Abstract
Each year, malignant melanoma accounts for 57 000 deaths globally. If current rates continue, there will be an estimated 510 000 new cases annually and 96 000 deaths by 2040. Melanoma and keratinocyte cancers (KCs) incur a large societal burden. Using a mathematical population model, we performed an economic evaluation of the SunSmart program in the state of Western Australia (WA), a primary prevention program to reduce the incidence of skin cancer, versus no program. A societal perspective was taken combining costs to the health system, patients and lost productivity. The model combined data from pragmatic trial evidence of sun protection, epidemiological studies and national cost reports. The main outcomes modelled were societal and government costs, skin cancer counts, melanoma deaths, life years and quality-adjusted life years. Over the next 20 years, the model predicted that implementing the WA SunSmart program would prevent 13 728 KCs, 636 melanomas and 46 melanoma deaths per 100 000 population. Furthermore, 251 life years would be saved, 358 quality-adjusted life years gained and AU$2.95 million in cost savings to society per 100 000 population would be achieved. Key drivers of the model were the rate reduction of benign lesions from sunscreen use, the costs of purchasing sunscreen and the effectiveness of reducing KCs in sunscreen users. The likelihood of WA SunSmart being cost-effective was 90.1%. For the WA Government, the estimated return on investment was $8.70 gained for every $1 invested. Primary prevention of skin cancer is a cost-effective strategy for preventing skin cancers.
Keywords: skin cancer, melanoma, keratinocyte carcinomas, primary prevention, cost-effectiveness, economic evaluation
Contribution to Health Promotion.
Solar ultraviolet radiation is the main cause of skin cancer, and skin cancer prevention is feasible and effective through sun protection behaviours.
Skin cancer rates are growing around the world, and health and cost burdens are large and preventable.
Our economic model combines the latest evidence to support the economic and health benefits of a population-based primary prevention intervention for skin cancer.
The high positive return on investment supports the business case for government investment in a state-wide SunSmart health promotion program for skin cancer prevention.
BACKGROUND
Skin cancer is a growing public health issue among fair-skinned populations around the world. Malignant melanoma is the most serious skin cancer and led to 57 000 deaths globally in 2020 (http://gco.iarc.fr/today/home). This is projected to rise to 96 000 deaths by 2040 with current rate increases of 3–4% per year in many European countries (http://gco.iarc.fr/today/home). Non-melanoma skin cancers [or basal and squamous cell carcinomas, collectively called keratinocyte cancers (KCs)] are less deadly but occur in far greater numbers than melanomas, mostly in aging populations. As nations with aging populations approach the peak ages for the development of skin malignancies, case numbers will continue to rise even if incidence rates plateau (Gordon and Rowell, 2015).
With increasing incidence of skin cancer, the healthcare costs of treating and managing skin cancers are expected to rise quickly (Gordon and Rowell, 2015; Urban et al., 2021; Olsen et al., 2022). In 2020–21, the Australian health sector spent AU$1.8 billion for skin cancer diagnosis and treatment, with non-melanoma and melanoma skin cancers accounting for AU$1.5 billion and AU$0.3 billion, respectively (Australian Institute of Health and Welfare, 2022). The financial burden of skin cancer is also borne by individuals through loss of income, out-of-pocket payments and travel costs for treatment, as well as by society generally through productivity losses. Rising healthcare costs are of major concern in most high-income countries, and the challenge for healthcare decision makers facing budgetary constraints is to implement cost-effective interventions (Turner et al., 2021).
Skin cancers are predominantly caused by ultraviolet (UV) radiation exposure, either naturally from the sun or artificially through indoor tanning devices (Gordon and Rowell, 2015). Preventative measures to decrease UV exposure offer an effective strategy to lower the risk of developing skin cancers (Montague et al., 2001; Greinnert et al., 2014). The main preventative initiatives centre around informing the public about the need to protect their skin in five ways including wearing clothing that covers the skin, applying sunscreen, avoiding the use of indoor tanning beds and rescheduling outdoor activities to avoid times when UV radiation is strongest (Gordon et al., 2020a).
