Abstract
Objectives
Rituximab is used for remission induction and the prevention of relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV). This study evaluated the incidence of safety events and compared time to first serious adverse event (SAE) between a rituximab cohort and a cohort treated with non-rituximab therapies in a real-life setting.
Methods
Rituximab surveillance study in vasculitis was a retrospective observational study of patients with AAV who received rituximab (MabThera) or other treatments between 2003 and 2017 at a specialist vasculitis clinic. The primary endpoint was time to first SAE.
Results
392 patients were enrolled: 247 in the rituximab and 145 in the control cohorts with a total follow up of 2217 person-years (mean study duration 5.7 years). Mean age was 61 years, 77% had granulomatosis with polyangiitis (GPA). There were differences in baseline characteristics (disease duration and prior immunosuppressive use) between groups. 134/247 patients (54%) in the rituximab and 58/145 (40%) of controls experienced at least one SAE. Time to first SAE was shorter in the rituximab group (hazard ratio (HR) 1.55, 95% CI 1.07–2.26, P = 0.022). Predictors of first SAE were higher vasculitis damage index and the presence of chronic pulmonary or kidney disease. The risk of serious infection was higher in the rituximab group (relative risk (RR) 2.12, 95% CI 1.31–3.43).
Conclusion
Over 40% of patients with AAV experienced at least one SAE. Although shorter time to first SAE and higher risk of infection were observed in the rituximab group, baseline imbalances necessitate a careful interpretation of these results.
Keywords: ANCA-associated vasculitis, rituximab, safety
Key messages.
Long term observation in a real-world setting is necessary to determine the adverse effects associated with the treatment of ANCA associated vasculitis.
Over 40% of patients with AAV experience at least one serious adverse event (SAE) during their disease course.
The risk of SAE, particularly serious infection, was higher in the rituximab group. This study provides real-world-data on the long-term safety of rituximab in patients with AAV.
Introduction
ANCA-associated vasculitis (AAV) is a rare disease that elicits systemic inflammation through specific autoantibodies [1]. The major subtypes are granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Historically, treatment regimens involving cyclophosphamide and glucocorticoids have controlled the disease and prevented death [2]. Evidence supports a central role for B-lymphocytes in the pathogenesis of GPA/MPA providing a rationale for the use of a B cell depleting agent, such as rituximab [3–6]. Rituximab had similar rates of adverse events to cyclophosphamide in the RAVE and RITUXVAS studies [7, 8]. Moreover, the efficacy of fixed interval repeat dose of rituximab has been confirmed for remission maintenance in the MAINRITSAN and RITAZAREM trials [9, 10]. Hence, rituximab has become a first-line treatment for both induction and maintenance of remission [11].
Serious adverse events (SAEs) following rituximab use in AAV, include infection [12–14], hypogammaglobulinemia [15], malignancy [16, 17], bone marrow suppression [18] and cardiovascular disease [19–21]. Infection is a primary concern, given its association with mortality especially in the first year of therapy [13, 22]. A prospective study of rituximab-treated AAV patients reported SAEs occurring in 39.2% and infections occurring in 14.4% over a follow-up period of 3.94 years [23].
Given improved AAV survival rates over recent decades [24], risk of relapse has to be balanced against therapy associated complications and the long-term safety of rituximab remains unclear. This study aimed to identify the incidence of safety events (SAEs and non-serious adverse events of special interest [AESIs]), the risk profile of rituximab (MabThera) and to compare time to adverse events, with up to 15 years follow-up, in patients with GPA/MPA who have been treated with rituximab versus other therapies.
Methods
Study design and participants
The RItuximab surveillance study in VASculitis was a retrospective observational study of patients with GPA/MPA (age≥18 years) who received a first-dose of rituximab (MabThera) or non-rituximab treatments between 2003 and 2017 in a specialist vasculitis clinic. Data was extracted from the UK and Ireland Vasculitis Rare Disease Group (UKIVAS) registry (REC reference: 10/H1102/77). The protocol was designed as a prospective study but was modified to a retrospective study in 2018 to maximize data on use of MabThera rituximab brand. Following the protocol amendment, participants initially recruited for the prospective study were re-consented form for inclusion in the UKIVAS registry. The study was approved by the Nottingham 1 Research Ethics Committee (REC reference: 16/EM/0355) and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent.
Data collection
The study collected data on age, gender, diagnosis, ANCA serotype, date of MabThera administration, previous immunosuppression exposure, co-morbidities, disease status (severe flare was defined as one or more Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) new/worse major items), Vasculitis Damage Index (VDI), safety events/SAEs, and laboratory data including eGFR, IgG, CD19 count, neutrophil count. Hypogammaglobulinemia and neutropenia was defined by IgG <5 g/l and absolute neutrophil count <1500 μl. IgG level was subclassified as severe <3 g/l and moderate <5 g/l. Data was collected at baseline and every 12–18 months until patient death, loss to follow-up, withdrawal of consent, or end of the study on 30 September 2018.
Definition
Safety events included SAEs and AESIs (Supplementary Table S1, available at Rheumatology Advances in Practice online). SAEs were defined as adverse events that were life-threatening, fatal, required hospitalization, or resulted in significant disability. Infections were defined by the Common Terminology Criteria for Adverse Events version 3.0 and infections graded ≥ 3 were considered serious infections. Additional safety events include hypogammaglobulinemia (<3 g/l) requiring treatment or change of treatment, hypogammaglobulinemia requiring Ig replacement, serious disease flares (major flare), infusion-related reaction, vaccination failure, and any other SAEs of unclear categorization.
Outcomes
The primary endpoint was time to first SAE. Secondary endpoints were time to first pre-categorized SAE, the incidence of safety events/SAEs, and longitudinal changes in laboratory tests. Exploratory analyses assessed time to second or multiple SAE.
