
| Women | Left olfactory cortex | 0.021 | [− 0.036, 0.079] | 0.463 | 0.566 |
Left hippocampus, right hippocampus, left amygdala, and right amygdala (in individuals aged ≥ 65 years) were significantly associated with the olfactory score according to the Benjamini–Hochberg FDR multiple testing procedure (FDR < 0.1). The procedure is as follows: 1. identify the test subject with the highest p-value among those whose p-valueBH is below an FDR cut-off and 2. consider any test subject with a smaller p-value than that as significant.
CI confidence interval, ICV intracranial volume, GMV grey matter volume, MoCA-J Montreal Cognitive Assessment-Japanese version, FDR false discovery rate.
Regression analyses were performed using olfactory score, age, sex, education duration, and smoking history as explanatory variables in the analysis without stratification.
Regression analyses were performed using olfactory score, age, sex, education duration, and smoking history as explanatory variables in the age group stratification analysis and using olfactory score, age, education duration, and smoking history as explanatory variables in the sex stratification analysis.
Association of the olfactory test score with the GMV, adjusting for the cognitive test score
We analysed the relationship between olfactory test scores and ICV-normalised GMVs, adjusting for the MoCA-J scores. Table 4 describes the standardised partial regression coefficients for the olfactory test scores in the multiple regression analyses of ICV-normalised GMVs, with the addition of the MoCA-J score as an explanatory variable to the regression in Table 3. The olfactory test score was significantly related to the left hippocampus, right hippocampus, and left amygdala (standardised coefficient [95% CI]: 0.051 [0.008–0.094], p = 0.020; 0.050 [0.007–0.093], p = 0.024; and 0.052 [0.006–0.098], p = 0.028, respectively) and also significantly related to the right amygdala in individuals aged ≥ 65 years (0.079 [0.012–0.146], p = 0.020). Those relationships were also significant with the B–H procedure (Table 4). The standardised partial regression coefficients for all explanatory variables are described in Supplementary Tables S1 and S2 (Table S1 shows the results without stratification for the left and right hippocampi, left amygdala, right parahippocampi, and right olfactory cortex. Table S2 shows the results of stratified analysis for the right amygdala, left parahippocampi, and left olfactory cortex).
Table 4.
Standardised partial regression coefficients for the olfactory test scores in the multiple regression analyses of ICV-normalised GMVs with the adjustment for the MoCA-J score.
| ROI | Standardised partial regression coefficient | ||||
|---|---|---|---|---|---|
| Estimate | [95% CI] | P-value | P-valueBH | ||
| No stratification | Left hippocampus | 0.051 | [0.008, 0.094] | 0.020 | 0.222 |
| Right hippocampus | 0.050 | [0.007, 0.093] | 0.024 | 0.086 | |
| Left amygdala | 0.052 | [0.006, 0.098] | 0.028 | 0.076 | |
| Right parahippocampus | 0.039 | [− 0.007, 0.085] | 0.099 | 0.182 | |
| Right olfactory cortex | 0.024 | [− 0.023, 0.072] | 0.321 | 0.504 | |
| Stratification by age groups | |||||
| Age < 65 years | Right amygdala | 0.015 | [− 0.048, 0.078] | 0.646 | 0.790 |
| Left parahippocampus | 0.006 | [− 0.055, 0.067] | 0.847 | 0.112 | |
| Age ≥ 65 years | Right amygdala | 0.079 | [0.012, 0.146] | 0.020 | 0.847 |
| Left parahippocampus | 0.064 | [− 0.008, 0.135] | 0.079 | 0.174 | |
| Stratification by sex | |||||
| Men | Left olfactory cortex | − 0.040 | [− 0.123, 0.043] | 0.343 | 0.472 |
| Women | Left olfactory cortex | 0.013 | [− 0.044, 0.071] | 0.651 | 0.716 |
Left hippocampus, right hippocampus, left amygdala, and right amygdala (in individuals aged ≥ 65 years) were significantly associated with the olfactory score according to the Benjamini–Hochberg FDR multiple testing procedure (FDR < 0.1). The procedure is as follows: 1. identify the test subject with the highest p-value among those whose p-valueBH is below an FDR cut-off and 2. consider any test subject with a smaller p-value than that as significant.