A systematic review published a decade ago revealed seven full economic evaluations for primary prevention initiatives (Gordon and Rowell, 2015). These various programs, including SunSmart programs, were either cost-saving or cost-effective to society. SunSmart programs are multi-component sun safety interventions designed to prevent skin cancers and their associated morbidity and mortality (Peraral, 2014). Since Gordon and Rowell’s review (Gordon and Rowell, 2015), there have been advances in research methods, data linkage processes, big data access and simulation modelling that allow for more accurate and improved analyses. As disease burden, risk factors and expenditure patterns change over time, it is important that economic analyses keep pace with these changes. For example, since 2013, many countries have adopted targeted therapies for advanced-stage melanoma, with strikingly higher treatment costs than those reported in earlier studies. Therefore, potentially the cost-benefit assessment of prevention initiatives may be undervalued.
Attracting and sustaining government investment in health promotion and preventive healthcare is challenging when competing with other interventions for pressing health problems, for both preventive and curative medicine. Attracting appropriate levels of investment in skin cancer prevention has remained a priority for Cancer Council. It is also relatively difficult to attract funding in skin cancer prevention versus other major risk factors such as tobacco use and obesity prevention. However, many health promotion interventions are effective and many also reduce health inequities (Chelak and Chakole, 2023). As national and global cancer plans prioritize health equity improvements for lower socio-economic populations with less access to health services, preventing skin cancer could be expected to benefit these communities the greatest (Chelak and Chakole, 2023). Preventing skin cancer requires appropriate and ongoing investment and support of governments and interest groups to promote and ensure sun protection messages, knowledge and interventions reach all communities and their benefits do not wane over time (Gordon et al., 2022a). It is proposed that the ideal investment in preventive health should reflect 5% of the cost of healthcare expenditure (Australian Government Department of Health, 2021). The purpose of this study was to conduct an economic evaluation of the SunSmart program in Western Australia (WA), a primary prevention program to reduce the incidence of skin cancer, versus the alternative of no state-wide primary prevention initiative, and provide economic evidence to inform decision-making and budget allocations.
METHODS
Study overview
A Markov cohort model was developed to assess the cost-effectiveness of the WA SunSmart program compared to the alternative of no program. The study population were Western Australians aged between 20 and 75 years, mean age 47 and modelled over 20 years. A societal cost perspective was taken combining costs to the health system, patients and lost productivity from premature melanoma deaths. The model combined high-quality data estimates from trial evidence of sun protection (van der Pols et al., 2006b; Green et al., 2012), epidemiological studies (Gershenwald et al., 2017; Pandeya et al., 2017), systematic reviews (Tran et al., 2018) and national cost reports (Independent Hospital Pricing Authority, 2020). The main outcomes modelled were societal and government costs, skin cancer counts, melanoma deaths, life years and quality-adjusted life years (QALYs). We also performed a benefit-cost analysis from the WA Government’s perspective to obtain the return on investment. This economic evaluation is compliant with the Consolidated Health Economic Evaluation Reporting Standards (Husereau et al., 2022) (Supplementary Data). Ethical approval was not required as the analysis involved available secondary data sources.
Comparative strategies
In each Australian state and territory, SunSmart programs are operated by the respective Cancer Councils using common principles. The WA SunSmart program includes (i) public education campaigns to improve awareness, knowledge and attitudes towards UV exposure and promote behaviour modification; (ii) promotion of shaded areas in public places; and (iii) policy-based initiatives such as in schools and early years settings and advocating a ban on commercial indoor tanning (Montague et al., 2001; Peraral, 2014). The comparator was a no-intervention strategy or counterfactual scenario in which the general population in WA was not exposed to the SunSmart program. WA is the largest state of Australia geographically (and about the size of Western Europe) and has a population of 2.9 million residents with mainly English, Australian, Irish, Scottish and Italian ancestries. Aboriginal and Torres Strait Islanders account for 3.1% of the WA population (Australian Bureau of Statistics [ABS], 2023b).