Statistical analysis
Continuous variables are presented as mean (s.d.), or as median and range. Categorical variables are presented as the number and percentage. Incidence rates for events were expressed as events/1000 person-years. Time 0 was defined as initiation of MabThera treatment for the rituximab cohort or the time of first disease flare/diagnosis for the control cohort. Patients who switched from Mabthera to a rituximab biosimilar during follow-up were censored at the time of switch. Time to SAE was described using Kaplan-Meier analysis. For recurrent SAEs, timing restarted after each event. For multiple different events timing began from baseline. To investigate the predictors of time to SAE, Cox proportional hazard analyses were performed, adjusting for age, disease duration, ANCA subtype, VDI and comorbidities. For the primary endpoint analysis, a sensitivity analysis was performed using the study end date instead of the time of switching to Truxima. Multiple events were analysed using frailty and Poisson regression models which used a generalized estimation equation with a log-link function. Unadjusted relative risk (RR) for SAEs by event category was described. Statistical analyses were conducted with R, version 4.1.1.
Results
Of 418 patients recruited, 392 were allocated: there were 247 patients in the rituximab group and 145 in the control group (Supplementary Fig. S1, available at Rheumatology Advances in Practice online).
Baseline characteristics
The total follow-up was 1370 patient years for the rituximab and 848 for the control group with a total of 2218 patient years (mean study duration 5.7 years). Mean age at study entry was 61 years [s.d. 16.3] and patients in the rituximab group were younger (58.3 years [s.d. 17.1] vs 65.6 years [s.d. 13.6]). Median disease duration at baseline was 25 months (range: 0–394) in the rituximab and 1.6 months (0–273) in the control group. The diagnosis was GPA in 203 patients (82%) in the rituximab and 99 (68%) in the control group. Hypertension was the most frequently reported comorbidity (41% in rituximab vs 39% in control). Although the percentage of prior exposure to cyclophosphamide and steroid was similar between groups, immune suppressive exposure was higher in the rituximab compared with the control group (azathioprine 58% vs 23%, methotrexate 25% vs 12% and mycophenolate 40% vs 19%, respectively). The median VDI at baseline was 1 (0–8) in the rituximab group and 0 (0–7) in the control group. Baseline BVAS was similar between groups, with remission rates of 71% and 74% and severe disease/flare rates of 3% and 2%, respectively. At baseline, median eGFR, was higher in the rituximab group (75 ml/min/1.73m2 [6–189] vs 61 ml/min/1.73 m2 [5–189]) and the median IgG and CD19 counts were lower in the rituximab group (IgG 8.6 g/l [2.9–23.2] vs 10.81 g/l [4.33–33.6], CD19 count 0.1 × 109/l [0–0.77] vs 0.12 × 109/l [0.02–0.82]). Chronic kidney disease (CKD) stage IV and V were less common in the rituximab group (31% vs 44%) (Table 1).
Table 1.
Patient demography at entry into the study
| All (n = 392) | Rituximab (n = 247) | Control (n = 145) | |
|---|---|---|---|
| Total follow-upa (per person-years) | 2217.5 | 1369.7 | 847.8 |
| Follow-up time: mean (s.d.), years | 5.7 (3.9) | 5.5 (3.5) | 5.8 (4.3) |
| Age, mean (s.d.), years | 61.0 (16.3) | 58.3 (17.1) | 65.6 (13.6) |
| Disease duration: median (range) (months) | 15.8 (0-393.7) | 25.0 (0-393.7) | 1.6 (0-272.6) |
| Female | 205/392 (52%) | 124/247 (50%) | 81/145 (56%) |
| BMI (kg/m2) | 27.7 (14.2-56.9) | 28 (14.2-56.9) | 26.9 (17.9-51.7) |
| Ethnicity | |||
| White British | 365/392 (93%) | 230/247 (93%) | 135/145 (93%) |
| Other White | 16/392 (4%) | 10/247 (4%) | 6/145 (4%) |
| Asian | 8/392 (2%) | 6/247 (2%) | 2/145 (1%) |
| Other | 3/392 (1%) | 1/247 (0%) | 2/145 (2%) |
| Diagnosis | |||
| MPA | 90/392 (23%) | 44/247 (18%) | 46/145 (32%) |
| GPA | 302/392 (77%) | 203/247 (82%) | 99/145 (68%) |
| ANCA type at diagnosis | |||
| Anti-proteinase3 | 157/392 (40%) | 104/247 (42%) | 53/145 (37%) |
| Anti-myeloperoxidase | 73/392 (19%) | 28/247 (11%) | 45/145 (31%) |
| Negative and/or unknown | 162/392 (41%) | 115/247 (47%) | 47/145 (32%) |
| Comorbidities | |||
| Pulmonary disease (chronic)b | 69/391 (18%) | 40/246 (16%) | 29/145 (20%) |
| Cardiac disease | 38/391 (10%) | 24/246 (10%) | 14/145 (10%) |
| Malignancy | 37/39 (9%) | 26/246 (11%) | 11/145 (8%) |
| Diabetes Mellitus | 24/391 (6%) | 18/246 (7%) | 6/145 (4%) |
| Hypertension requiring treatment | 159/391 (41%) | 102/246 (41%) | 57/145 (39%) |
| Kidney disease | 61/391 (16%) | 38/246 (15%) | 23/145 (16%) |
| Kidney disease stage | |||
| II | 6/61 (10%) | 4/38 (11%) | 2/23 (9%) |
| III | 33/61 (54%) | 22/38 (58%) | 11/23 (48%) |
| IV | 10/61 (16%) | 5/38 (13%) | 5/23 (22%) |
| V | 12/61 (20%) | 7/38 (18%) | 5/23 (22%) |
| Prior immune suppressive treatment | |||
| Azathioprine | 177/392 (45%) | 144/247 (58%) | 33/145 (23%) |
| Cyclophosphamide | 273/392 (70%) | 180/247 (73%) | 93/145 (64%) |
| Steroid | 386/392 (98%) | 246/247 (100%) | 140/145 (97%) |
| Methotrexate | 0/392 (20%) | 62/247 (25%) | 18/145 (12%) |
| Mycophenolate | 127/392 (32%) | 99/247 (40%) | 28/145 (19%) |
| VDI Score | 1 (0,8) | 1 (0,8) | 0 (0,7) |
| BVAS Disease status | |||
| Severe Disease/Flare | 4/169 (2%) | 3/119 (3%) | 1/50 (2%) |
| Limited Disease/Flare | 12/169 (7%) | 9/119 (8%) | 3/50 (6%) |
| Persistent Disease | 31/169 (18%) | 22/119 (18%) | 9/50 (18%) |
| Remission | 122/169 (72%) | 85/119 (71%) | 37/50 (74%) |
| Laboratory data at entry | |||
| eGFRc (ml/min/1.73m2) | 69 (5–189) | 75 (6–189) | 61 (5–189) |
| IgGd (g/l) | 9.36 (2.9–33.6) | 8.6 (2.9–23.2) | 10.81 (4.33–33.6) |
| CD19 count (×109/l)e | 0.1 (0–0.82) | 0.1 (0–0.77) | 0.12 (0.02–0.82) |
Continuous variables are expressed as median (range) unless otherwise indicated, and categorical variables as number of sample size/total number (percentages).
