Abstract
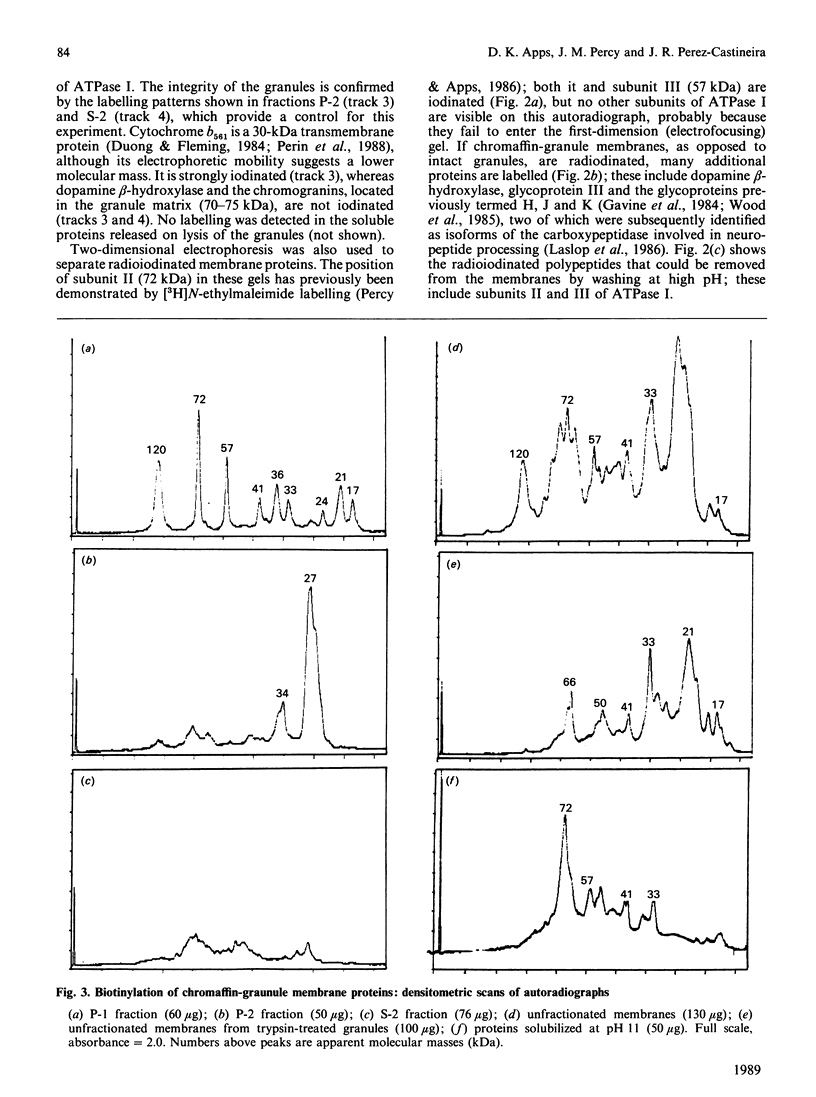
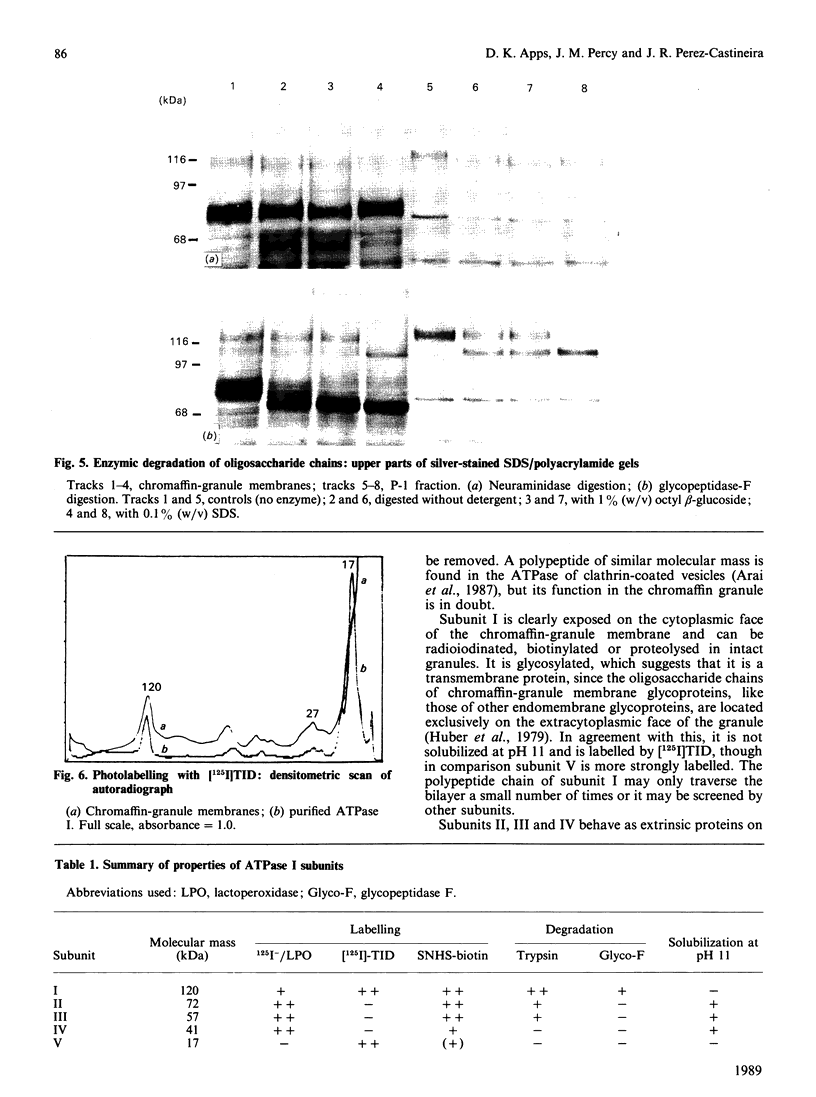
Proteins exposed on the cytoplasmic face of isolated chromaffin granules were labelled by lactoperoxidase-catalysed radioiodination and by non-enzymic biotinylation. Granule membranes were then prepared, and the H+-translocating ATPase isolated by fractionation with Triton X-114. The labelling of individual ATPase subunits was assessed by polyacrylamide-gel electrophoresis, followed by autoradiography or by blotting and decoration with 125I-labelled streptavidin. Subunits of 72, 57 and kDa were strongly labelled, and could be removed from the membrane at pH 11: they are therefore extrinsic proteins. The 120 kDa subunit was also labelled, but it was not solubilized at pH 11. Photolabelling with a hydrophobic probe indicated that this subunit penetrates the bilayer, and enzymic degradation studies showed the presence of N-linked oligosaccharides; this subunit therefore spans the chromaffin-granule membrane. Labelling of the 17 kDa subunit occurred predominantly on the extracytoplasmic (matrix) face of the granule membrane. These results are consistent with this V-type ATPase having a structure that is generally similar to that of mitochondrial (F-type) ATPases, although the attachment of the 120 kDa subunit may be asymmetrical.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. doi: 10.1146/annurev.cb.02.110186.001143. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Boisclair M. D., Gavine F. S., Pettigrew G. W. Unusual redox behaviour of cytochrome b-561 from bovine chromaffin granule membranes. Biochim Biophys Acta. 1984 Jan 30;764(1):8–16. doi: 10.1016/0005-2728(84)90134-8. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Pryde J. G., Sutton R. Characterization of detergent-solubilized adenosine triphosphatase of chromaffin granule membranes. Neuroscience. 1983 Jul;9(3):687–700. doi: 10.1016/0306-4522(83)90185-9. [DOI] [PubMed] [Google Scholar]
- Arai H., Berne M., Terres G., Terres H., Puopolo K., Forgac M. Subunit composition and ATP site labeling of the coated vesicle proton-translocating adenosinetriphosphatase. Biochemistry. 1987 Oct 20;26(21):6632–6638. doi: 10.1021/bi00395a011. [DOI] [PubMed] [Google Scholar]
