Abstract
Background
We evaluated the radiologic, pulmonary functional, and antibody statuses of coronavirus disease 2019 (COVID-19) patients 6 and 18 months after discharge, comparing changes in status and focusing on risk factors for residual computed tomography (CT) abnormalities.
Methods
This prospective cohort study was conducted on COVID-19 patients discharged between April 2020 and January 2021. Chest CT, pulmonary function testing (PFT), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) measurements were performed 6 and 18 months after discharge. We evaluated factors associated with residual CT abnormalities and the correlation between lesion volume in CT (lesionvolume), PFT, and IgG levels.
Results
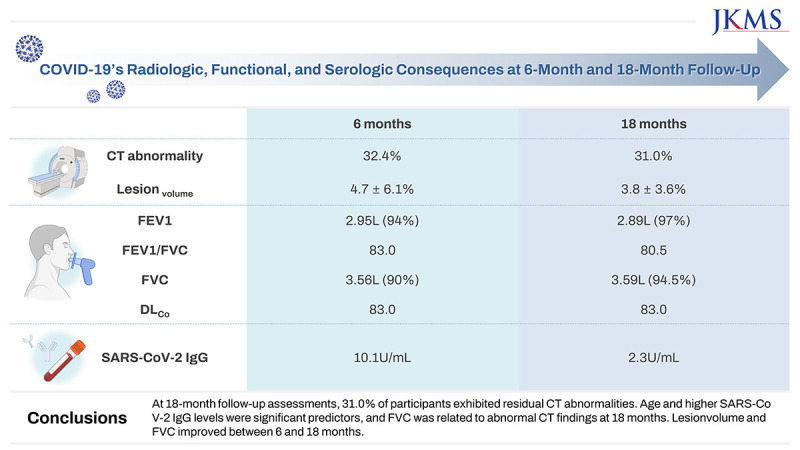
This study included 68 and 42 participants evaluated 6 and 18 months, respectively, after hospitalizations for COVID-19. CT abnormalities were noted in 22 participants (32.4%) at 6 months and 13 participants (31.0%) at 18 months. Lesionvolume was significantly lower at 18 months than 6 months (P < 0.001). Patients with CT abnormalities at 6 months showed lower forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC), and patients with CT abnormalities at 18 months exhibited lower FVC. FVC significantly improved between 6 and 18 months of follow-up (all P < 0.0001). SARS-CoV-2 IgG levels were significantly higher in patients with CT abnormalities at 6 and 18 months (P < 0.001). At 18-month follow-up assessments, age was associated with CT abnormalities (odds ratio, 1.17; 95% confidence interval, 1.03–1.32; P = 0.01), and lesionvolume showed a positive correlation with IgG level (r = 0.643, P < 0.001).
Conclusion
At 18-month follow-up assessments, 31.0% of participants exhibited residual CT abnormalities. Age and higher SARS-CoV-2 IgG levels were significant predictors, and FVC was related to abnormal CT findings at 18 months. Lesionvolume and FVC improved between 6 and 18 months.
Trial Registration
Clinical Research Information Service Identifier: KCT0008573
Keywords: COVID-19, Complications, Computed Tomography, Pulmonary Function Tests, Serologic Tests
Graphical Abstract
INTRODUCTION
More than 774 million cases and 7.0 million deaths from coronavirus disease 2019 (COVID-19) infection have been reported as of October 2023 (https://covid19.who.int/; accessed February 6, 2024), with the first official cases occurring in December 2019. The incidence has been declining, and the World Health Organization (WHO) declared an end to the COVID-19 global health emergency in May 2023. Therefore, now is the time to focus on long-term follow-up, including the radiological and functional pulmonary sequelae of COVID-19 infection, which could have a large long-term impact on healthcare systems.
Previous clinical studies and meta-analyses have reported antibody status and/or radiological and pulmonary functional outcomes 6 and 12 months after COVID-19 infection or hospital discharge.1,2,3,4,5,6,7,8,9,10 These studies indicated that long-term computed tomography (CT) abnormalities and a decline in pulmonary function test (PFT) results could be common in hospitalized patients, especially in severe and critical cases.2,3,4,5,8,9,10 However, the long-term outcomes of lung sequelae shown on CT and their impact on pulmonary function are unknown. These studies emphasized that vigilant observation and longer follow-up periods are warranted, as these sequelae may last a long time.
Therefore, we aimed to evaluate the radiologic, functional, and antibody statuses of COVID-19 patients 6 and 18 months after discharge, including the changes between 6 and 18 months, as well as the risk factors associated with residual CT abnormalities.
METHODS
Study design and participants
We designed a prospective cohort study of patients with COVID-19. Inclusion criteria were: 1) 18 years of age or older, 2) COVID-19 diagnosis confirmed by a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction test, and 3) hospitalization in any institution between April 2020 and January 2021. Among the patients who met the inclusion criteria, those who voluntarily agreed to participate in this study were enrolled by visiting our COVID-19 outpatient clinic. The exclusion criteria were patients who did not want to participate in the study or those who expressed a desire to discontinue participation during the study. For each participant, follow-up visits at 6 and 18 months after discharge were scheduled for chest CT, PFT, and SARS-CoV-2 immunoglobulin G (IgG). Supplementary Fig. 1 shows the flowchart of study enrollment and recruitment.
Chest CT protocol
All low-dose chest CT scans were conducted by one dedicated CT scanner (Revolution; GE HealthCare, Chicago, IL, USA). CT scan parameters were: kVp, 120; mA, 85; matrix, 512 × 512; scan mode, helical; collimation, 64 × 0.625 mm; slice thickness, 2.5 mm; beam width, 40 mm; focal spot size, small; pitch, 0.984; and rotation time, 0.5 seconds. Coronal and sagittal reconstructions were performed.
Quantitative and qualitative analysis of chest imaging
All chest images were reviewed by two board-certified thoracic radiologists, with 20 and 13 years of experience, respectively, blinded to all clinical and functional data for the evaluation of CT abnormalities, including ground-glass opacity (GGO), parenchymal bands, anterior bronchiectasis, traction bronchiectasis, perilobular opacification, and reticulation, according to previous reports.11,12 Among the participants, 64 of 68 (94.1%) had chest radiographs (CXRs) at admission, which were also evaluated according to the extent of lung involvement: grade 0, no lesion; grade 1, 1–25%; grade 2, 25–50%; grade 3, 50–75%; and grade 4, 75–100%. These 6-month CTs were compared side-by-side with 18-month CTs to evaluate whether there had been improvements or stability.