Total follow-up was assessed until 30 September 2018.
Pulmonary disease (chronic) includes asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease/pulmonary fibrosis, bronchiectasis and emphysema.
Data were missing for 12 patients.
Data were missing for 19 patients.
Data from 125 patients in the rituximab group and 11 patients in the control group.
MPA: microscopic polyangiitis; GPA: granulomatosis with polyangiitis; VDI: Vasculitis Damage Index.
Rituximab infusions
The mean rituximab treatment duration was 5.5 years over 1370 patient years and patients received a mean of 7 (range 1 to 23) infusions during the follow-up period. The dose was typically 1 g.
Safety: safety events and SAEs
There were 533 safety events in 164 patients (66%) in the rituximab and 170 in 78 patients (54%) in the control groups. 386 SAEs occurred in 134 (54%) in the rituximab and 114 in 58 (40%) in the control groups. The incident rate of SAEs for the rituximab group was 270 per 1000 patient years (95% CI 244.7–298.7) compared with 129 per 1000 patient years (95% CI 107.2–154.8) for the control group (Table 2, Supplementary Tables S2 and S3, available at Rheumatology Advances in Practice online). There were 149 serious infections in 83 patients (21%); 121 occurred in 65 (26%) in the rituximab compared with 28 in 18 (12%) in the control groups, incidence rates: 84.8 per 1000 patient years (95% CI 70.9–101.3) and 31.6 per 1000 patient years (95% CI 21.8–45.8), respectively. Lower respiratory tract infections were the most common serious infection (93/149 events [62%]); 79 in 40 patients (16%) in the rituximab and 14 in 12 (8%) in the control groups (Table 3). There were seven opportunistic infections (five in the rituximab and one in the control group) (Supplementary Table S4, available at Rheumatology Advances in Practice online). 200/245 (81%) in the rituximab and 105/145 (72%) in the control group received antibiotic prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX).
Table 2.
Total number and incidence rate of safety events (serious adverse events [SAEs] and non-serious adverse events of special interest [AESIs]) and SAEs by event category for rituximab and control groups
| Rituximab (N = 247) |
Control (N = 145) |
Rituximab (N = 247) | Control (N = 145) | |||
|---|---|---|---|---|---|---|
| Event type | Events n | Patients n (%) | Events n | Patients n (%) | IR per 1000 person-years (95% CI)a | IR per 1000 person-years (95% CI)a |
| All safety events | 533 | 164 (66%) | 170 | 78 (54%) | 373.3 (342.9, 406.4) | 192.1 (165.3, 223.2) |
| Infection | 124 | 67 (27%) | 29 | 19 (13%) | 86.9 (72.8, 103.6) | 32.8 (22.8, 47.2) |
| Cardiovascular disorder | 42 | 36 (15%) | 22 | 18 (12%) | 29.4 (21.7, 39.8) | 24.9 (16.4, 37.8) |
| Haematological events | 35 | 31 (13%) | 9 | 9 (6%) | 24.5 (17.6, 34.1) | 10.2 (5.3, 19.5) |
| Malignant events | 28 | 21 (9%) | 21 | 16 (11%) | 19.6 (13.5, 28.4) | 23.7 (15.5, 36.4) |
| Renal Insufficiency | 21 | 15 (6%) | 20 | 15 (10%) | 14.7 (9.6, 22.6) | 22.6 (14.6, 35) |
| PML | 1 | 1 (0.4%) | 0 | 0 (0%) | 0.7 (0.1, 5) | 0 (0, NaN) |
| Additional safety events | 282 | 122 (49%) | 69 | 48 (33%) | 197.5 (175.8, 222) | 78.0 (61.6, 98.7) |
| All SAEs | 386 | 134 (54%) | 114 | 58 (40%) | 270.4 (244.7, 298.7) | 128.8 (107.2, 154.8) |
| Serious infection | 121 | 65 (26%) | 28 | 18 (12%) | 84.8 (70.9, 101.3) | 31.6 (21.8, 45.8) |
| Cardiovascular disorder | 28 | 23 (9%) | 17 | 15 (10%) | 19.6 (13.5, 28.4) | 19.2 (11.9, 30.9) |
| Haematological events | 9 | 8 (3%) | 2 | 2 (1%) | 6.3 (3.3, 12.1) | 2.3 (0.6, 9.0) |
| Malignant events | 11 | 11 (4%) | 8 | 8 (6%) | 7.7 (4.3, 13.9) | 9.0 (4.5, 18.1) |
| Renal Insufficiency | 19 | 14 (6%) | 19 | 15 (10%) | 13.3 (8.5, 20.9) | 21.5 (13.7, 33.7) |
| PML | 1 | 1 (0.4%) | 0 | 0 (0%) | 0.7 (0.1, 5) | 0 (0, NaN) |
| Additional safety eventsb | 197 | 95 (38%) | 40 | 30 (21%) | 138.0 (120, 158.7) | 45.2 (33.2, 61.6) |
Total follow-up period was 1369.7 per 1000 person-years for the rituximab and 847.8 per 1000 person-years for the control group.