The program logic model for the two strategies (primary prevention program versus no program) and health outcomes has been presented elsewhere (Gordon et al., 2020a). In brief, through its activities, the SunSmart program promotes a greater awareness of sun protective behaviour, leading to greater adoption of protective behaviour and more regular sunscreen use, a reduction in the incidence of skin lesions, KCs and melanomas, proportionately fewer thicker and thin melanomas compared to the absence of the program, and long-term improvements in survival and quality of life (Gordon et al., 2020a).
Markov model structure
A decision-analytic model with Markov chains was created in TreeAge Pro 2023 R2 (TreeAge Software Inc., Williamstown, MA, USA) (Supplementary Figure S1). The model modified a published model similarly assessing skin cancer primary prevention (Gordon et al., 2020a). Face validity of that model was assessed by senior doctors working in skin cancer medicine, and review of clinical practice guidelines and publications (Gordon et al., 2020a). The model had annual cycles and ran for 20 years, with duration altered in sensitivity analyses.
The model starts with the decision branches of WA SunSmart implemented versus no WA SunSmart. In each strategy, the cohort cycles within the following possible health states: (i) lesion-free, (ii) benign skin lesions, (iii) KCs, (iv) melanoma (separated into in situ; stage I and II melanoma, stage III and IV melanoma), (v) post-melanoma diagnosis (separately for in situ; stage I and II melanoma, stage III and IV melanoma), (vi) melanoma deaths and (vii) other deaths. Melanomas were categorized into three broad groups to enable an efficient model structure but allowing for different survival and cost values appropriate for disease stage. The mixed-age cohort could move between health states according to different probabilities of developing skin lesions or skin cancers, or they could remain in the same state (e.g. lesion-free). The model included the occurrence of people having multiple benign lesions and/or skin cancers over time and risk increased with age (following epidemiological trends). In any annual cycle, people could die from melanoma or other causes at any time. Deaths from KCs were not included because at a population level, these are very rare and well below rates of background mortality from common chronic diseases. Transitioning between thin and thicker melanoma health states also did not occur as it was assumed patients would be treated with curative excision following diagnosis within 1 year (one cycle). Table 1 summarizes all model input values.
Table 1:
Model inputs, one-way sensitivity values and sources
| Description | Base value | Sensitivity values | Source/notes | |
|---|---|---|---|---|
| Low | High | |||
| Starting age (mean) | 47 | 40 | 60 | Mean age 47 in Nambour trial—random selection of adults aged 18–60 years, normal distribution |
| Background mortality | By age | not applicable | not applicable | WA Life Tables (ABS, 2023b) |
| Costs (inflated to 2023 AUD) | ||||
| WA SunSmart program per capita | $0.44 | $0.40 | $0.49 | Financial records, Cancer Council WA, WA Department of Health, Healthway, ±10% |
| Healthcare diagnosis and treatment of: | ||||
| Benign lesion | $175.57 | $159.61 | $195.08 | Medicare Benefit Scheme and Pharmaceutical Benefit Scheme items (Australian Government Department of Health and Aged Care) |
| KCs | $568 | $489 | $709 | (Gordon et al., 2022a) |
| In situ melanoma | $891.90 | $810.82 | $991.00 | Medicare Benefit Scheme items (Supplementary Data), ±10% mean |
| Stage I and II melanoma in first year | $7895 | $7177 | $8772 | (Gordon et al., 2022b) |
| Stage I and II melanoma after year 1 | $81.70 | $74.27 | $90.78 | Medicare Benefit Scheme item 104 on 6-monthly consultations (Australian Government Department of Health and Aged Care) |
| Stage III and IV melanoma in first year | $86 692 | $78 811 | $96 325 | (Gordon et al., 2022b) |
| Stage III and IV melanoma after year 1 | $17 187 | $15 551 | $19 006 | (Gordon et al., 2022b) (10% patients with ongoing therapies after year 1) |