- Arai H., Terres G., Pink S., Forgac M. Topography and subunit stoichiometry of the coated vesicle proton pump. J Biol Chem. 1988 Jun 25;263(18):8796–8802. [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman B. J., Bowman E. J. H+-ATPases from mitochondria, plasma membranes, and vacuoles of fungal cells. J Membr Biol. 1986;94(2):83–97. doi: 10.1007/BF01871190. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988 Feb 18;331(6157):622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Cidon S., Nelson N. Purification of N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J Biol Chem. 1986 Jul 15;261(20):9222–9227. [PubMed] [Google Scholar]
- Duong L. T., Fleming P. J. The asymmetric orientation of cytochrome b561 in bovine chromaffin granule membranes. Arch Biochem Biophys. 1984 Jan;228(1):332–341. doi: 10.1016/0003-9861(84)90074-2. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavine F. S., Pryde J. G., Deane D. L., Apps D. K. Glycoproteins of the chromaffin granule membrane: separation by two-dimensional electrophoresis and identification by lectin binding. J Neurochem. 1984 Nov;43(5):1243–1252. doi: 10.1111/j.1471-4159.1984.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Immunoaffinity purification and characterization of vacuolar H+ATPase from bovine kidney. J Biol Chem. 1987 Nov 15;262(32):15780–15789. [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Proton-translocating ATPase from bovine kidney medulla: partial purification and reconstitution. Am J Physiol. 1988 Jan;254(1 Pt 2):F71–F79. doi: 10.1152/ajprenal.1988.254.1.F71. [DOI] [PubMed] [Google Scholar]
- Hiram Y., Nir A., Zinder O. Tensile strength of the chromaffin granule membrane. Biophys J. 1982 Jul;39(1):65–69. doi: 10.1016/S0006-3495(82)84491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber E., König P., Schuler G., Aberer W., Plattner H., Winkler H. Characterization and topography of the glycoproteins of adrenal chromaffin granules. J Neurochem. 1979 Jan;32(1):35–47. doi: 10.1111/j.1471-4159.1979.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Hurley W. L., Finkelstein E., Holst B. D. Identification of surface proteins on bovine leukocytes by a biotin-avidin protein blotting technique. J Immunol Methods. 1985 Dec 17;85(1):195–202. doi: 10.1016/0022-1759(85)90287-x. [DOI] [PubMed] [Google Scholar]
