Abstract
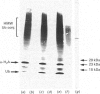
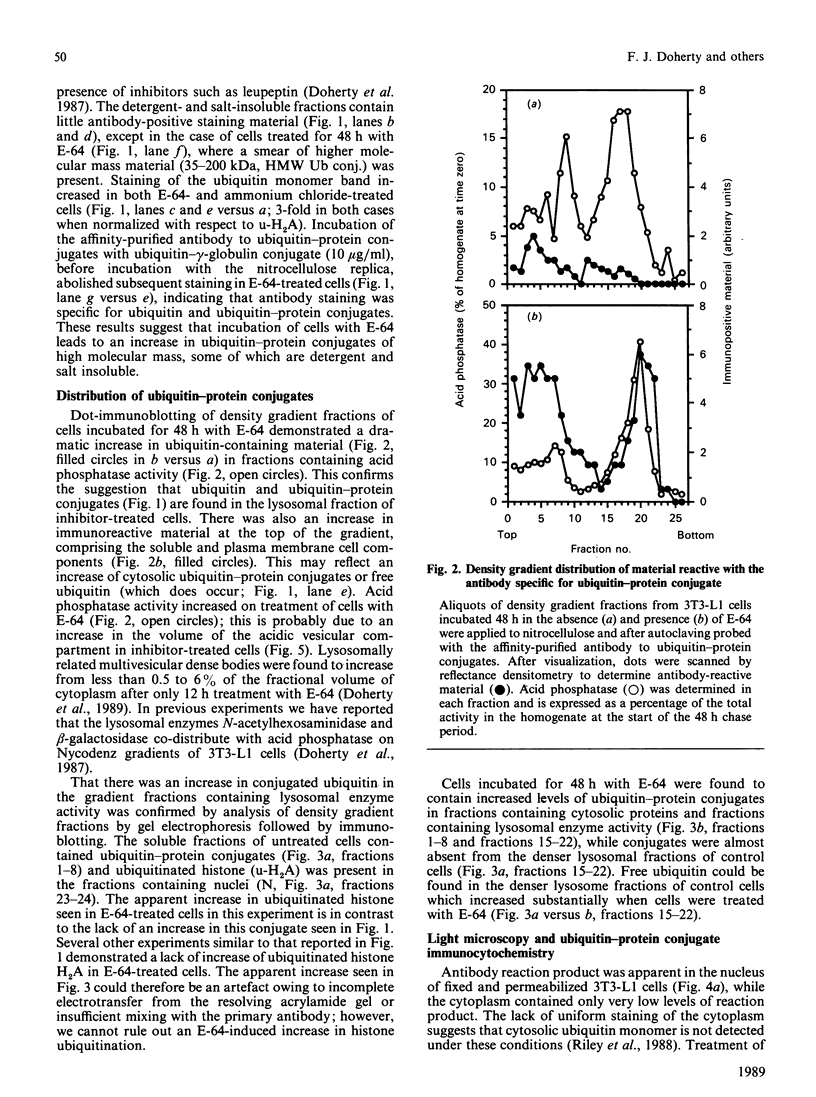
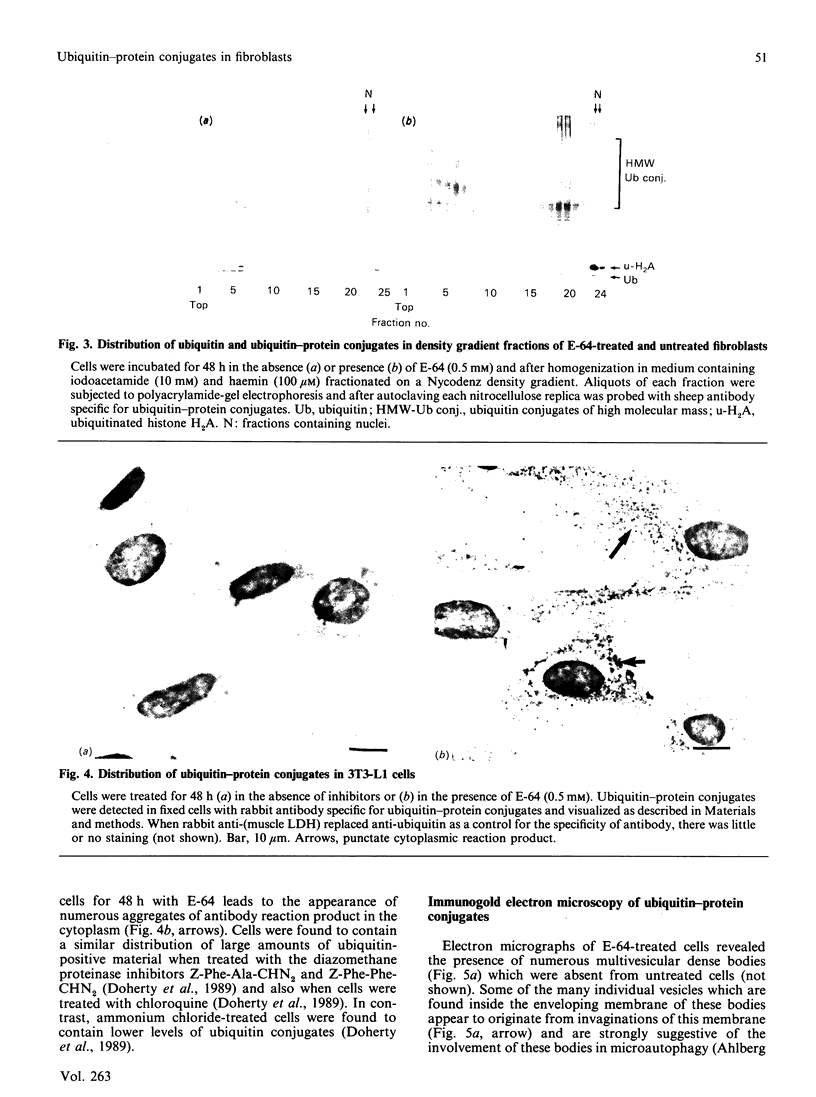
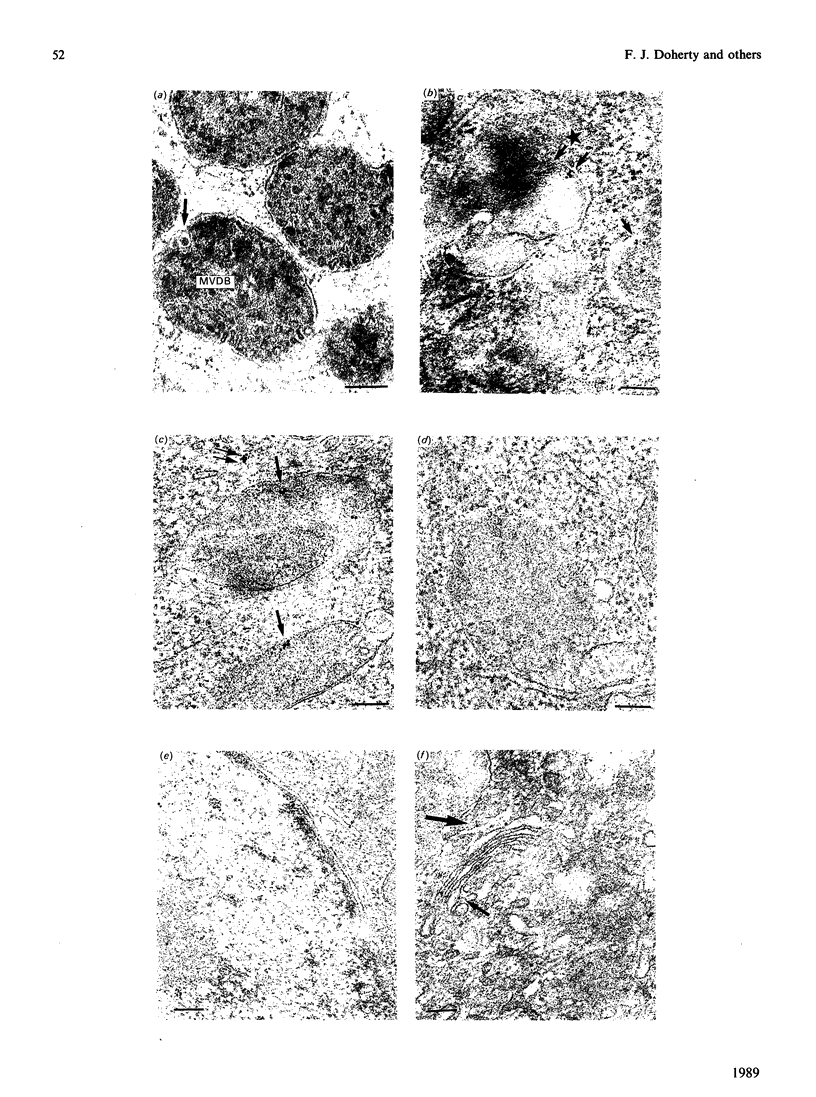
Mouse fibroblasts (3T3-L1 cells) accumulate detergent- and salt-insoluble aggregates of proteins conjugated to ubiquitin when incubated in the presence of inhibitors of lysosomal cysteine cathepsins, including E-64. These ubiquitin-protein conjugates co-fractionate with lysosomes on density gradients and are found in multivesicular dense bodies which by electron microscopy appear to be engaged in microautophagy. Both E-64 and ammonium chloride increase the intracellular concentration of free ubiquitin, but only E-64 leads to the formation of insoluble lysosomal ubiquitin-protein conjugates. The results are discussed in relation to the possible intracellular roles of ubiquitin conjugation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlberg J., Glaumann H. Uptake--microautophagy--and degradation of exogenous proteins by isolated rat liver lysosomes. Effects of pH, ATP, and inhibitors of proteolysis. Exp Mol Pathol. 1985 Feb;42(1):78–88. doi: 10.1016/0014-4800(85)90020-6. [DOI] [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson N., Rechsteiner M. Microinjection of ubiquitin: intracellular distribution and metabolism in HeLa cells maintained under normal physiological conditions. J Cell Biol. 1987 Mar;104(3):537–546. doi: 10.1083/jcb.104.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson N., Rogers S., Rechsteiner M. Microinjection of ubiquitin: changes in protein degradation in HeLa cells subjected to heat-shock. J Cell Biol. 1987 Mar;104(3):547–555. doi: 10.1083/jcb.104.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Molecular determinants of protein half-lives in eukaryotic cells. FASEB J. 1987 Nov;1(5):349–357. doi: 10.1096/fasebj.1.5.2824267. [DOI] [PubMed] [Google Scholar]