CI confidence interval, ICV intracranial volume, GMV grey matter volume, MoCA-J Montreal Cognitive Assessment-Japanese version, FDR false discovery rate.
Regression analyses were performed using olfactory score, MoCA-J score, age, sex, education duration, and smoking history as explanatory variables in the analysis without stratification.
Regression analyses were performed using olfactory score, MoCA-J score, age, sex, education duration, and smoking history as explanatory variables in the age group stratification analysis and using olfactory score, MoCA-J score, age, education duration, and smoking history as explanatory variables in the sex stratification analysis.
The olfactory test scores showed an effect size almost equal to that of the MoCA-J scores (e.g., estimates of standardised partial regression coefficients for olfactory test scores in the left and right hippocampus were 0.051 and 0.050, respectively, while those for the MoCA-J scores in the left and right hippocampus were 0.049 and 0.045, respectively, as shown in Supplementary Table S1).
Discussion
In this study, the higher the limits of distinguishable odour intensities, the larger the ICV-normalised GMVs. Furthermore, we found that the relationship between the olfactory test score and ICV-normalised GMVs of the left hippocampus, right hippocampus, and left amygdala were significant, adjusting for cognitive function test data that screen for cognitive impairment in people aged 31–91 years. Moreover, we confirmed that the olfactory test scores were significantly related to ICV-normalised GMVs of the right amygdala in older adults (age ≥ 65 years); better olfactory test scores were associated with a larger volume of the right amygdala. There was no statistically significant difference according to age group, sex, education duration, or smoking history in the associations of the olfactory test scores with the ICV-normalised GMVs of the left and right hippocampi and amygdala.
Su et al.6 reported a correlation between the olfactory test data and hippocampal volume in patients with Alzheimer’s disease (AD) and MCI based on a meta-analysis. Kubota et al.7 showed a relationship between hippocampal volume reduction and decreased olfactory function in 27 older participants and 27 younger healthy controls, although it was not limited to the GMV. In a longitudinal study of participants aged ≥ 50 years, Tian et al.8 reported that higher odour-identification scores were associated with prior and subsequent slower brain atrophy in the hippocampus. Kashibayashi et al.9 reported a significant correlation between the olfactory detection scores and left parahippocampal gyrus volume in 70 patients with chief complaints of memory impairment who were diagnosed with amnestic MCI or AD, with a clinical dementia rating of 0.5. These findings partially support our results, although the analyses in these studies were performed with no adjustment for the MoCA-J score, and more than five odours were required for the olfactory test (only two odours were required in our olfactory test).
The association between the cognitive function score and brain atrophy might be weak for people with higher levels of cognitive reserve because they tend to retain cognitive function even when their brains become atrophied. Our results demonstrated a statistically significant relationship between olfactory test data and ICV-normalised GMVs of certain brain regions, even while adjusting for MoCA-J scores. This suggests that olfactory test data can capture the degree of brain atrophy that cannot be explained by cognitive function test scores to screen for cognitive impairment and may indirectly indicate the presence of participants with cognitive reserve. This interpretation supports the potential importance of olfactory testing for identifying subtle brain changes that are otherwise missed in standard cognitive assessments.
All estimates of the regression coefficients for education duration were negative (Supplementary Tables S1 and S2); that is, the longer the education duration, the smaller the ICV-normalised GMVs, although some of these were not significantly related. A previous study10 reported that longer education could increase the overall thickness and volume of the cerebral cortex of the brain (with respect to the total ICV and education duration in our data, the Pearson’s correlation coefficient was 0.178; p < 0.001). An increase in the overall thickness and volume of the cerebral cortex of the brain may result in a higher total ICV. In contrast, previous research11 showed that educational achievement was positively associated with the volume of specific sub-regions within the hippocampus and amygdala in 110 older adults without dementia who were at higher risks for cognitive decline and functional impairment. Considering these previous findings, the negative relationship between the education duration and ICV-normalised GMV in this study could be attributed to the association between the education duration and total ICV. The negative relationship could arise because the degree of increase in the total ICV relative to the education duration is larger than that in the ICV-normalised GMV of each region.