| Cost of hospitalization for adverse events for melanoma treatment | $3412 | $3102 | $3791 | (Gordon et al., 2022b) |
| Palliative care in last months of life | $11 835 | $10 759 | $13 150 | Australian-revised Diagnostic Related Group for palliative care (Independent Hospital Pricing Authority, 2020) |
| Costs to individuals | ||||
| Cost of sunscreen in SunSmart users | $27.00 | $24.55 | $30.00 | Three bottles per year per person, 500 ml retail brand SPF 50+ $9.00 each—regular use |
| Cost of sunscreen in low users | $9.00 | $8.18 | $10.00 | One bottle per year per person, 500 ml retail brand SPF 50+ $9.00 each as current background use |
| Out-of-pocket costs for in situ melanoma | $511.11 | $464.64 | $567.90 | (Gordon et al., 2018) |
| Out-of-pocket costs for thin melanoma | $1545.53 | $1405.02 | $1717.25 | (Gordon et al., 2018) |
| Out-of-pocket costs for thick melanoma | $3072.75 | $2793.41 | $3414.17 | (Gordon et al., 2018) |
| Cost of productivity losses for premature melanoma death | $365 061 | $331 873 | $405 623 | (Carter et al., 2016) |
| Probabilities | ||||
| Benign lesions | 0.383 | 0.2911 | 0.4753 | (Janda et al., 2014) |
| KCs | By age group | n/a | n/a | (Pandeya et al., 2017) |
| Melanoma | By age group | n/a | n/a | Cancer Data in Australia 2023, 2019 figures for Australia used for WA |
| Multiple benign lesions | 0.0631 | 0.0574 | 0.0701 | 65% over 10 years converted to annual probability (Gordon, Leung, Johns, McNoe, Lindsay, Merollini, Elliott, Neale, Olsen, Pandeya and Whiteman, 2022) |
| Multiple KCs | By age group | n/a | n/a | (Pandeya et al., 2017) |
| Subsequent melanomas | 0.0186 | 0.0083 | 0.0288 | 5-year rate, 95% CI (Green et al., 2022) |
| Proportion of all melanomas | ||||
| Stage I and II melanoma | 0.3478 | 0.3313 | 0.3661 | (Cancer Council Victoria and Victorian Cancer Registry, 2023) ±10% (Supplementary Data) |
| Stage III and IV melanoma | 0.032 | 0.0305 | 0.0337 | As above |
| In situ melanomas | 1-(invasive proportions above) | |||
| Adverse events needing hospitalization following thick melanoma treatment | 0.22 | 0.15 | 0.28 | (Gordon, Leung, Johns, McNoe, Lindsay, Merollini, Elliott, Neale, Olsen, Pandeya and Whiteman, 2022) |
| Death from melanoma—Stage I and II | 10 years = 0.105 | n/a | n/a | 10.5% mortality by 10 years (Gershenwald et al., 2017), weighted by stage |
| Death from melanoma—Stage III and IV | 10 years = 0.485 | n/a | n/a | 48.5% mortality by 10 years (Green et al., 2011), weighted by stage |
| Rate ratios | ||||
| Ratio of in situ to invasive melanoma | 1.63 | 1.48 | 1.81 | (Cancer Council Victoria and Victorian Cancer Registry, 2023) ±10% (Supplementary Data) |
| Benign lesions—regular sunscreen use | 0.76 | 0.66 | 0.86 | (Darlington et al., 2003) |
| KCs—regular sunscreen users | 0.65 | 0.45 | 0.94 | Based on squamous cell carcinomas (van der Pols et al., 2006b) |
| Melanoma—regular sunscreen users | 0.50 | 0.45 | 0.55 | (Green et al., 2012) |
| Health utilities | ||||
| Benign lesion and lesion-free | 0.91 | n/a | n/a | (Gordon et al., 2023) |
| KC | −0.01 | −0.015 | −0.005 | (Gordon et al., 2023) (disutility applied) |
| Multiple KCs | −0.03 | −0.04 | −0.02 | (Gordon et al., 2023) (disutility applied) |
| In situ, stage I and II melanoma | −0.03 | −0.04 | −0.02 | (Tran et al., 2018) (disutility applied) |
| Stage III and IV melanoma | −0.23 | −0.26 | −0.20 | (Tran et al., 2018) (disutility applied) |
Effectiveness of WA SunSmart
For the SunSmart strategy, effectiveness was based on available data from the community-based Nambour Skin Cancer Prevention Trial (n = 1621) that evaluated daily application of Sun Protection Factor (SPF) 15+ sunscreen to face, arms and hands versus discretionary use of sunscreen (Pandeya et al., 2005; Green et al., 2011). Although encouraging regular sunscreen use is only one component of the SunSmart program, the evidence of sunscreen use behaviour in preventing skin cancer is robust and other sun protection behaviours occur concurrently with sunscreen use (https://ncci.canceraustralia.gov.au/prevention/sun-exposure/sunburn-and-sun-protection). In the