Additional safety events include hypogammaglobulinemia (<3 g/l) requiring treatment or change of treatment, hypogammaglobulinemia requiring Ig replacement therapy, serious disease flares (major flare), serious infusion-related reaction, vaccination failure, and any other SAEs of unclear categorization.
IR: incidence rate; PML: progressive multifocal leukoencephalopathy.
Table 3.
Adverse events associated with serious infections and the prevalence of antibiotic prophylaxis (trimethoprim/sulfamethoxazole (TMP/SMX)) for rituximab and control groups
| Rituximab (N = 247) |
Control (N = 145) |
|||
|---|---|---|---|---|
| Event type | Events n | Patients n (%) | Events n | Patients n (%) |
| Serious infection | 121 | 65 (26%) | 28 | 18 (12%) |
| Lower respiratory tract infection | 79 | 40 (16%) | 14 | 12 (8%) |
| Hypogammaglobulinemia (<3 g/l) requiring treatment or change of treatment | 22 | 21 (9%) | 3 | 3 (2%) |
| Hypogammaglobulinemia requiring Ig replacement | 19 | 19 (8%) | 1 | 1 (0.7%) |
| Use of TMP/SMX | – | 200/245 (81%) | – | 105/145 (72%) |
Ig: immunoglobulin; TMP/SMX: trimethoprim/sulfamethoxazole.
There were 42 cardiovascular events in 36 patients (15%) in the rituximab and 22 in 18 (12%) in the control groups. In this category, venous thrombotic events were common: 15 events in 14 (6%) in the rituximab and 11 in 10 (7%) in the control groups (Table 2, Supplementary Table S2, available at Rheumatology Advances in Practice online).
49 malignancies occurred during the study: 28 in the rituximab (non-melanoma skin cancer (NMSC) (17), other (11)) and 21 in the control group (NMSC (14), other (7)) (Table 2, Supplementary Tables S2 and S5, available at Rheumatology Advances in Practice online). There were no differences in incidence rates of malignancy, cardiovascular events, and renal insufficiency between groups. One male in the rituximab group had progressive multifocal leukoencephalopathy (PML).
In the category of additional safety events, 21 (9%) in the rituximab group developed hypogammaglobulinemia (IgG <3 g/l) requiring withdrawal or reduction of immunosuppressive agents and 19 (8%) received Ig replacement for hypogammaglobulinemia compared with 3 (2%) and 1 (0.7%) patient in the control group. Eighteen (7.3%) in the rituximab and 2 (1.4%) in the control group developed neutropenia. Of these, 6/18 in the rituximab group required antibiotics and/or hospitalization for infectious symptoms, and one in the rituximab group received granulocyte colony-stimulating growth factor (GCSF). No serious neutropenia occurred in the control group (Supplementary Table S2 and S3, available at Rheumatology Advances in Practice online). The main cause of ‘Additional safety events’ in the rituximab group was ‘Any other SAE(s) of unclear categorization’, which included planned hospital admissions for bronchoscopy with treatment or admissions for vasculitis-related complications such as acute kidney injury or epistaxis (Table 2, Supplementary Table S3, available at Rheumatology Advances in Practice online). Notably, more patients in the rituximab group experienced subglottic or tracheal stenosis requiring multiple admissions for dilatation and/or intravenous medication than in the control group.
Six patients died during the study period but were excluded from the analysis due to lack of re-consent following changes in the study design.
Time to first SAE
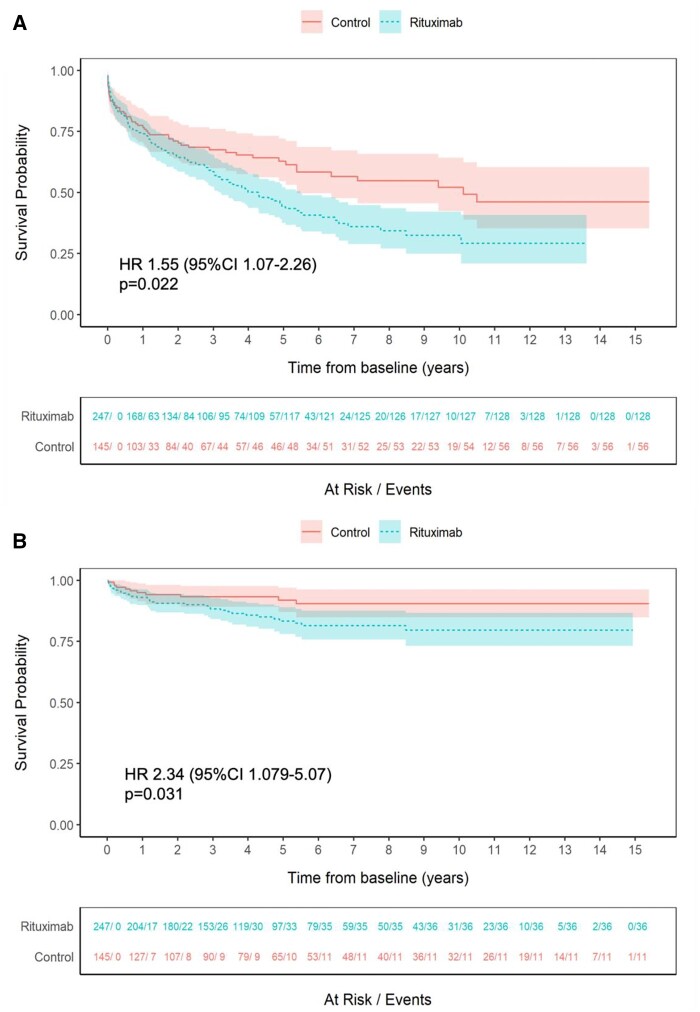
Time to first SAE was shorter in the rituximab than in the control groups (HR 1.55, 95% CI 1.07–2.26, P = 0.022) (Fig. 1A). Covariates at baseline associating with a shorter time to first SAE were higher VDI (HR 1.14, 95% CI 1.02–1.27, P = 0.022), pulmonary (HR 1.58, 95% CI 1.07–2.34, P = 0.023) and chronic kidney disease stage V (HR 3.37, 95% CI 1.66–6.84, P < 0.001) (Supplementary Table S6, available at Rheumatology Advances in Practice online). The sensitivity analysis with study end date had no effect on the outcomes.