All CT images were also quantitatively analyzed in terms of the Hounsfield unit (HU) distribution of the pixels, with normalized histograms computed via commercial software (IntelliSpace Portal; Philips, Andover, MA, USA) (Supplementary Fig. 2). The HU of fibrotic lung lesions are typically within −200 and −600 HU, according to a previous study.13 Some lesions were identified outside of this definition if the examining radiologist regarded them as lung sequelae. The lung lesion and whole-lung volumes were measured, and the lesion volume percentage (lesionvolume) was calculated: Volume of Lung Lesion/Whole-Lung Volume × 100.
PFT and SARS-CoV-2 IgG level
PFT and SARS-CoV-2 IgG measurements were performed on patients who had a follow-up outpatient visit at 6 and 18 months after discharge, respectively. Spirometry was performed according to the American Thoracic Society and European Respiratory Society standards.14 This study measured forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and diffusing capacity of the lungs for carbon monoxide (DLCO), and the spirometry results were also expressed as a percentage of the measured to the predicted values. SARS-CoV-2 IgG level was measured using the commercial kit manufactured by Sysmex, which is based on the chemiluminescent enzyme immunoassay method.
Statistical analysis
The Student’s t-test or Mann-Whitney U test was used to analyze continuous variables, and Pearson’s χ2 test with Yates’ continuity correction or the Fisher exact test was used for categorical variables. After univariate analysis, variables were subjected to multivariate analysis by logistic regression. We included the significant variables from a simple logistic regression in the multivariate analysis. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). The diagnostic performance of SARS-CoV-2 IgG level for predicting CT abnormalities was analyzed using receiver operating characteristic (ROC) curves and area under the curve (AUC). The correlations between lesionvolume and IgG level or PFT were analyzed using Spearman’s rank correlation. Lesionvolume and PFT were compared between 6 and 18 months within subjects by paired t-test or Wilcoxon signed-rank test. A P value < 0.05 was considered significant. All statistical analyses were performed with R (version 4.3.0; The R Foundation, Vienna, Austria).
Ethics statement
This prospective cohort study protocol was reviewed and approved by the Institutional Review Board of Korea University Ansan Hospital (approval number: 2020AS0122). Informed consent was submitted by all subjects when they were enrolled. This prospective cohort study was registered at the Clinical Research Information Service (CRIS, http://cris.nih.go.kr), number KCT0008573.
RESULTS
Demographic characteristics
During the study period, 87 COVID-19 patients initially decided to participate in the study, but 68 patients actually underwent follow-up CT, and 68 patients participated in the study. Table 1 shows the demographic and clinical characteristics at 6 months and 18 months after hospitalization for COVID-19. In the 6-month follow-up group, 37 (54.4%) participants had mild COVID-19 disease, 20 (29.4%) had moderate disease, and 10 (14.7%) had severe disease, according to the COVID-19 WHO ordinal scale. Furthermore, 15 (22.1%) underwent invasive ventilation therapy, and 35 (51.5%) had a CXR of at least grade 1. Among the 42 participants who attended 18-month follow-up visits, 20 (47.6%) had mild disease, 16 (38.1%) had moderate disease, and six (14.3%) had severe disease. In addition, 10 patients (23.8%) had invasive ventilation therapy, and 27 (64.3%) had at least a grade 1 CXR. No patient had underlying lung disease, such as asthma, chronic obstructive pulmonary disease, or lung cancer.
Table 1. Demographic and clinical characteristics of the study population at 6 and 18 months after COVID-19 infection.
| Characteristics | Value at 6 mon (n = 68) | Value at 18 mon (n = 42) | |
|---|---|---|---|
| Age, yr | 39 (29–58) | 43 (27–60) | |
| Male sex | 28 (41.2) | 18 (42.9) | |
| BMI, kg/m2 | 23.5 ± 3.4 | 24.4 ± 3.2 | |
| Charlson Comorbidity Index | 0 (0–2) | 0 (0–2) | |
| Comorbidities | |||
| Cerebrovascular accident | 1 (1.4) | 0 (0.0) | |
| Hypertension | 12 (17.6) | 9 (21.4) | |
| Coronary artery disease | 1 (1.4) | 1 (2.4) | |
| Diabetes | 9 (13.2) | 7 (16.7) | |
| Symptoms at admission | |||
| None | 4 (5.9) | 4 (9.5) | |
| Cough | 32 (47.1) | 17 (40.5) | |
| Sputum | 13 (19.1) | 8 (19.0) | |
| Fever/chills | 41 (60.3) | 22 (52.4) | |
| Rhinorrhea | 11 (16.2) | 8 (19.0) | |
| Dyspnea | 7 (10.3) | 5 (11.9) | |
| Diarrhea | 11 (16.2) | 6 (14.3) | |
| Dysosmia | 36 (52.9) | 16 (38.1) | |
| Dysgeusia | 27 (39.7) | 14 (33.3) | |
| Sore throat | 15 (22.1) | 8 (19.0) | |
| CXR grade at admission | |||
| Grade 0 (no lesion) | 29 (42.6) | 15 (35.7) | |
| Grade 1 (0–25%) | 16 (23.5) | 12 (28.6) | |
| Grade 2 (25–50%) | 4 (5.9) | 3 (7.1) | |
| Grade 3 (50–75%) | 3 (4.4) | 2 (4.8) | |
| Grade 4 (75–100%) | 12 (17.6) | 10 (23.8) | |
| Smoking status at admission | |||
| Current smoker | 4 (5.9) | 1 (2.4) | |
| Ex-smoker | 1 (1.4) | 4 (9.5) | |
| Nonsmoker | 22 (32.4) | 19 (45.2) | |
| Ordinal severity scale during admission | |||
| Mild | 37 (54.4) | 20 (47.6) | |
| Moderate | 20 (29.4) | 16 (38.1) | |
| Severe | 10 (14.7) | 6 (14.3) | |
| Highest level of ventilatory support | |||
| Oxygen therapy | 46 (67.6) | 26 (61.9) | |
| Invasive ventilation | 15 (22.1) | 10 (23.8) | |
| No respiratory support | 6 (8.8) | 6 (14.3) | |
| Treatment | |||
| Steroid | 11 (16.2) | 9 (21.4) | |
| Remdesivir | 8 (11.8) | 6 (14.3) | |
| Convalescent therapy | 3 (4.4) | 1 (2.4) | |
| Hydroxychloroquine | 7 (10.3) | 5 (11.9) | |
| Ritonavir/lopinavir | 5 (7.4) | 2 (4.8) | |
| Duration of hospital stay | 31.5 (22.8–39.3) | 34.0 (21.3–38.0) | |
Values are presented as numbers of participants (%), mean ± standard deviation, or median (interquartile range).