trial, evidence on skin cancer outcomes was collected using dermatological examinations for the first 5 years and then through record linkage to histopathology reports of skin lesions for 15 years (van der Pols et al., 2006b; Green et al., 2011). The trial population were aged 20–75 at baseline and 47 years was the mean age. The trial demonstrated reduced incidence of invasive and in situ melanomas (Green et al., 2011) and squamous cell carcinomas (van der Pols et al., 2006b) at 5 years. Incidence of basal cell carcinomas was also reduced but not statistically significant while rates of actinic keratoses (benign skin lesions) declined also. Twelve years after the trial ended, routine sunscreen use was sustained in people randomized to the regular application group (van der Pols et al., 2006a).
Model inputs and sources
Probabilities in the model were derived from WA population data where available (e.g. background mortality rates, population size, melanoma incidence) or from Australian population data (e.g. stage distribution of melanoma, the proportion of in situ to invasive melanoma, KC incidence) (Table 1). All rates were converted to annual probabilities using mathematical formulae. We obtained epidemiological data by age group where available because the risks of developing skin cancer increase with age. Health utilities are similar to quality-of-life scores. These were applied to each health state in the model (e.g. when a person develops a KC or melanoma), to adjust survival time by quality of life and generate QALYs. Health utilities were poorer for more advanced stages of melanoma and were obtained from a recent meta-analysis (Tran et al., 2018). Quality-of-life effects also occur for patients with KCs where people face multiple cancers, anxiety, disfiguration, pain, infection and other symptoms (Gaulin et al., 2015; Gordon et al., 2023). A utility score of 0.91 was assigned to patients with no skin lesions or skin cancers and utility reductions of 0.01 and 0.03 for the first and each additional KC diagnosed and treated (Gordon et al., 2023).
The study took a societal cost perspective and included previous years’ WA SunSmart per capita program costs, healthcare costs for diagnosis, treatment and follow-up, patient out-of-pocket expenses for sunscreen and medical treatments, and productivity losses to society for each premature melanoma death. Costs are presented from the government’s perspective also, excluding patient costs. Per capita costs to implement the WA SunSmart program ($0.44) were applied in each year of the model and sourced from financial records of Cancer Council WA, including related expenditure by WA Department of Health and Healthway (a government-funded health promotion agency in WA: https://www.healthway.wa.gov.au/our-organisation/vision-goals-and-purpose/). Costs were included for public education campaigns, skin cancer prevention and early detection resources, initiatives targeting settings, sponsorship programs, administrative expenses and miscellaneous costs involved in program implementation. All resources were valued in 2023 Australian dollars and inflated where applicable using the Health Price Index (ABS, 2023a). Specific details on model input calculations and sources are provided in the Supplementary Data.
Analyses
Mean costs, skin cancer counts and other outcomes for the two strategies (WA SunSmart versus no program) were calculated with 5000 Monte Carlo simulations and the differences across strategies were compared. Outcomes were presented per person, per 100 000 persons and per WA population for adults. The WA adult population is 2.167 million (ABS, 2023b). Future costs and QALYs were discounted at 3% per year to provide present values (Sanders et al., 2016). In addition, using the modelled outputs, we performed a benefit-cost analysis to assess the return on investment for the WA Government in funding SunSmart. Here, the cost of the WA SunSmart program for the current state population was subtracted from the expected cost savings resulting from avoided healthcare and productivity losses over 20 years (Supplementary Data).