All estimates of the regression coefficients for sex in the analyses without stratification were negative; that is, ICV-normalised GMVs were smaller in men than in women, although some aspects were not significantly related. These results could be strongly influenced by the fact that the total ICV is larger in men than in women (average total ICV: 1581.2 and 1395.0 cm3 for men and women, respectively). The results of this study showed that the average ICV of men was 13.3% greater than that of women.
As shown in Fig. 1, the correlation coefficients between the olfactory test score and ICV-normalised GMVs were significant (p < 0.001) in all eight regions. The observed significance in every region is believed to be due to confounding factors such as age. The results of multiple regression analyses with explanatory variables, confounding factors and the olfactory test score, are considered to more appropriately evaluate the statistical significance of the relationship between the olfactory test score and ICV-normalised GMVs.
Our findings indicate that when the total score for spearmint and stuffy socks, which contributed most to the distinction between suspected and unsuspected cognitive impairment12, was used as the olfactory test score, the olfactory test scores and ICV-normalised GMVs of the hippocampi and amygdala obtained with the adjustment for MoCA-J scores were significantly associated. Previous studies12,13 reported that the strength of the association between olfactory test scores and cognitive function test scores varied with different odours. Therefore, the relationship between olfactory test scores and ICV-normalised GMVs of cognitive brain regions may also differ depending on the odour.
This study has some limitations. In general, individual differences in the ICV-normalised GMV exist. However, this study could not rule out their influence. In recent years, various genetic olfactory receptors have shown distinct activation patterns in response to odorants, leading to individual variations in odour sensitivity14. Certain viruses, including those responsible for common cold and influenza, may harm olfactory cells. Individuals may sometimes experience prolonged loss of smell lasting for several months or even more than a year following exposure to such viruses15,16. Our study might have included participants with temporary olfactory impairment or organic diseases, such as sinusitis and nasal allergies. These factors may have negatively influenced the outcomes of the analyses regarding the relationship between olfactory test scores and GMVs. This study used the MoCA-J, which may have a ceiling effect. Therefore, it is unclear whether the same results would be obtained if a cognitive function assessment without a ceiling effect (e.g., logical memory test) was used. The whole-brain voxel-wise approach of the correlation with the olfactory score could provide a more spatial resolution of the ROIs; however, we could not acquire such findings for more spatial resolution because we adopted the ROI-level approach in the framework of confirmatory hypothesis testing. In this study, we focused on memory-related regions where atrophy is often observed in dementia and only examined the potential of capturing the degree of the atrophy in memory-related regions more effectively by adding olfactory tests to cognitive function tests. However, dementia does not always involve atrophy in memory-related regions, and the brain abnormalities caused by dementia extend beyond the medial temporal regions in some cases, which are the first to be affected. If we could demonstrate, using a voxel-wise approach or ROI-based approach covering the whole brain, that the memory-related ROIs had the strongest association with olfactory test score, this study would significantly provide scientific evidence for establishing an early and accurate method for estimating dementia-related atrophy using olfactory testing.
In conclusion, this is the first population-based study to evaluate an association between olfactory test scores using multiple odour intensities with a few odours and the GMV of the hippocampi, amygdala, parahippocampal gyri, and the olfactory cortex, which show atrophy in the early stages of dementia onset, under conditions that removed the effect of the differences in cognitive decline (participants aged 31–91 years). Olfactory test scores using multiple odour intensities have a significant association with ICV-normalised GMVs of the left and right hippocampi and left amygdala with the adjustment for cognitive function test data. Furthermore, olfactory test scores were significantly related to the right amygdala in adults aged ≥ 65 years. The findings of this study indicate the possibility of a more accurate estimation of the GMVs of the hippocampus and amygdala, especially in older adults (aged ≥ 65 years), by combining the olfactory test with the cognitive function test to screen for cognitive impairment.