Figure 1.
Time to first SAE and serious infection for the rituximab and control groups. (A) Time to first SAE. (B) Time to first serious infection. Shaded areas represent 95% CIs
Time to first pre-categorized SAE
Time to first serious infection was shorter in the rituximab than in the control group (HR 2.34, 95% CI 1.079–5.07, P = 0.031). No between-group differences were observed for other categories of SAE (HR 0.62, 95% CI 0.205–1.86, P = 0.390 for cardiovascular disorders; HR 12.8, 95% CI 1.275–128.76, P = 0.03 for haematological events; HR 1.03, 95% CI 0.220–4.86, P = 0.966 for malignant events; HR 1.51, 95% CI 0.421–5.38, P = 0.529 for renal insufficiency; HR 1.39, 95% CI 0.76–2.53, P = 0.282 for additional safety events) (Fig. 1B, Supplementary Figs S2 and S6, available at Rheumatology Advances in Practice online). The results for haematological events may be compromised by the wide confidence intervals, with the potential for overfitting caused by a limited number of events.
Time to second or multiple SAE
Time to second SAE was shorter in the rituximab group (HR 2.58, 95% CI 1.43–4.65, P = 0.002) (Supplementary Fig. S7, available at Rheumatology Advances in Practice online). Chronic kidney disease stage V (HR 8.07, 95% CI 3.57–18.28, P < 0.001) was a predictor of second SAE. For multiple SAEs, in the frailty model, the rituximab group had a shorter time between SAEs (HR 1.86, 95% CI 1.31–2.64, P < 0.001) and a shorter time from baseline to multiple SAEs (HR 2.45, 95% CI 1.35–4.44, P < 0.003). Poisson regression model showed a similar trend (Supplementary Tables S7–S10, available at Rheumatology Advances in Practice online).
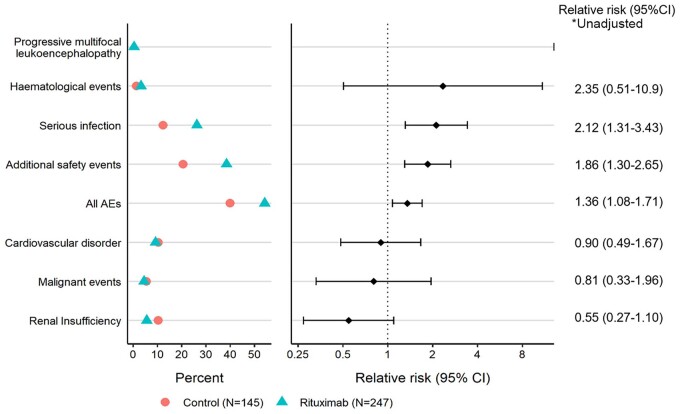
Unadjusted relative risk (RR) for SAEs by event category demonstrated an increased risk for infection (RR 2.12, 95% CI 1.31–3.43) and additional safety events (RR 1.86, 95% CI 1.30–2.65) for the rituximab group, while there were no differences in risk for other categories (Fig. 2).
Figure 2.
Unadjusted relative risk of SAEs by event category between rituximab and control groups
IgG levels and neutrophil counts during follow-up
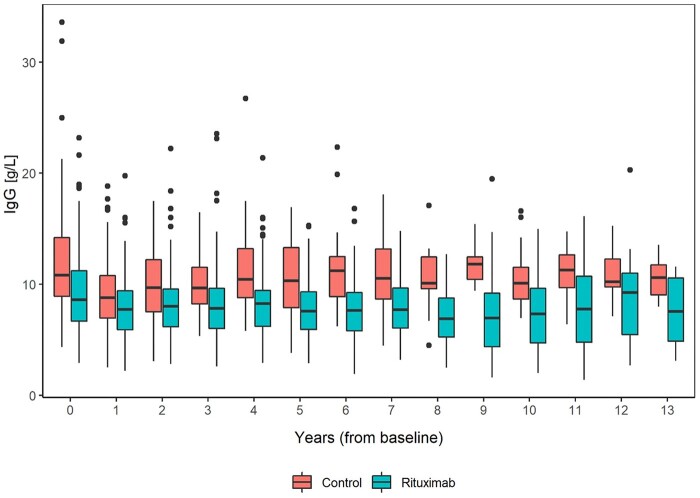
IgG levels decreased over the first 3 years, with greater recovery of IgG in the control group and a larger difference between groups. More patients in the rituximab group developed IgG <5 g/l, with a small number of patients falling below 3 g/l. Although the number of events were limited, this trend continued over the longer term (Fig. 3, Supplementary Fig. S8, available at Rheumatology Advances in Practice online). There were no differences in neutrophil counts between groups (Supplementary Fig. S9, available at Rheumatology Advances in Practice online).
Figure 3.