To address uncertainty in the model inputs, distributions were assigned around the mean values. Beta distributions were applied for probabilities and gamma distributions for costs (Supplementary Data). Mean costs and 95% uncertainty intervals (95% UIs) were estimated using Monte Carlo simulation. To assess the key drivers of mean outcomes, one-way sensitivity analyses on each variable addressed how potential uncertainty of input values would vary the main findings. High and low values for each variable included published 95% confidence intervals or using 10% margins of error. Assessing variation in model inputs simultaneously, probabilistic sensitivity analyses were performed and presented in an incremental cost-effectiveness scatterplot. A threshold of AU$50 000 per QALY gained was used as a benchmark for cost-effective healthcare in Australia (Harris et al., 2008). Internal coherence checks were conducted and all inputs checked with two modellers.
RESULTS
Societal perspective
Over the next 20 years, implementing the WA SunSmart program, at current levels, could prevent 13 728 KCs, 636 melanomas and 46 melanoma deaths per 100 000 population. Furthermore, 251 life years would be saved, 358 QALYs gained and $2.95 million in societal cost savings achieved per 100 000 population (Table 2). Extrapolating to the wider adult WA population indicates over 297 504 KCs and 13 774 melanomas could be avoided, saving the WA economy $63.9 million.
Table 2:
Projected cohort outcomes of WA SunSmart program compared with no program over 20 years
| Outcomes | Mean per person | Mean per 100 000 persons |
Benefits (WA SunSmart versus no SunSmart) |
|||
|---|---|---|---|---|---|---|
| WA SunSmart | No SunSmart | WA SunSmart | No SunSmart | Per 100 000 population | Per WA population | |
| n | n | n | n | Avoided skin cancers (n) | ||
| KCs | 0.3218 | 0.4589 | 32 179 | 45 907 | 13 728 | 297 504 |
| In situ melanomas | 0.0043 | 0.0083 | 429 | 823 | 394 | 8544 |
| Stage I and II melanomas | 0.0024 | 0.0046 | 240 | 461 | 221 | 4790 |
| Stage III and IV melanomas | 0.0002 | 0.0004 | 22 | 42 | 20 | 440 |
| All melanomas | 0.0069 | 0.0133 | 691 | 1327 | 636 | 13 774 |
| Melanoma deaths avoided (n) | ||||||
|---|---|---|---|---|---|---|
| Melanoma deaths | 0.0007 | 0.0012 | 69 | 115 | 46 | 998 |
| Life years saved (n) | ||||||
|---|---|---|---|---|---|---|
| Life years | 19.174 | 19.172 | 1 917 440 | 1 917 189 | 251 | 5449 |
| Cost savings ($) | ||||||
|---|---|---|---|---|---|---|
| Costs—societal | $2260 | $2289 | $226.0 million | $228.9 million | $2.95 million | $63.9 million |
| Costs—government | $1840 | $2143 | $184.0 million | $214.3 million | $30.4 million | $655.4 million |
| QALYs gained (n) | ||||||
|---|---|---|---|---|---|---|
| QALYs | 13.443 | 13.439 | 1 344 332 | 1 343 973 | 358 | 7765 |
Government perspective
From a government cost perspective (both Federal and State governments), total costs of a strategy of implementing WA SunSmart for 20 years were estimated at $184.0 million per 100 000 persons for the WA SunSmart program compared with $214.3 million without the program; corresponding melanoma counts were 691 and 1327, respectively. At the WA population level, total 20-year cost savings of $655.4 million were predicted for governments from avoided healthcare costs and productivity losses (or mean annual cost savings of $33 million). Cost savings to the WA economy was far lower ($63.9 million) due to the substantial financial contributions from citizens for sunscreen and out-of-pocket healthcare expenses for skin cancers and other lesions.