The following are our plans for future research. There may be a lag of several years to a decade or more between olfactory dysfunction and brain atrophy. Therefore, olfactory test data obtained several years earlier may be more relevant to atrophy of grey matter, such as the hippocampus and amygdala, rather than olfactory test data obtained on the same day that the brain MRI was performed. We are considering a similar analysis to this study, using historical olfactory test data obtained by performing MRI on the same participants several years later. We will also analyse the association among changes in the GMVs for the ROIs, changes in cognitive function data, and olfactory test data used in this study. Furthermore, we plan to investigate the association between the brain structure and the olfactory score at greater spatial resolution (e.g., correlation maps across the longitudinal axis of the hippocampus), including brain regions other than memory-related regions.
Methods
Participants
In Japan, since July 2014, the Tohoku University Tohoku Medical Megabank Organization has been actively collecting data on MRI, cognitive function tests that screen for cognitive impairment, age, sex, education duration, and smoking history from participants (age ≥ 20 years) of the ongoing Health Surveillance of the Brain and Psychological State Programme. Parallel to the data collection procedures of this programme, from 27 August 2019 to 30 March 2021, we conducted olfactory testing to collect olfactory test data using our own equipment12. The study was approved by the Institutional Review Board of Tohoku University. This study was conducted according to the principles of the Declaration of Helsinki17. The participants were men and women in good physical condition on the day of the health survey. We assessed participants’ health condition through self-report and a brief interview to confirm no abnormal health conditions such as fever, cough, abdominal pain, or feeling unwell, which was considered ‘good’ condition. The following persons were excluded: pregnant women, women suspected of being pregnant, and those with metallic implants without a doctor’s certificate for MRI safety.
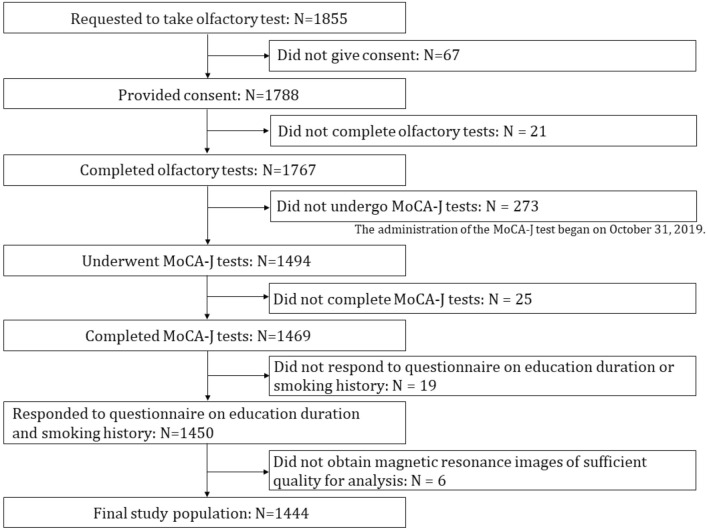
Among 1855 people invited to participate in the olfactory test, 1788 provided consent; however, only 1444 participants completed the olfactory and cognitive function tests that could screen for cognitive impairment; underwent MRI and provided images of sufficient quality for analysis; responded to a questionnaire on age, sex, education duration, and smoking history; and did not withdraw their consent (Fig. 2). Age, sex, educational level, and smoking history are generally considered to be related to cognition and olfaction12.
Figure 2.
Flowchart depicting participant screening and inclusion.