COVID-19 = coronavirus disease 2019, BMI = body mass index, CXR = chest radiograph.
CT findings at 6- and 18-month follow-up assessments
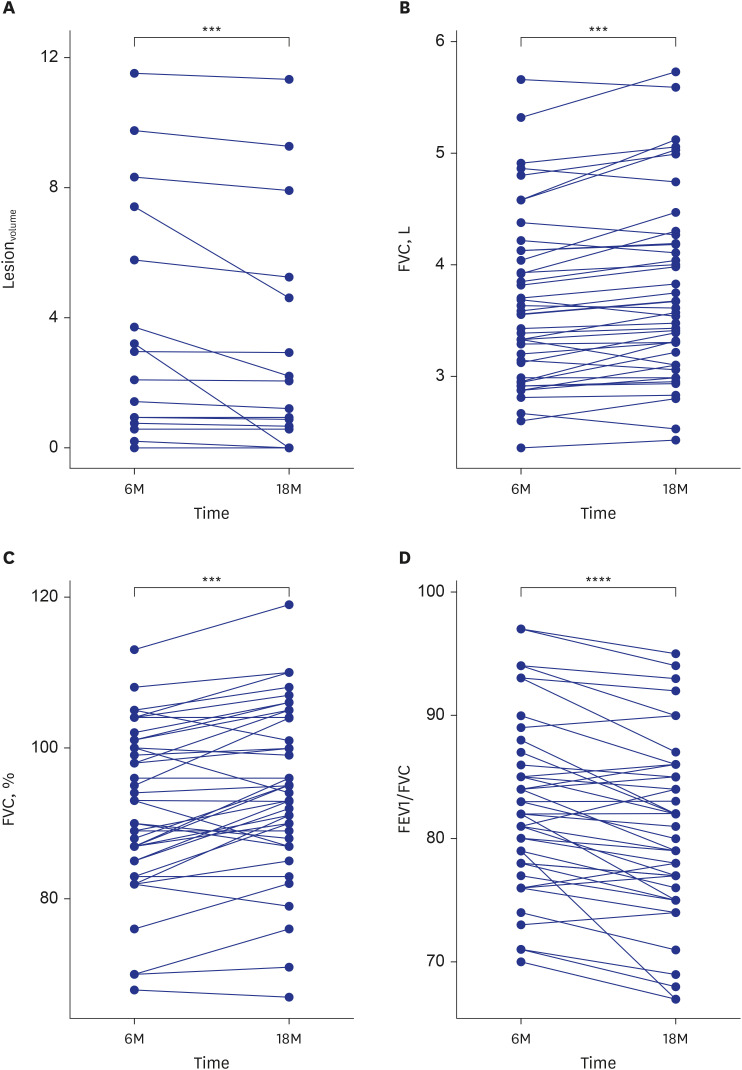
CT findings of the study population are described in Supplementary Table 1. At 6 and 18 months after hospitalization for COVID-19, 22 participants (32.4%) and 13 (31.0%) had persistent CT abnormalities, respectively. Among 35 participants who had at least a grade 1 CXR, 21 (60%) had residual CT abnormalities at 6 months. At 18 months, 20 participants (29.4%) had GGO and parenchymal bands, 18 (26.5%) had anterior bronchiectasis, 14 (20.6%) had traction bronchiectasis, 19 (27.9%) had perilobular opacification, and 19 (27.9%) had reticulation. In addition, 13 (31.0%) had decreased but persistent lung lesions at 18 months, and three (7.1%) showed improvement in all lesions compared with 6 months. The mean lesionvolume on CT was 4.7 ± 6.1% (range, 0.2–23.4%) at 6 months and 3.8 ± 3.6% (range, 0.6–11.3%) at 18 months, representing a significant decrease between the two time points (P < 0.001; Fig. 1A).
Fig. 1. Graphs comparing changes between 6 months (6M) and 18 months (18M), with specific changes in (A) lesionvolume, (B) FVC (L), (C) FVC (%), and (D) FEV1/FVC.
lesionvolume = lesion volume percentage, FVC = forced vital capacity, FEV1 = forced expiratory volume in 1 second.
Comparisons were performed with the paired t-test and Wilcoxon signed rank test.
***P < 0.001; ****P < 0.0001.
PFT and SARS-CoV-2 IgG levels at 6- and 18-month follow-up assessments
PFTs of study participants are shown in Supplementary Table 1. Sequential PFTs at 6 and 18 months showed significant improvement in FVC (3.56 L [90.0%] vs. 3.59 L [94.5%], P < 0.001) and FEV1/FVC (83.0 vs. 80.5), but no significant changes in FEV1 or DLCO (2.95 L vs. 2.89 L; 83.0 vs. 83.0; all P > 0.05) (Fig. 1B-D).
The median SARS-CoV-2 IgG levels at 6- and 18-month follow-up were 10.1 U/mL (interquartile range [IQR], 4.6–28.8 U/mL) and 2.3 U/mL (IQR, 1.0–12.1 U/mL), respectively—a statistically significant decrease (P < 0.001) (Supplementary Table 2).