WA Government investment
Using a return on investment approach, the 20-year cost of SunSmart for the WA Government was $19.1 million (at 44 cents per capita) compared with government benefits worth $166.1 million over the same period. The estimated return on investment was $8.70 for every $1 invested.
Sensitivity analysis
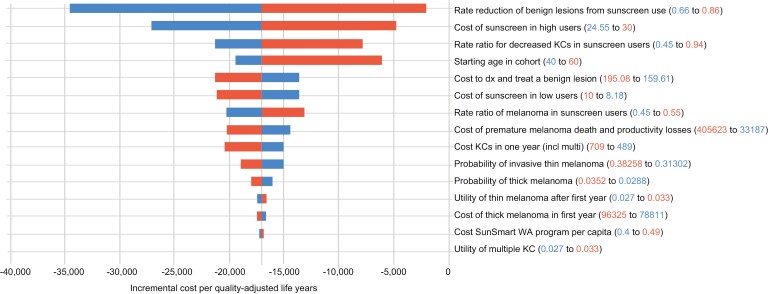
One-way sensitivity analyses indicated that the most important model inputs were the rate reduction of benign lesions from sunscreen use (0.66–0.86), the costs of purchasing sunscreen in regular users (range $24.55–$30.00 per year) under the WA SunSmart strategy, the effectiveness of reducing KCs in sunscreen users (relative risk 0.45–0.94), age, and diagnosis and treatment costs of benign lesions ($160–$195 per lesion) (Figure 1). These variables changed the ‘base-case’ incremental cost per QALY ratio for WA SunSmart versus no program between –$40 000 and –$2500, but SunSmart was superior (cost-saving and higher benefits) to no intervention in all sensitivity analyses. Probabilistic sensitivity analysis indicated the likelihood of WA SunSmart producing cost savings and higher QALYs (win win) was 59.8% (Supplementary Data). Applying a willingness-to-pay threshold of AU$50 000 per QALY, the probability of WA SunSmart being cost-effective or dominant over no program was 90.1%.
Fig. 1:
Tornado diagram of one-way sensitivity analysis comparing the WA SunSmart program with no program.
DISCUSSION
Our findings show that investment in a multi-component sun safety program, that encourages sun protection practices, substantially reduces skin cancers and their associated health system and broader societal costs. Primary prevention of skin cancer is a highly cost-effective investment in preventing melanoma death despite the rapid advances in immunotherapies lengthening survival prospects of people with advanced-stage disease. Taking into account the latest evidence from epidemiological studies on incidence, mortality, quality of life impacts and the potential effectiveness of sun protection, it is evident that primary prevention has favourable and strong outcomes for the health system and wider economy.
A key assumption in our analysis was that the effectiveness of WA SunSmart was similar to the findings of the Nambour randomized controlled trial for reductions in new skin cancers. The Nambour trial was a pragmatic community-based trial with little exclusion criteria and ran for 4.5 years with close monitoring of participants (Green et al., 1999). Participants in the intervention arm (only) were given sunscreen but this discontinued in the 15-year follow-up period afterwards where people purchased sunscreen themselves (van der Pols et al., 2006a). The sunscreen product disseminated to participants was also SPF 15+, far lower than what is standard nowadays of SPF 50+. The parallels with SunSmart WA are that extensive activities were undertaken to avoid sunburn, encourage people to wear sunscreen and protect their skin from sun damage and provide the health education for self-managing optimal sun protection behaviours. The effectiveness of long-term sunscreen use was observed many years after the intervention when habit-forming was established and outside the formal trial conditions (van der Pols et al., 2006a). The additional sun protection behaviours, such as wearing hats, protective clothing and sunglasses, are also shown to correlate reasonably well with sunscreen use despite the Nambour trial’s focus on sunscreen behaviours (https://ncci.canceraustralia.gov.au/prevention/sun-exposure/sunburn-and-sun-protection). Notwithstanding these points, we acknowledge our findings heavily rely on behaviour changes reported in trial evidence which may be different to WA SunSmart. This uncertainty was addressed through our probabilistic sensitivity analyses covering a broad range of values for the effectiveness variable (including very little difference in effects across strategies).