Study procedures and data collection
Our olfactory test used six types of living odours of stable single compounds including four pleasant odours (vanillin, cyclohexanone, γ-undecalactone, and (−)-carvone) and two unpleasant odours (n-valeric acid and 4-methyl-3-hexenoic acid) as reference to the odours used in T&T olfactometer (Daiichi Yakuhin Sangyo, Tokyo, Japan), the only olfactory test kit that is covered by insurance in Japan. For each odour, three odour intensity levels (indicated by numbers in parentheses) were determined with triethyl citrate as the dilution solvent, as follows: Vanilla: vanillin (2, 3, 4), wet rag: 4-methyl-3-hexenoic acid (3, 4, 5), caramel: cyclotene (2, 3, 4), spearmint: ( −)-carvone (2, 3, 4), stuffy socks: n-valeric acid (3, 4, 5), and yellow peach: γ-undecalactone (3, 4, 5). According to standards specified by the Japanese Ministry of the Environment, two panellists judged the odour intensity on a six-point scale (0, none; 1, very weak; 2, weak; 3, moderate; 4, strong; and 5, very strong). The Aroma Shooter® (Aromajoin Corporation) testing device was used to release the odour, and one Aroma Shooter was filled with the same odour of three different intensities. After the odour was emitted from the device, the participants were directed to select 1 of the 12 pictures displayed on a computer screen that they believed best matched the perceived scent. Each odour was tested at all three intensities. In addition to the 12 pictures, the option ‘I do not know’ was provided on the screen. We administered tests at all odour concentrations and scored points by considering the coincidental possibility of choosing the correct answer12. We previously reported that the combination of spearmint and stuffy socks contributed the most to the distinction between suspected and unsuspected cognitive impairment, as assessed using the MoCA-J criteria, among all combinations from the six odours12. Therefore, the total score for spearmint and stuffy socks was utilised as the olfactory test score in this study.
The MoCA-J is a cognitive function test for screening and detecting cognitive impairment and is typically employed in the diagnostic processes of medical and clinical psychology in Japan18.
MRI data pre-processing
Brain T1-weighted imaging was performed using 3.0-Tesla MRI machines19. We used a standalone version of the Statistical Parametric Mapping SPM12 (Institute of Neurology, University College London, London, UK) to pre-process the images as follows: (1) conversion of Digital Imaging and Communications in Medicine files to Neuroimaging Informatics Technology Initiative images; (2) reorientation of the images; (3) segmentation of the brain structural images into grey matter, white matter, and cerebrospinal fluid applying the Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL) technique20 and creation of a group template; (4) spatial normalisation to the Montreal Neurological Institute (MNI) template based on the segmented information from DARTEL to mitigate the impact of individual differences in brain size and shape; and (5) spatial smoothing with an 8-mm full-width at half-maximum Gaussian kernel21.
For the ROI-based approach, masking data for each ROI were created using SPM12 with WFU_pickatlas software 3.0.5b (Wake Forest University, Winston-Salem, NC, USA), referencing the Automated Anatomical Labeling atlas22. Using the masking data, the GMV in each ROI was calculated using SPM12.
Statistical analyses
Age, sex, education duration, and smoking history were used as adjustment variables to examine the association of the olfactory test and cognitive function test scores with the GMV, which are currently considered related to cognition and olfaction. In the analyses, sex was set to 0 and 1 for women and men, respectively, and smoking history was set to 0 and 1 for more than or less than 100 cigarettes smoked in the participant’s lifetime. The education duration was defined as the number of years from the first grade of elementary school.
Participant characteristics were first assessed across quartiles of the olfactory test scores, and the Jonckheere–Terpstra and Cochran–Armitage trend tests were conducted on the distribution of continuous and categorical variables, respectively, in the four groups. Pearson’s correlation analyses were conducted between olfactory data and the ICV-normalised GMV of the eight ROIs. We then illustrated a box plot that expressed the distribution of ICV-normalised GMVs in the eight ROIs using olfactory data. Next, the necessities of stratification were considered by referencing statistical analysis as follows, since those necessities were not clear. To assess the appropriateness of stratification by age group (younger or older adults; < 65 or ≥ 65 years), multiple regression analyses were performed using age, sex, education duration, smoking history, olfactory test score, the interaction term between age group (coded as 0 and 1 for individuals aged < 65 and ≥ 65 years, respectively), and the olfactory test score as explanatory variables and the ICV-normalised GMV of each ROI as the objective variable, with each variable centred. The appropriateness of stratification by sex, education duration (up to high school graduation or beyond; ≤ 12 and > 12 years; assigned 0 for the former and 1 for the latter), and smoking history were similarly examined. Based on the abovementioned results, multiple regression analyses of the ICV-normalised GMVs were conducted with appropriate stratification. Regression analyses were performed with and without the MoCA-J scores as explanatory variables. The association between the olfactory test score and the ICV-normalised GMVs was evaluated using the B–H procedure5 for multiple testing correction (false discovery rate < 0.1).