- Doherty F. J., Wassell J. A., Mayer R. J. A putative protein-sequestration site involving intermediate filaments for protein degradation by autophagy. Studies with microinjected purified glycolytic enzymes in 3T3-L1 cells. Biochem J. 1987 Feb 1;241(3):793–800. doi: 10.1042/bj2410793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Fernig D. G., Mayer R. J. Degradation of nuclear proteins: studies on transplanted B82 cell karyoplast proteins. FEBS Lett. 1987 Jan 5;210(2):165–168. doi: 10.1016/0014-5793(87)81329-7. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Grinde B. The thiol proteinase inhibitors, Z-Phe-PheCHN2 and Z-Phe-AlaCHN2, inhibit lysosomal protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1983 May 4;757(1):15–20. doi: 10.1016/0304-4165(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The dynamics of ubiquitin pools within cultured human lung fibroblasts. J Biol Chem. 1987 Jan 5;262(1):345–351. [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Kanda F., Sykes D. E., Yasuda H., Sandberg A. A., Matsui S. Substrate recognition of isopeptidase: specific cleavage of the epsilon-(alpha-glycyl)lysine linkage in ubiquitin-protein conjugates. Biochim Biophys Acta. 1986 Mar 7;870(1):64–75. doi: 10.1016/0167-4838(86)90009-9. [DOI] [PubMed] [Google Scholar]
- Lenkinski R. E., Chen D. M., Glickson J. D., Goldstein G. Nuclear magnetic resonance studies of the denaturation of ubiquitin. Biochim Biophys Acta. 1977 Sep 27;494(1):126–130. doi: 10.1016/0005-2795(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Lennox G., Lowe J., Morrell K., Landon M., Mayer R. J. Anti-ubiquitin immunocytochemistry is more sensitive than conventional techniques in the detection of diffuse Lewy body disease. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):67–71. doi: 10.1136/jnnp.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988 May;155(1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Lowe J., Lennox G., Jefferson D., Morrell K., McQuire D., Gray T., Landon M., Doherty F. J., Mayer R. J. A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localisation of ubiquitin. Neurosci Lett. 1988 Nov 22;94(1-2):203–210. doi: 10.1016/0304-3940(88)90296-0. [DOI] [PubMed] [Google Scholar]
- Lowe J., Morrell K., Lennox G., Landon M., Mayer R. J. Rosenthal fibres are based on the ubiquitination of glial filaments. Neuropathol Appl Neurobiol. 1989 Jan-Feb;15(1):45–53. doi: 10.1111/j.1365-2990.1989.tb01148.x. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., Croall D. E., DeMartino G. N. ATP-stimulated proteolysis in soluble extracts of BHK 21/C13 cells. Evidence for multiple pathways and a role for an enzyme related to the high-molecular-weight protease, macropain. Arch Biochem Biophys. 1988 Apr;262(1):273–285. doi: 10.1016/0003-9861(88)90189-0. [DOI] [PubMed] [Google Scholar]
- Meyer E. M., West C. M., Chau V. Antibodies directed against ubiquitin inhibit high affinity [3H]choline uptake in rat cerebral cortical synaptosomes. J Biol Chem. 1986 Nov 5;261(31):14365–14368. [PubMed] [Google Scholar]
- Raboy B., Parag H. A., Kulka R. G. Conjugation of [125I]ubiquitin to cellular proteins in permeabilized mammalian cells: comparison of mitotic and interphase cells. EMBO J. 1986 May;5(5):863–869. doi: 10.1002/j.1460-2075.1986.tb04296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A., Bain J. L., Ellis S., Haas A. L. Quantitation and immunocytochemical localization of ubiquitin conjugates within rat red and white skeletal muscles. J Histochem Cytochem. 