Participants with vs. without residual CT abnormalities at 6- and 18-month follow-up
Tables 2 and 3 compare the demographic and clinical characteristics, PFTs, and SARS-CoV-2 IgG levels between participants with and without persistent CT abnormalities (CT-positive and CT-negative, respectively) at 6- and 18-month follow-up. Participants who were CT-positive at both the 6- and 18-month follow-up were significantly older than CT-negative participants (59 vs. 31 years and 62 vs. 32 years, both P < 0.001). Hypertension (50% vs. 2.2% and 53.8% vs. 6.9%) and diabetes (40.9% vs. 0% and 46.2% vs. 3.7%) were significantly more common in CT-positive patients than in CT-negative patients at both the 6- and 18-month follow-up (all P < 0.01). The mean Charlson Comorbidity Index (CCI) was significantly higher in CT-positive participants than in CT-negative participants at both the 6- and 18-month follow-up (3 vs. 0 and 3 vs. 0, P < 0.001).
Table 2. Characteristics of participants with and without abnormal CT findings at 6 months.
| Characteristics | CT-positive group (n = 22) | CT-negative group (n = 46) | P value | |
|---|---|---|---|---|
| Age, yr | 59 (55–66) | 31 (26–39) | < 0.001 | |
| Male sex | 12 (54.5) | 16 (34.8) | 0.199 | |
| BMI, kg/m2 | 24.6 ± 3.3 | 23.0 ± 3.3 | 0.066 | |
| Charlson Comorbidity Index | 3 (1–3.75) | 0 (0–0) | < 0.001 | |
| Comorbidities | ||||
| Cerebrovascular accident | 0 (0.0) | 1 (2.2) | > 0.999 | |
| Hypertension | 11 (50.0) | 1 (2.2) | < 0.001 | |
| Coronary artery disease | 1 (2.2) | 0 (0.0) | 0.324 | |
| Diabetes | 9 (40.9) | 0 (0.0) | < 0.001 | |
| Symptoms at admission | ||||
| None | 1 (4.5) | 3 (6.5) | > 0.999 | |
| Cough | 9 (40.9) | 23 (50.0) | 0.658 | |
| Sputum | 6 (27.3) | 7 (15.2) | 0.324 | |
| Fever/chills | 14 (63.6) | 27 (58.7) | 0.901 | |
| Rhinorrhea | 0 (0.0) | 11 (23.9) | 0.012 | |
| Dyspnea | 6 (27.3) | 1 (2.2) | 0.004 | |
| Diarrhea | 0 (0.0) | 11 (23.9) | 0.012 | |
| Dysosmia | 3 (13.6) | 33 (71.7) | < 0.001 | |
| Dysgeusia | 3 (13.6) | 24 (52.2) | 0.006 | |
| Sore throat | 1 (4.5) | 14 (30.4) | 0.026 | |
| CXR grade at admission | < 0.001 | |||
| Grade 0 (no lesion) | 0 (0.0) | 29 (67.4) | ||
| Grade 1 (0–25%) | 4 (19.0) | 12 (27.9) | ||
| Grade 2 (25–50%) | 2 (9.5) | 2 (4.7) | ||
| Grade 3 (50–75%) | 3 (14.3) | 0 (0.0) | ||
| Grade 4 (75–100%) | 12 (57.1) | 0 (0.0) | ||
| Pulmonary function test | ||||
| FEV1, L | 2.8 ± 0.7 | 3.0 (2.6–3.5) | 0.013 | |
| FEV1, % | 93.1 ± 8.7 | 94.0 (89–100) | 0.301 | |
| FVC, L | 3.5 ± 0.9 | 3.6 (3.1–4.2) | 0.079 | |
| FVC, % | 89.8 ± 11.8 | 93.2 ± 8.8 | 0.194 | |
| FEV1/FVC | 80.2 ± 5.3 | 84.3 ± 7.2 | 0.024 | |
| DLCO | 86.4 ± 12.9 | 82.9 ± 10.4 | 0.246 | |
| Ordinal severity scale during admission | < 0.001 | |||
| Mild | 0 (0.0) | 37 (82.2) | ||
| Moderate | 12 (54.5) | 8 (17.8) | ||
| Severe | 10 (45.5) | 0 (0.0) | ||
| Highest level of ventilatory support | < 0.001 | |||
| No respiratory support | 0 (0.0) | 6 (13.3) | ||
| Oxygen therapy | 7 (31.8) | 39 (86.7) | ||
| Invasive ventilation | 15 (68.2) | 0 (0.0) | ||
| Residual symptoms at 6 mon | ||||
| None | 10 (45.5) | 26 (56.5) | 0.551 | |
| Cough | 2 (9.1) | 1 (2.2) | 0.243 | |
| Sputum | 2 (9.1) | 1 (2.2) | 0.243 | |
| Chest pain/palpitation | 1 (4.5) | 6 (13.0) | 0.414 | |
| Dyspnea | 2 (9.1) | 2 (4.3) | 0.590 | |
| Dysosmia | 1 (4.5) | 5 (10.9) | 0.656 | |
| Dysgeusia | 1 (4.5) | 2 (4.3) | > 0.999 | |
| Insomnia | 0 (0.0) | 2 (4.3) | > 0.999 | |
| Hair loss | 2 (9.1) | 2 (4.3) | 0.590 | |
| Memory loss/poor concentration | 1 (4.5) | 5 (10.9) | 0.656 | |
| Depression | 1 (4.5) | 0 (0.0) | 0.324 | |
| Headache | 1 (4.5) | 4 (8.7) | > 0.999 | |
| Mild abdominal pain | 1 (4.5) | 1 (2.2) | 0.546 | |
| Fatigue | 4 (18.2) | 1 (2.2) | 0.035 | |
| Myalgia | 0 (0.0) | 1 (2.2) | > 0.999 | |
| Sore throat | 1 (4.5) | 1 (2.2) | 0.546 | |
| Duration of hospital stay | 33.5 (25.3–40) | 27.0 (19.3–37) | 0.084 | |
| Smoking status at admission | > 0.999 | |||
| Current smoker | 0 (0.0) | 1 (2.2) | ||
| Ex-smoker | 1 (4.5) | 3 (6.5) | ||
| Nonsmoker | 18 (81.8) | 28 (60.9) | ||
| Antibody at 6 mon | ||||
| SARS-CoV-2 IgG, U/mL | 25.2 (13.7–43.3) | 7 (3.0–13.4) | < 0.001 | |
Values are presented as numbers of participants (%), mean ± standard deviation, or median (interquartile range).