All statistical analyses, except for the Jonckheere–Terpstra test, were performed using statistical packages, including Statsmodels and Scipy.stats, in Python 3.7.4. The Jonckheere–Terpstra test was performed using the Clinfun package in R software, as the package is unavailable in Python. Statistical significance was set at p < 0.05.
Ethics declarations
The study was approved by the Institutional Review Board of Tohoku University. This study was conducted according to the principles of the Declaration of Helsinki17.
Supplementary Information
Acknowledgements
We would like to thank all the participants in the Health Surveillance of the Brain and Psychological State Programme. We thank the members of Tohoku University and Tohoku Medical Megabank Organization for supporting our study and allowing us to use their equipment. We would like to thank Naoko Tsutsumi for performing the olfactory tests. The Toyota Group provided research funding to Toyota Central R&D Labs, Inc. These funders provided financial support in the form of salaries to the authors (SS, TI, MT, and KH). Except for the olfactory test data, the study data were collected as part of the Health Surveillance of the Brain and Psychological State Program of Tohoku University Tohoku Medical Megabank Organization, which was subsidised by the government [Grant Number: JP21tm0124005]. Toyota Central R&D Labs provided a collaborative study grant to Tohoku University.
Author contributions
S.S. and A.H. are the co-corresponding authors of this study. S.S. mainly analysed the data and wrote the manuscript. A.H. coordinated the cohort study, supervised the experimental procedures, and provided expertise in epidemiological statistical analyses. T.I. designed the protocol for olfactory assessment and contributed to the data analysis. S.M. and N.M. provided expertise and insights on brain and cognitive functions and managed MRI scans. N.F. and S.K. coordinated the cohort study. M.T. and K.H. assessed and fine-tuned the odour testing apparatus. F.N., K.K., S.K., I.N.M., M.K., A.N., and T.S. contributed to the data analysis. N.N., S.O., T.N., M.T., R.I., R.H., K.N., I.C., and I.K. contributed to the collection and management of the data. All the authors made a substantial contribution to the drafting of the manuscript and have approved this submission. All participants provided written informed consent.
Data availability
The data that support the findings of this study are available from Tohoku Medical Megabank Organization; however, restrictions apply to the availability of these data, which were used with permission for the current study and are not publicly available. Data are available from the corresponding author upon reasonable request and with permission from the Tohoku Medical Megabank Organization.
Competing interests
Beyond their role as funders, Toyota Group Companies and Toyota Central R&D Labs, Inc. did not play any additional role in the design or conduct of the study, data collection, analysis, interpretation, or preparation of the manuscript. Olfactory test data were collected through a collaborative study between Toyota Central R&D Laboratories, Inc., and Tohoku University. All other data in this study were collected as part of the Health Surveillance of the Brain and Psychological State Program of the Tohoku University Tohoku Medical Megabank Organization that is subsidised by the government. Toyota Central R&D Labs, Inc., and Tohoku University have pending Japanese patents (Japanese Patent Application No. 2024-048577) that pertain to the research topic.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuichi Sato, Email: shuichi-sato@mosk.tytlabs.co.jp.
Atsushi Hozawa, Email: hozawa@megabank.tohoku.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69726-4.