- Ingalls H. M., Goodloe-Holland C. M., Luna E. J. Junctional plasma membrane domains isolated from aggregating Dictyostelium discoideum amebae. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4779–4783. doi: 10.1073/pnas.83.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslop A., Fischer-Colbrie R., Hook V., Obendorf D., Winkler H. Identification of two glycoproteins of chromaffin granules as the carboxypeptidase H. Neurosci Lett. 1986 Dec 23;72(3):300–304. doi: 10.1016/0304-3940(86)90530-6. [DOI] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989 Feb 25;264(6):3577–3582. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Purification and properties of a vanadate- and N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J Biol Chem. 1988 Jun 15;263(17):8521–8527. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. The purified ATPase from chromaffin granule membranes is an anion-dependent proton pump. J Biol Chem. 1987 Jul 5;262(19):9175–9180. [PubMed] [Google Scholar]
- Percy J. M., Apps D. K. Proton-translocating adenosine triphosphatase of chromaffin-granule membranes. The active site is in the largest (70 kDa) subunit. Biochem J. 1986 Oct 1;239(1):77–81. doi: 10.1042/bj2390077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy J. M., Pryde J. G., Apps D. K. Isolation of ATPase I, the proton pump of chromaffin-granule membranes. Biochem J. 1985 Nov 1;231(3):557–564. doi: 10.1042/bj2310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Slaughter C. A., Südhof T. C. The structure of cytochrome b561, a secretory vesicle-specific electron transport protein. EMBO J. 1988 Sep;7(9):2697–2703. doi: 10.1002/j.1460-2075.1988.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of catecholamines by resealed chromaffin-grnaule "ghosts". Biochem J. 1974 Nov;144(2):311–318. doi: 10.1042/bj1440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde J. G., Phillips J. H. Fractionation of membrane proteins by temperature-induced phase separation in Triton X-114. Application to subcellular fractions of the adrenal medulla. Biochem J. 1986 Jan 15;233(2):525–533. doi: 10.1042/bj2330525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R., Apps D. K. Isolation of a DCCD-binding protein from bovine chromaffin-granule membranes. FEBS Lett. 1981 Jul 20;130(1):103–106. doi: 10.1016/0014-5793(81)80675-8. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Stone D. K. Isolation and reconstitution of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1986 Feb 25;261(6):2492–2495. [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]