CT = chest tomography, BMI = body mass index, CXR = chest radiograph, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity of the lungs for carbon monoxide, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, IgG = immunoglobulin G.
Table 3. Characteristics of participants with and without abnormal CT findings at 18 months.
| Characteristics | CT-positive group (n = 13) | CT-negative group (n = 29) | P value | |
|---|---|---|---|---|
| Age, yr | 62.0 (58.0–71.0) | 32.0 (25.0–45.0) | < 0.001 | |
| Male sex | 9 (69.2) | 9 (31.0) | 0.048 | |
| BMI, kg/m2 | 25.0 ± 2.8 | 24.2 ± 3.4 | 0.318 | |
| Charlson Comorbidity Index | 3 (2–4) | 0 (0–1) | < 0.001 | |
| Comorbidities | ||||
| Hypertension | 7 (53.8) | 2 (6.9) | 0.002 | |
| Coronary artery disease | 1 (7.7) | 0 (0.0) | 0.310 | |
| Diabetes | 6 (46.2) | 1 (3.7) | 0.001 | |
| Symptoms at admission | ||||
| Cough | 6 (46.2) | 11 (37.9) | 0.871 | |
| Sputum | 4 (30.8) | 4 (13.8) | 0.226 | |
| Fever/chills | 9 (69.2) | 13 (44.8) | 0.143 | |
| Rhinorrhea | 0 (0.0) | 8 (27.6) | 0.043 | |
| Dyspnea | 3 (23.1) | 2 (6.9) | 0.162 | |
| Diarrhea | 0 (0.0) | 6 (20.7) | 0.153 | |
| Dysosmia | 1 (7.7) | 15 (51.7) | 0.007 | |
| Dysgeusia | 1 (7.7) | 13 (44.8) | 0.032 | |
| CXR grade at admission | < 0.001 | |||
| Grade 0 (no lesion) | 0 (0.0) | 15 (51.7) | ||
| Grade 1 (0–25%) | 0 (0.0) | 12 (41.4) | ||
| Grade 2 (25–50%) | 1 (7.7) | 2 (6.9) | ||
| Grade 3 (50–75%) | 2 (15.4) | 0 (0.0) | ||
| Grade 4 (75–100%) | 10 (76.9) | 0 (0.0) | ||
| Pulmonary function test | ||||
| FEV1, L | 2.8 ± 0.5 | 3.1 ± 0.6 | 0.107 | |
| FEV1, % | 96.4 ± 14.1 | 94.2 ± 9.7 | 0.562 | |
| FVC, L | 3.5 ± 0.6 | 3.7 ± 0.8 | 0.256 | |
| FVC, % | 85.9 ± 11.6 | 94.3 ± 9.3 | 0.016 | |
| FEV1/FVC | 80.6 ± 5.5 | 83.7 ± 7.3 | 0.188 | |
| DLCO | 87.5 ± 15.1 | 83.7 ± 10.9 | 0.360 | |
| Ordinal severity scale during admission | < 0.001 | |||
| Mild | 0 (0.0) | 20 (69.0) | ||
| Moderate | 7 (53.8) | 9 (31.0) | ||
| Severe | 6 (46.2) | 0 (0.0) | ||
| Highest level of ventilatory support | < 0.001 | |||
| No respiratory support | 0 (0.0) | 6 (20.7) | ||
| Oxygen therapy | 3 (23.1) | 23 (79.3) | ||
| Invasive ventilation | 10 (76.9) | 0 (0.0) | ||
| Residual symptoms at 18 mon | ||||
| None | 9 (69.2) | 21 (77.8) | 0.700 | |
| Dyspnea | 1 (7.7) | 1 (3.7) | > 0.999 | |
| Chest pain | 1 (7.7) | 3 (11.1) | > 0.999 | |
| Headache | 0 (0.0) | 1 (3.7) | > 0.999 | |
| Dysosmia | 0 (0.0) | 1 (3.7) | > 0.999 | |
| Duration of hospital stay | 33.0 ± 12.8 | 28.2 ± 12.6 | 0.267 | |
| Smoking status at admission | ||||
| Current smoker | 0 (0.0) | 1 (3.4) | ||
| Ex-smoker | 1 (8.3) | 3 (10.3) | ||
| Nonsmoker | 11 (91.7) | 25 (86.2) | ||
| Antibody at 6 mon | ||||
| SARS-CoV-2 IgG, U/mL | 40.3 (17.3–60.6) | 7.1 (4.1–18) | < 0.001 | |
| Antibody at 18 mon | ||||
| SARS-CoV-2 IgG, U/mL | 25.1 (8–59.2) | 1.4 (0.7–2.4) | < 0.001 | |
Values are presented as numbers of participants (%), mean ± standard deviation, or median (interquartile range).
CT = chest tomography, BMI = body mass index, CXR = chest radiograph, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, DLCO = diffusing capacity of the lungs for carbon monoxide, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, IgG = immunoglobulin G.
Dyspnea at admission was significantly more common in CT-positive participants than in CT-negative participants at the 6-month follow-up assessments (27.3% vs. 2.2%, P = 0.004). Among residual symptoms at 6 months, fatigue was significantly more common in CT-positive participants than in CT-negative participants (18.2% vs. 2.2%, P = 0.035). Dyspnea as a residual symptom at the 6- and 18-month follow-up assessments was found in 4 (5.9%) and 2 (4.8%) participants, respectively.
Regarding disease severity at admission, significantly more CT-positive participants than CT-negative participants were in the severe (45.5% vs. 0% and 46.2% vs. 0%) and moderate (54.5% vs. 17.8% and 53.8% vs. 31.0%) groups at the 6- and 18-month follow-up assessments. All patients in the CT-positive group at 6 and 18 months needed oxygen therapy during admission and underwent invasive ventilation at that time.
FEV1 and FEV1/FVC were significantly lower in CT-positive participants than in CT-negative participants at 6 months (2.8 L vs. 3.3 L and 80.2 vs. 84.3, all P < 0.05). At 18 months, FVC was significantly lower in CT-positive participants than in CT-negative participants (85.9% vs. 94.3%, P = 0.016).