References
- 1.Matsuda, H. Voxel-based morphometry of brain MRI in normal aging and Alzheimer’s disease. Aging Dis.4, 29–37 (2013). [PMC free article] [PubMed] [Google Scholar]
- 2.Stern, Y. Cognitive reserve. Neuropsychologia47, 2015–2028 (2009). 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dintica, C. S. et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology92, e700–e709 (2019). 10.1212/WNL.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajiwara, R., Tominaga, T. & Takashima, I. Olfactory information converges in the amygdaloid cortex via the piriform and entorhinal cortices: Observations in the guinea pig isolated whole-brain preparation. Eur. J. Neurosci.25, 3648–3658 (2007). 10.1111/j.1460-9568.2007.05610.x [DOI] [PubMed] [Google Scholar]
- 5.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol.57, 289–300 (1995). 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 6.Su, M. W. et al. The correlation between olfactory test and hippocampal volume in Alzheimer’s disease and mild cognitive impairment patients: A meta-analysis. Front. Aging Neurosci.13, 755160 (2021). 10.3389/fnagi.2021.755160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubota, S. et al. Hippocampus and parahippocampus volume reduction associated with impaired olfactory abilities in subjects without evidence of cognitive decline. Front. Hum. Neurosci.14, 556519 (2020). 10.3389/fnhum.2020.556519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian, Q. et al. Associations of olfaction with longitudinal trajectories of brain volumes and neuropsychological function in older adults. Neurology100, e964–e974 (2023). 10.1212/WNL.0000000000201646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashibayashi, T. et al. Correlation between regional brain volume and olfactory function in very mild amnestic patients. J. Neurol. Sci.411, 116686 (2020). 10.1016/j.jns.2020.116686 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell, B. L. et al. Educational attainment polygenic scores are associated with cortical total surface area and regions important for language and memory. NeuroImage212, 116691 (2020). 10.1016/j.neuroimage.2020.116691 [DOI] [PubMed] [Google Scholar]
- 11.Tang, X., Varma, V. R., Miller, M. I. & Carlson, M. C. Education is associated with sub-regions of the hippocampus and the amygdala vulnerable to neuropathologies of Alzheimer’s disease. Brain Struct. Funct.222, 1469–1479 (2017). 10.1007/s00429-016-1287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato, S. et al. Association between olfactory test data with multiple levels of odour intensity and suspected cognitive impairment: A cross-sectional study. J. Alzheimers Dis.95, 1469–1480 (2023). 10.3233/JAD-230318 [DOI] [PubMed] [Google Scholar]
- 13.Fukumoto, T., Ezaki, T. & Urakami, K. Verification of the association between cognitive decline and olfactory dysfunction using a dementia screening kit in subjects with Alzheimer’s dementia, mild cognitive impairment, and normal cognitive function (DESK study): a multicenter, open-label, interventional study. NeurologicalSci1(29), 100439 (2022). 10.1016/j.ensci.2022.100439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeger, S. R. et al. A Mendelian trait for olfactory sensitivity affects odor experience and food selection. Curr. Biol.23, 1601–1605 (2013). 10.1016/j.cub.2013.07.030 [DOI] [PubMed] [Google Scholar]
- 15.de Haro-Licer, J., Roura-Moreno, J., Vizitiu, A., González-Fernández, A. & González-Ares, J. A. Long term serious olfactory loss in colds and/or flu. Acta Otorrinolaringol. Esp.64, 331–338 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Welge-Lüssen, A. & Wolfensberger, M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol.63, 125–132 (2006). [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA310, 2191–2194 (2013). 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc.53, 695–699 (2005). 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 19.Taira, M. et al. Tohoku Medical Megabank brain magnetic resonance imaging study: Rationale, design, and background. JMA J.6, 246–264 (2023). 10.31662/jmaj.2022-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage38, 95–113 (2007). 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Nemoto, K. Understanding voxel-based morphometry. Brain Nerve.69, 505–511 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Tzourio-Mazoyer, N. et al. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage15, 273–289 (2002). 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Tohoku Medical Megabank Organization; however, restrictions apply to the availability of these data, which were used with permission for the current study and are not publicly available. Data are available from the corresponding author upon reasonable request and with permission from the Tohoku Medical Megabank Organization.