1988 Jun;36(6):621–632. doi: 10.1177/36.6.2835410. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Ciechanover A., Brandt R. A., Geuze H. J. Immunoelectron microscopic localization of ubiquitin in hepatoma cells. EMBO J. 1988 Oct;7(10):2961–2966. doi: 10.1002/j.1460-2075.1988.tb03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Tolleshaug H., Høyvik H. Autophagy and protein degradation in isolated rat hepatocytes. Biochem Soc Trans. 1985 Dec;13(6):1007–1010. doi: 10.1042/bst0131007. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kasai Y., Senshu M., Iwashita S., Imahori K. Thiol protease-specific inhibitor E-64 arrests human epidermoid carcinoma A431 cells at mitotic metaphase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):146–150. doi: 10.1073/pnas.85.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- Swerdlow P. S., Finley D., Varshavsky A. Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal Biochem. 1986 Jul;156(1):147–153. doi: 10.1016/0003-2697(86)90166-1. [DOI] [PubMed] [Google Scholar]
- Tweto J., Doyle D. Turnover of the plasma membrane proteins of hepatoma tissue culture cells. J Biol Chem. 1976 Feb 10;251(3):872–882. [PubMed] [Google Scholar]
- Vierstra R. D., Sullivan M. L. Hemin inhibits ubiquitin-dependent proteolysis in both a higher plant and yeast. Biochemistry. 1988 May 3;27(9):3290–3295. doi: 10.1021/bi00409a025. [DOI] [PubMed] [Google Scholar]
- Wilkinson K. D. Protein ubiquitination: a regulatory post-translational modification. Anticancer Drug Des. 1987 Oct;2(2):211–229. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]