IgG levels throughout the study in the rituximab and control groups. The boxes indicate the medians and interquartile ranges, the vertical lines indicate the 1.5 interquartile range. Circles indicate the outliers
Discussion
This retrospective study assessed the frequency and type of safety events associated with rituximab (MabThera) in GPA/MPA patients in a real world setting with long-term follow-up compared with a control group treated with non-rituximab immunosuppressive therapies. We demonstrated a high incidence of safety events/SAEs in both treatment groups with a shorter time to first SAE and serious infection in the rituximab group. In addition, the rituximab group was more prone to second and multiple SAEs. An increased risk of serious infections and additional safety events was observed in the rituximab group. There were differences in baseline characteristics between the groups, reflecting that from 2003 rituximab was initially used for refractory disease. In particular, disease duration and prior immunosuppressive use varied between groups, limiting the value of the comparisons between groups and attribution of the difference in safety events to rituximab treatment alone.
Serious infection is a major cause of adverse events and mortality in GPA/MPA [13, 25]. In a meta-analysis of rituximab-treated AAV patients, the incidence of severe infections was 6.5 per 100 person-years (95% CI 2.9–11.4), compared with 3.9 per 100 person-years in rituximab-treated rheumatoid arthritis [26, 27]. A complication of rituximab in GPA/MPA is hypogammaglobulinemia, with increased infection risk <3–4 g/l [15, 28–30]. We observed a trend of greater falls in IgG for the first 1–3 years in patients treated with rituximab, consistent with previous reports [14, 27]. The rates of hypogammaglobulinemia were not clearly different between the treatment groups, however, more in the rituximab group developed IgG <5 g/l. Earlier reports have defined predictors of hypogammaglobulinemia (IgG <5 g/l) and/or use of Ig replacement therapy at 60 months following rituximab, namely, prior use of cyclophosphamide, glucocorticoid use at 12 months, a lower nadir IgG within 12 months of starting rituximab and female sex [15]. Lower IgG at the time of first rituximab was correlated with lower nadir IgG levels post rituximab [31]. Importantly, the cumulative dose of rituximab has not been associated with moderate/severe hypogammaglobulinemia [15, 32]. In this study, 73% of patients in the rituximab group had prior cyclophosphamide use and a lower baseline IgG, which suggests an increased risk of serious infections related to hypogammaglobulinemia. As some patients subsets are at high risk of prolonged hypogammaglobulinemia even after therapy withdrawal, it is recommended to monitor IgG levels before and after rituximab treatment and consider early interventions, such as prophylactic antibiotics and Ig replacement therapy [15].
Late-onset neutropenia is a complication of rituximab that occurred in 12% of patients a median time of 86 days after rituximab and was frequently associated with infection [18]. We found neutropenia more prevalent in the rituximab group also increasing infective risk. Neutropenia was typically transient lasting 1–2 weeks and tended not to recur. We did not study vaccine responses and a poor vaccine response is a predictor of all-cause mortality in AAV [33].
Although the highest rate of cardiovascular events have been observed in the first year of treatment due to active disease [14, 19, 20], these events remain of concern long term [13]. Furthermore, venous thromboembolic events occurred in 9.7% of AAV patients, ranging from 6.3% to 13.7%, consistent with our study [34]. We did not observe any differences in thromboembolic or cardiovascular events between groups.
A previous study observed that the malignancy risk in cyclophosphamide-treated AAV patients was 4.6-fold higher (95% CI 1.16–39.98) than in rituximab-treated patients during a follow-up of 5.6 years [16]. We observed no differences in the rate of malignancies between groups. This difference might result from reduced cyclophosphamide in recent regimens and its prior use in the rituximab group. In accordance with previous studies [17, 35], NMSC was the most frequent malignancy, underscoring the need for patient education to minimize exposure to ultraviolet radiation. Furthermore, prolonged use of azathioprine is associated with a higher risk of NMSC in numerous inflammatory diseases. Mycophenolate mofetil also contributes to an increased risk of NMSC, although to a lesser extent [36].
PML, an opportunistic infection caused by JC virus, is a rare safety risk of rituximab. One case of non-fatal PML was occurred in the rituximab group, despite increased use of previous immune suppressants, known risk factors for PML [37].
Although the proportion of patients experiencing SAEs in this study is comparable to the RAVE and RITUXVAS rituximab induction trials, those studies found no differences in adverse event rates between rituximab and cyclophosphamide groups [7, 8]. Similarly, in the MAINRITSAN and RITAZAREM rituximab maintenance trials, comparing rituximab to azathioprine for maintenance therapy, no differences in SAEs were observed between groups [9, 10]. In our study, the incidence rate of SAEs for the rituximab cohort was 270.4 per 1000 patient years (95% CI 244.7–298.7), similar to a prospective study with 278.4 per 1000 patient years (95% CI 225–341) with 14.4% of patients over 3.94 years [23]. The higher proportion of patients developing SAEs in the rituximab group may be attributed to the imbalance in baseline characteristics.
Risk factors for rituximab-related SAE were not identified from RCTs where no major between-group difference were observed, whereas risk factors for serious infections have been identified as pulmonary disease and low GFR, with mortality linked to high VDI at diagnosis, consistent with our findings for time to first SAE [14, 38, 39]. Given that infection and additional safety events were the common complications among SAEs with no apparent differences in other categories, and that the risks of these two events were higher in the rituximab group in our study, it is possible that hypogammaglobulinemia leading to infection and admission for bronchoscopy with treatment or for other reason were associated with a shorter time to first SAE. These findings highlight the importance of prophylaxis with TMP/SMX, particularly in view of the potential beneficial impact on respiratory tract infections beyond the prevention of Pneumocystis jirovecii pneumonia [40, 41], although the optimal duration of prophylaxis remains uncertain.
For the second SAE, patients developed one SAE were prone to experience subsequent events in the rituximab group, likely due to serious infections and additional safety events. In keeping with our study, renal dysfunction showed an increased infection risk due to impaired immunity and low drug clearance resulting in high toxicity of immunosuppressive drugs [14].