The median SARS-CoV-2 IgG levels at both 6 and 18 months were significantly higher in CT-positive participants than in CT-negative participants (25.2 vs. 7.0 U/mL and 25.1 vs. 1.4 U/mL, all P < 0.001). At 18 months, the median SARS-CoV-2 IgG level at 6 months was also significantly higher in CT-positive participants than in CT-negative participants (40.3 vs. 7.1 U/mL, P < 0.001).
Factors associated with persistent CT abnormalities
In the univariate analysis, age, CCI, FEV1, and FEV1/FVC were associated with a greater likelihood of CT abnormalities at 6 months (Supplementary Table 2). At 18 months, age, CCI, FVC, and SARS-CoV-2 IgG level were associated with a greater likelihood of CT abnormalities in the univariate analysis. In the multivariable analysis, age was associated with persistent CT abnormalities at 6- and 18-month follow-up assessments (OR, 1.14; 95% CI, 1.04–1.22; P < 0.001 and OR, 1.17; 95% CI, 1.03–1.32; P = 0.014, respectively).
SARS-CoV-2 IgG levels for predicting residual CT abnormalities
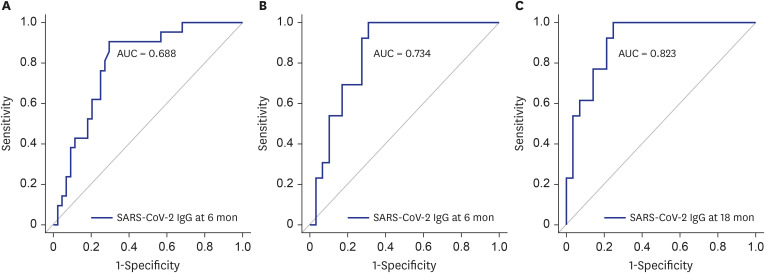
The ROC curves of SARS-CoV-2 IgG levels for predicting CT abnormalities at 6 or 18 months are shown in Fig. 2. The AUC of the ROC curve of SARS-CoV-2 IgG levels for predicting CT abnormalities at 6 months was 0.688 (95% CI, 0.798–0.908; P < 0.001). The cut-off IgG value for predicting CT abnormalities at 6 months was 10.25 (sensitivity, 70.5%; specificity, 90.5%). The AUC of the ROC curve of SARS-CoV-2 IgG levels at 6 and 18 months for predicting CT abnormalities at 18 months was 0.734 (95% CI, 0.849–0.964; P < 0.001) and 0.823 (95% CI, 0.823–0.909; P < 0.001). The cut-off SARS-CoV-2 IgG values at 6 and 18 months for predicting CT abnormalities at 18 months were 10.1 (sensitivity, 69.0%; specificity, 100%) and 2.35 (sensitivity, 75.0%; specificity, 100%).
Fig. 2. The ROC curves of SARS-CoV-2 IgG levels for predicting CT abnormalities at 6 or 18 months. (A) ROC curves were used to test the performance of SARS-CoV-2 IgG level at 6 months for predicting residual CT abnormalities at 6 months, showing an AUC of 0.688 (95% CI, 0.798–0.908; P < 0.001). The cut-off IgG level at 6 months for predicting CT abnormalities at 6 months was 10.25 (sensitivity, 70.5%; specificity, 90.5%). (B) The ROC curve of IgG level at 6 months for predicting residual CT abnormalities at 18 months showed an AUC of 0.734 (95% CI, 0.849–0.964; P < 0.001). The cut-off value was 10.1 (sensitivity, 69.0%; specificity, 100%). (C) The ROC curve of IgG level at 18 months for predicting residual CT abnormalities at 18 months showed an AUC of 0.823 (95% CI, 0.823–0.909; P < 0.001). The cut-off value was 2.35 (sensitivity, 75.0%; specificity, 100%).
ROC = receiver operating characteristic, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, IgG = immunoglobulin G, CT = computed tomography, AUC = area under the curve, CI = confidence interval.
The correlations between lesionvolume and PFT/SARS-CoV-2 IgG levels
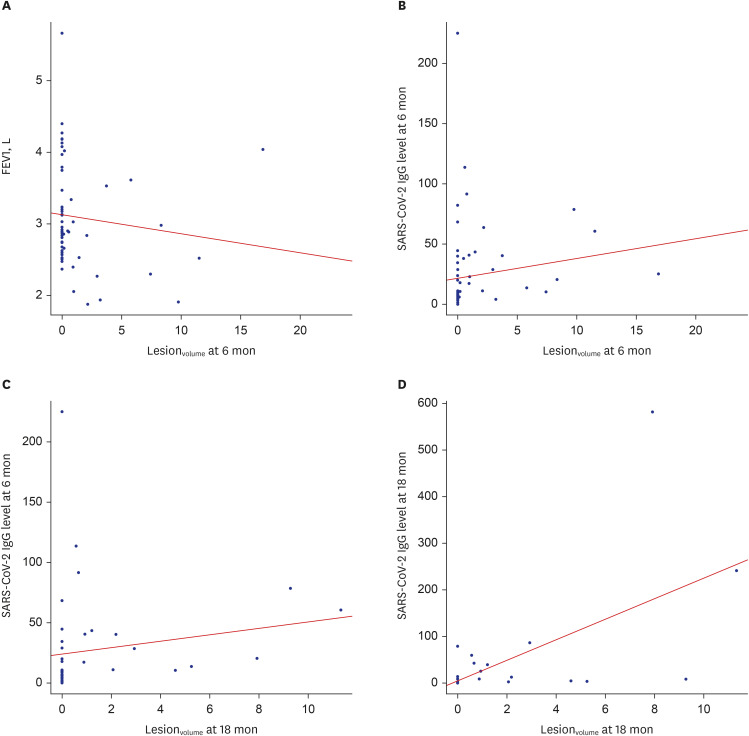
At 6-month follow-up assessments, lesionvolume showed a negative correlation with FEV1 (r = −0.31, P = 0.011) and a positive correlation with SARS-CoV-2 IgG level (r = 0.482, P < 0.001). At 18-month follow-up assessments, lesionvolume showed a positive correlation with SARS-CoV-2 IgG level at 6 months (r = 0.534, P < 0.001) and at 18 months (r = 0.643, P < 0.001; Fig. 3).
Fig. 3. The correlations between lesionvolume and PFT, as well as between lesionvolume and SARS-CoV-2 IgG levels.
(A) lesionvolume at 6 months and FEV1 (L) at 6 months, (B) lesionvolume at 6 months and SARS-CoV-2 IgG level at 6 months, (C) lesionvolume at 18 months and SARS-CoV-2 IgG level at 6 months, and (D) lesionvolume at 18 months and SARS-CoV-2 IgG level at 18 months.
lesionvolume = lesion volume percentage, PFT = pulmonary function testing, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, IgG = immunoglobulin G, FEV1 = forced expiratory volume in 1 second.
DISCUSSION
In our study, 32.4% of participants at 6 months and 31.0% of participants at 18 months had persistent CT abnormalities. The mean lesionvolume was significantly decreased, and FVC significantly improved between the 6- and 18-month follow-up assessments. Dyspnea was found in four participants at 6 months and two at 18 months. Eighteen-month follow-up data are scarce, but our findings are in line with those few studies. Guo et al.15 reported that 35.8% of participants displayed a reticular pattern, and 29.5% showed GGO at the 18.5-month follow-up CT. PFT parameters slightly improved during follow-up, and 4.7% of COVID-19 convalescents experienced dyspnea at 18.5 months. Barini et al.16 also reported that radiological findings did not improve at 18 months in 16.5% of participants. Although these studies did not quantitatively analyze the lesionvolume of CT abnormalities and involved fewer patients who received ventilator care than our study, residual CT abnormalities 18 months after COVID-19 infection were seen in less than 40% of participants, and dyspnea in less than 6%. PFT results at 18 months could be improved compared with the 6-month results.
In our results, statistically significant variables for patients with residual CT-positive findings at 6 and 18 months were consecutively similar, which could explain the clinical features of COVID-19 pneumonia. Among those with residual CT abnormalities, no participants had mild disease combined with CXR grades of 3 or 4 at admission. Additionally, none of the participants without residual CT abnormalities at both the 6- and 18-month follow-up assessments required ventilatory support. This indicates that residual CT abnormalities were significantly more common in patients who were severely ill at admission. However, multivariate analysis could not prove the effect of clinical severity on residual CT findings because the variables in this study were somewhat dichotomous, and the sample size was insufficient to discriminate between the two groups. In this study, residual CT abnormalities at the 6- and 18-month follow-up assessments may be related to the severity of COVID-19.
Residual CT abnormalities may also be caused by barotrauma from invasive ventilation, lung injury, acute respiratory distress syndrome (ARDS), or direct fibrotic stimuli from viruses, but the pathophysiology is unclear.17 Previous reports demonstrated that invasive ventilation may be a risk factor for residual lung abnormalities at 6 weeks to 12 months of follow-up after COVID-19 diagnosis.5,18 As lung fibrosis is known to complicate diffuse alveolar injury or ARDS, and fibrosis in ARDS might be caused by barotrauma,19 some features seen on CT after COVID-19 might have resulted from mechanical ventilation or associated lung injury rather than COVID-19 itself.18 In this study, all patients who underwent invasive ventilation at the time of COVID-19 pneumonia had residual CT abnormalities until the 18-month follow-up point.
It is well-known that severe or critical disease leads to greater residual CT and PFT abnormalities compared with mild or moderate infection, although most previous studies followed patients for less than 7 months.20 Our study showed that disease severity is still an important factor for residual CT abnormalities, even at 18 months of follow-up. Age was also a risk factor for lung sequelae at both 6 and 18 months in our study, which is in line with earlier studies that had shorter than 12 months of follow-up.6,21 As a previous serial CT follow-up study in patients with severe acute respiratory syndrome (SARS) reported, lung sequelae could be stable for the following 15 years after lesion absorption, with recovery occurring within the first 12 months.22 Follow-up, especially for severe or critical infection, ventilatory support, and older age, should extend for longer than 18 months to plan long-term management.
FEV1 and FEV1/FVC at 6 months, as well as FVC at 18 months after hospitalization, were significantly lower in participants with residual CT abnormalities, which differed from previous studies. It has been suggested that restrictive pulmonary alteration and decreased DLCO, which is the most sensitive factor reflecting changes in pulmonary function, are consequences of SARS-CoV infection.23,24 Previous studies also reported that DLCO was reduced after COVID-19 infection and improved over time.25,26 The cause behind decreased FEV1 in COVID-19 is unclear. However, considering the previous report showing a relationship between the modified Medical Research Council dyspnea scale and improved FEV1 through rehabilitation treatment, we can infer that FEV1 is related to functional lung capacity.27,28 In our study, there was no change in DLCO between 6 and 18 months. It is possible that our study population had already recovered their DLCO, which was damaged during COVID-19 infection.
The median SARS-CoV-2 IgG levels at both 6 and 18 months were significantly higher in participants with CT abnormalities than in those without CT abnormalities, and lesionvolume showed a positive correlation with SARS-CoV-2 IgG level at both 6 and 18 months in our study. The IgG cut-off values for predicting CT abnormalities were also shown. In previous studies, the proportion of IgG-negative patients was higher among non-severe cases than in severe cases, and there was a correlation between IgG conversion and disease severity at 12 months.1 Patients with severe infection had higher SARS-CoV-2 IgG levels than those with mild cases.29 These results suggest that disease severity is associated with immune responses. Additionally, IgG was detected in survivors at 18 months.30 Our results also demonstrated the correlation between SARS-CoV-2 IgG levels and CT abnormalities 18 months after hospitalization for COVID-19, with IgG detectable in COVID-19 survivors at that time.
This study had some limitations. First, we only had 17 CT scans at admission, and 94.1% of participants had initial CXRs. We analyzed CXR grade according to lesion extent. Second, due to a wide 95% CI range in univariate analysis, some factors could not be included in the multivariate analysis. This wide range might be due to the relatively small sample size or potential selection bias from patients’ voluntary participation or refusal to undertake follow-up CT. Lastly, SARS-CoV-2 antibody titers may be affected by COVID-19 vaccination, but the data regarding vaccination history were limited in this study.