There are limitations in this study. First, the baseline variables were not matched between treatment groups. A long disease duration prior to rituximab implied that rituximab was often used for patients with relapsing or refractory disease who would have both a long history of immunosuppressive and steroid treatment with accrued vasculitis related damage. These factors would have had an influence on safety risks, and we cannot exclude unmeasured confounders and selection bias. Second, the absence of data on achieving remission after baseline might lead to different outcomes; however, the remission status at baseline was comparable in both groups. Third, this study did not assess detailed therapies other than rituximab during follow-up. Thus, we cannot exclude the possibility that choices of maintenance therapy may have had the effects on assessed outcomes. Finally, the relative risk for SAEs by event category was not adjusted, which may have affected the results.
This study has several strengths as it includes all eligible patients with GPA/MPA referred to a tertiary vasculitis centre which supports the reliability of the estimates of the risks for safety events and reflects real world setting. Treatment has changed over the period of observation and continues to evolve since the observation period was completed. Rituximab is now the first immunosuppressive agent to be used to induce and maintain remission [11]. This means that patients with GPA/MPA now treated with rituximab may have less prior immunosuppressive exposure, potentially reducing future safety events.
High adverse event rates with rituximab and other regimens, impacting patient health, mortality, and healthcare costs, underscore the need for safer therapies. Glucocorticoids are an important contributor to risk and lower dose regimens have proven equally effective in the PEXIVAS and LOVAS studies [42, 43]. Alternative use of avacopan has also led to lower glucocorticoid use and may thus improve safety. Currently, no alternatives to rituximab or cyclophosphamide exist for induction therapy, thus future patients are likely to encounter the risks we have described. Alternative immunomodulators, including obinutuzumab are in clinical trials in AAV, and, if effective, may offer a different risk profile.
In conclusion, this study reported that over 40% of patients with GPA/MPA experience at least one SAE during their disease course. Infection remains the most common complication of therapy. Although the risk of first and multiple SAE was higher in the rituximab group, with an increased risk of infection by unadjusted analysis, baseline imbalances due to the study design were a major cause of bias, making it challenging to draw firm conclusions. Nevertheless, this study provides a real-world data on the long-term safety of rituximab in patients with GPA/MPA.
Supplementary Material
Acknowledgements
We thank all the patients and medical staffs who were involved in this study. This work was presented at the European Alliance of Associations for Rheumatology meeting (Milano, Italy, 2023) and at the American College of Rheumatology Convergence (San Diego, United states, 2023). David Jayne is supported by the NIHR Cambridge Biomedical Research Centre.
Contributor Information
Lisa Uchida, Department of Medicine, University of Cambridge, Cambridge, UK.
Rachel B Jones, Vasculitis and Lupus Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Rona M Smith, Department of Medicine, University of Cambridge, Cambridge, UK; Vasculitis and Lupus Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Marianna Nodale, Department of Medicine, University of Cambridge, Cambridge, UK; Cambridge Clinical Trials Unit, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Simon Bond, Cambridge Clinical Trials Unit, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Claudia Loechel, Vasculitis and Lupus Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Maria King, Vasculitis and Lupus Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Raashid Luqmani, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
David Gray, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Joe Barrett, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
David R W Jayne, Department of Medicine, University of Cambridge, Cambridge, UK; Vasculitis and Lupus Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
Data can be requested from the corresponding author on reasonable request.
Funding
This study was supported by Cambridge Biomedical Research Centre and Hoffman La Roche (BE29950).
Disclosure statement: R.J. reports research grants from GlaxoSmithKline, Roche and CSL Vifor, honoraria from Roche, and consultant fees from GlaxoSmithKline and CSL Vifor. RS has received research grants from GlaxoSmithKline and Union Therapeutics. R.L. has received grants from CSL Vifor, BMS-Celgene and GlaxoSmithKline; honoraria from AbbVie, CSL Vifor, GlaxoSmithKline and Infla Rx; participation on advisory board for AbbVie. D.J. has received grants from GlaxoSmithKline and CSL Vifor; consulting fees from AstraZeneca, GlaxoSmithKline, Novartis, Roche, Takeda and CSL Vifor; honoraria from GlaxoSmithKline and CSL Vifor; participation on advisory boards for Chinook, GlaxoSmithKline and Hansa and stock options from Aurinia. The remaining authors have declared no conflicts of interest.
References
- 1. Kitching AR, Anders H-J, Basu N. et al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020;6:71. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman GS, Kerr GS, Leavitt RY. et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–98. [DOI] [PubMed] [Google Scholar]
- 3. Wolff SM. Wegener’s granulomatosis. Vol. 81. Annals of Internal Medicine, 1974:513.Philadelphia, PA: American College of Physicians. 10.7326/0003-4819-81-4-513 (12 August 2024, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Aries PM, Hellmich B, Voswinkel J. et al. Lack of efficacy of rituximab in Wegener’s granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis 2006;65:853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith KGC, Jones RB, Burns SM, Jayne DRW.. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum 2006;54:2970–82. [DOI] [PubMed] [Google Scholar]
- 6. Jones RB, Ferraro AJ, Chaudhry AN. et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2009;60:2156–68. [DOI] [PubMed] [Google Scholar]
- 7. Jones RB, Tervaert JWC, Hauser T. et al. ; European Vasculitis Study Group. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363:211–20. [DOI] [PubMed] [Google Scholar]
- 8. Stone JH, Merkel PA, Spiera R. et al. ; RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillevin L, Pagnoux C, Karras A. et al. ; French Vasculitis Study Group. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. [DOI] [PubMed] [Google Scholar]