In conclusion, 32.4% of participants at 6 months and 31.0% of participants at 18 months had persistent CT abnormalities. FEV1 and FEV1/FVC at 6 months, as well as FVC at 18 months, were significantly lower. SARS-CoV-2 IgG levels at both 6 and 18 months were significantly higher in participants with CT abnormalities than in those without CT abnormalities. The lesion volume in CT and FVC were significantly better at 18 months than at 6 months. There were correlations between PFT, IgG level, and residual CT abnormalities. Age was a significant risk factor for CT abnormalities at 18 months, and disease severity, initial CXR grade, and ventilatory support were also important factors.
Footnotes
Funding: This study was supported by a research program funded by the Korea Disease Control and Prevention Agency, Korea University Ansan Hospital (O2000831), and Sysmex Korea Company (I2005491).
Disclosure: The authors have no potential conflicts of interest to disclose.
Data Sharing Statement: Data sharing statement is provided in Supplementary Data 1.
- Conceptualization: Kim C, Seok H, Choi WS.
- Investigation: Kim C, Seok H, Kim J, Choi H, Kim C.
- Supervision: Choi WS.
- Validation: Park DW, van Assen M, De Cecco CN, Hwang SH, Yong HS, Oh YW.
- Writing - original draft: Kim C, Seok H.
- Writing - review & editing: Kim C, Seok H, Choi WS.
SUPPLEMENTARY MATERIALS
Data sharing statement
Chest CT, pulmonary function test, and SARS-CoV-2 IgG findings at 6-month and 18-month after COVID-19 infection
Admission factors associated with abnormality at 6 and 18 months
Study flowchart shows enrollment and recruitment.
CT quantitative analysis. CT abnormalities are shown in green, and normal lung parenchyma in pink. In this case, whole-lung volume was 4,813.6 mL, and lesion volume was 27.8 mL; therefore, lesionvolume was 0.58%.
References
- 1.Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin Y, et al. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front Med (Lausanne) 2021;8:717194. doi: 10.3389/fmed.2021.717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, Yang C, An X, Xiong Y, Shang Y, He J, et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis. 2021;112:173–182. doi: 10.1016/j.ijid.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11(1):22666. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe A, So M, Iwagami M, Fukunaga K, Takagi H, Kabata H, et al. One-year follow-up CT findings in COVID-19 patients: a systematic review and meta-analysis. Respirology. 2022;27(8):605–616. doi: 10.1111/resp.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faverio P, Luppi F, Rebora P, D’Andrea G, Stainer A, Busnelli S, et al. One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study. Respir Res. 2022;23(1):65. doi: 10.1186/s12931-022-01994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso D, Guido G, Zerunian M, Polidori T, Lucertini E, Pucciarelli F, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology. 2021;301(2):E396–E405. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luger AK, Sonnweber T, Gruber L, Schwabl C, Cima K, Tymoszuk P, et al. Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology. 2022;304(2):462–470. doi: 10.1148/radiol.211670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bongiovanni M, Barilaro G, Bini F. Twelve-month clinical, functional, and radiological outcomes in patients hospitalized for SARS-CoV-2 pneumonia. J Med Virol. 2023;95(2):e28524. doi: 10.1002/jmv.28524. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Yim JJ, Park J. Pulmonary function and chest computed tomography abnormalities 6-12 months after recovery from COVID-19: a systematic review and meta-analysis. Respir Res. 2022;23(1):233. doi: 10.1186/s12931-022-02163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MC, Little BP. Chronic pulmonary manifestations of COVID-19 infection: imaging evaluation. Radiology. 2023;307(2):e222379. doi: 10.1148/radiol.222379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanne JP, Little BP, Schulte JJ, Haramati A, Haramati LB. Long-term lung abnormalities associated with COVID-19 pneumonia. Radiology. 2023;306(2):e221806. doi: 10.1148/radiol.221806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sul B, Flors L, Cassani J, Morris MJ, Reifman J, Altes T, et al. Volumetric characteristics of idiopathic pulmonary fibrosis lungs: computational analyses of high-resolution computed tomography images of lung lobes. Respir Res. 2019;20(1):216. doi: 10.1186/s12931-019-1189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Wang H, Xiao M, Guan X, Lei Y, Diao T, et al. Long-term outcomes of COVID-19 convalescents: an 18.5-month longitudinal study in Wuhan. Int J Infect Dis. 2023;127:85–92. doi: 10.1016/j.ijid.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barini M, Percivale I, Danna P, Longo V, Costantini P, Paladini A, et al. 18 Months computed tomography follow-up after Covid-19 interstitial pneumonia. J Public Health Res. 2022;11(2):2782. doi: 10.4081/jphr.2022.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA. CT of post-acute lung complications of COVID-19. Radiology. 2021;301(2):E383–E395. doi: 10.1148/radiol.2021211396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijayakumar B, Tonkin J, Devaraj A, Philip KE, Orton CM, Desai SR, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2022;303(2):444–454. doi: 10.1148/radiol.2021211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210(1):29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 20.Huntley CC, Patel K, Bil Bushra SE, Mobeen F, Armitage MN, Pye A, et al. Pulmonary function test and computed tomography features during follow-up after SARS, MERS and COVID-19: a systematic review and meta-analysis. ERJ Open Res. 2022;8(2):00056-2022. doi: 10.1183/23120541.00056-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Ding C, Yu L, Guo W, Feng X, Yu L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021;19(1):191. doi: 10.1186/s12916-021-02056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8(1):8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnham EL, Hyzy RC, Paine R, 3rd, Coley C, 2nd, Kelly AM, Quint LE, et al. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med. 2013;41(2):445–456. doi: 10.1097/CCM.0b013e31826a5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie L, Liu Y, Xiao Y, Tian Q, Fan B, Zhao H, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí-Moix A, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-Month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101(6):593–601. doi: 10.1159/000522118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11:610300. doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Fontanet A, Zhang PH, Zhan L, Xin ZT, Baril L, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data sharing statement
Chest CT, pulmonary function test, and SARS-CoV-2 IgG findings at 6-month and 18-month after COVID-19 infection
Admission factors associated with abnormality at 6 and 18 months
Study flowchart shows enrollment and recruitment.
CT quantitative analysis. CT abnormalities are shown in green, and normal lung parenchyma in pink. In this case, whole-lung volume was 4,813.6 mL, and lesion volume was 27.8 mL; therefore, lesionvolume was 0.58%.