- 10. Smith RM, Jones RB, Specks U. et al. Rituximab versus azathioprine for maintenance of remission for patients with ANCA-associated vasculitis and relapsing disease: an international randomised controlled trial. Ann Rheum Dis 2023;82:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellmich B, Sanchez-Alamo B, Schirmer JH. et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis 2023;83:30–47. [DOI] [PubMed] [Google Scholar]
- 12. Garcia-Vives E, Segarra-Medrano A, Martinez-Valle F, Agraz I, Solans-Laque R.. Prevalence and risk factors for major infections in patients with antineutrophil cytoplasmic antibody-associated vasculitis: influence on the disease outcome. J Rheumatol 2020;47:407–14. [DOI] [PubMed] [Google Scholar]
- 13. Flossmann O, Berden A, de Groot K. et al. ; European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. [DOI] [PubMed] [Google Scholar]
- 14. Mohammad AJ, Segelmark M, Smith R. et al. Severe infection in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 2017;44:1468–75. [DOI] [PubMed] [Google Scholar]
- 15. Tieu J, Smith RM, Gopaluni S. et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol 2021;12:671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Daalen EE, Rizzo R, Kronbichler A. et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis 2017;76:1064–9. [DOI] [PubMed] [Google Scholar]
- 17. Heijl C, Harper L, Flossmann O. et al. ; European Vasculitis Study Group (EUVAS). Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European Vasculitis Study Group clinical trials. Ann Rheum Dis 2011;70:1415–21. [DOI] [PubMed] [Google Scholar]
- 18. Knight A, Sundström Y, Börjesson O. et al. Late-onset neutropenia after rituximab in ANCA-associated vasculitis. Scand J Rheumatol 2016;45:404–7. [DOI] [PubMed] [Google Scholar]
- 19. Houben E, Penne EL, Voskuyl AE. et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology 2018;57:555–62. [DOI] [PubMed] [Google Scholar]
- 20. Liapi M, Jayne D, Merkel PA, Segelmark M, Mohammad AJ.. Venous thromboembolism in ANCA-associated vasculitis: a population-based cohort study. Rheumatology 2021;60:4616–23. [DOI] [PubMed] [Google Scholar]
- 21. Stassen PM, Derks RPH, Kallenberg CGM, Stegeman CA.. Venous thromboembolism in ANCA-associated vasculitis—incidence and risk factors. Rheumatology 2008;47:530–4. [DOI] [PubMed] [Google Scholar]
- 22. Little MA, Nightingale P, Verburgh CA. et al. ; European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. [DOI] [PubMed] [Google Scholar]
- 23. Merkel PA, Niles JL, Mertz LE. et al. Long-term safety of rituximab in granulomatosis with polyangiitis and in microscopic polyangiitis. Arthritis Care Res 2021;73:1372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelveg-Kristensen KE, Szpirt W, Carlson N. et al. Increasing incidence and improved survival in ANCA-associated vasculitis—a Danish nationwide study. Nephrol Dial Transplant 2022;37:63–71. [DOI] [PubMed] [Google Scholar]
- 25. Tudesq J-J, Cartron G, Rivière S. et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun Rev 2018;17:115–24. [DOI] [PubMed] [Google Scholar]
- 26. Thery-Casari C, Euvrard R, Mainbourg S. et al. Severe infections in patients with anti-neutrophil cytoplasmic antibody-associated vasculitides receiving rituximab: a meta-analysis. Autoimmun Rev 2020;19:102505. [DOI] [PubMed] [Google Scholar]
- 27. van Vollenhoven RF, Emery P, Bingham CO. et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol 2010;37:558–67. [DOI] [PubMed] [Google Scholar]
- 28. Marco H, Smith RM, Jones RB. et al. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet Disord 2014;15:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cortazar FB, Pendergraft WF III, Wenger J. et al. Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheumatol 2017;69:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah S, Jaggi K, Greenberg K, Geetha D.. Immunoglobulin levels and infection risk with rituximab induction for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Kidney J 2017;10:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts DM, Jones RB, Smith RM. et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 2015;57:60–5. [DOI] [PubMed] [Google Scholar]
- 32. Venhoff N, Effelsberg NM, Salzer U. et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One 2012;7:e37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. David Morgan M, Richter A, Al-Ali S. et al. Association of low B cell count and IgG levels with infection, and poor vaccine response with all-cause mortality in an immunosuppressed vasculitis population. Arthritis Care Res 2016;68:853–60. [DOI] [PubMed] [Google Scholar]
- 34. Moiseev S, Kronbichler A, Makarov E. et al. Association of venous thromboembolic events with skin, pulmonary and kidney involvement in ANCA-associated vasculitis: a multinational study. Rheumatology 2021;60:4654–61. [DOI] [PubMed] [Google Scholar]
- 35. Rahmattulla C, Berden AE, Wakker S-C. et al. Incidence of malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis diagnosed between 1991 and 2013. Arthritis Rheumatol 2015;67:3270–8. [DOI] [PubMed] [Google Scholar]
- 36. Kreher MA, Noland MMB, Konda S, Longo MI, Valdes-Rodriguez R.. Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part I: Calcineurin inhibitors, thiopurines, IMDH inhibitors, mTOR inhibitors, and corticosteroids. J Am Acad Dermatol 2023;88:521–30. [DOI] [PubMed] [Google Scholar]
- 37. Berger JR, Malik V, Lacey S, Brunetta P, Lehane PB.. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J Neurovirol 2018;24:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Md Yusof MY, Vital EM, McElvenny DM. et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2019;71:1812–23. [DOI] [PubMed] [Google Scholar]
- 39. Koo G, Ha JW, Ahn SS. et al. Earliest total vascular damage index scores independently predict all-cause mortality in patients with ANCA-associated vasculitis. Clin Exp Rheumatol 2024;42:795–802. [DOI] [PubMed] [Google Scholar]
- 40. Odler B, Riedl R, Gauckler P. et al. ; RAVE−ITN Research Group. Risk factors for serious infections in ANCA-associated vasculitis. Ann Rheum Dis 2023;82:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kronbichler A, Kerschbaum J, Gopaluni S. et al. Trimethoprim—sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 2018;77:1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh M, Merkel PA, Peh C-A. et al. ; PEXIVAS Investigators. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020;382:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furuta S, Nakagomi D, Kobayashi Y. et al. ; LoVAS Collaborators. Effect of reduced-dose vs high-dose glucocorticoids added to rituximab on remission induction in ANCA-associated vasculitis: a randomized clinical trial. JAMA 2021;325:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be requested from the corresponding author on reasonable request.