Previous studies of prevention and early detection of skin cancer, such as the SunSmart program in Australia, have demonstrated strong economic credentials (Gordon et al., 2022a). Two Australian studies (Doran et al., 2015; Shih et al., 2017) have shown that SunSmart and a public education campaign aimed at preventing skin cancer had positive returns of investment of between $3 and $4 for every $1 invested, respectively. This analysis demonstrates the return on investment could be substantially higher now at $8.70, and likely due to significantly higher healthcare costs of treating and diagnosing skin cancers compared with earlier studies. Recent work also supports the cost-effectiveness of primary prevention interventions in reducing development of skin cancers (Gordon et al., 2022a). However, none were performed in the WA setting and transferring findings of economic evaluations across jurisdictions may be difficult, given differences in local conditions, populations and implementation strategies (Garcia-Mochon et al., 2021). Government funding of the WA SunSmart has varied over time and has been influenced by budgetary pressures and political will. At times, the level of funding has been viewed as inadequate to undertake all key activities of the intended SunSmart program. It is therefore possible that had WA SunSmart received sufficient or greater investment, better health outcomes could have been realized.
The cost of sunscreen for individuals was a key driver of the model. Sunscreen purchasing and use are encouraged by SunSmart but the financial costs are borne by individuals. The sunscreen market in Australia has expanded in retail value and volume over time; however, consumption is still inadequate given Australia’s high ambient UV exposure (Gordon et al., 2022a). In most states, including WA, the average UV Index ranges from high to extreme throughout most of the year (Elliott et al., 2023). Calls are made for governments to reduce the prices of approved sunscreen products and other protective equipment, or make sunscreen freely available in outdoor settings like beach entrances, to increase general uptake of sun protection behaviours (Gordon et al., 2022a). This may be increasingly important since annual sunburn rates continue to be high in Australia; 55% of respondents aged 18 years and over reported being sunburnt at least once during the summer (Cancer Council Western Australia, 2022).
Over the last decade, there have been economic evaluations on primary prevention of skin cancer both within Australia (Doran et al., 2016; Shih et al., 2017; Gordon et al., 2020a; Gordon et al., 2020b; Law et al., 2023) and in the USA (Guy et al., 2017; Eskander et al., 2021), England (Eden et al., 2022), Denmark (Køster et al., 2020), Canada (Mofidi et al., 2021) and Belgium (Pil et al., 2016). However, most of these are directed at assessing regulations to restrict the use of indoor tanning devices, with or without education campaigns warning about the dangers of sunbeds, while fewer have covered health promotion of sun protection behaviours. One economic study also assessed genetic testing and provision of genomic risk information to persons to improve their sun protection behaviours, but this departs from a whole population approach (Law et al., 2023). For public health campaign evaluations, findings showed that in Belgium every €1 invested returned €3.60 (Pil et al., 2016), and in Denmark every €1 invested returned €2.18 (Køster et al., 2020). In a US study targeting construction workers and involving provision of shade structures and personal protective equipment, every US$1 invested returned $0.49 and $0.35, respectively (Mofidi et al., 2021).
This study is limited due to several factors. Health promotion programs are difficult to evaluate as they can have multiple and long-term outcomes perhaps only tenuously linked to the program. This requires a program logic model that reasonably attributes observational data to outcomes. WA data were not easily available for some data inputs and therefore required the use of other Australian sources. We were not able to perform health equity analyses breaking down the data by socio-economic outcomes as data inputs precluded this. Potentially, the benefits will be even greater than reported here if health promotion efforts can lift sun protection uptake in socially and economically disadvantaged populations (i.e. through tailored intervention), who have different challenges in engaging in health promotion activities (Coupe et al., 2018). Against these limitations, we have constructed a comprehensive model, used the latest data, employed a societal perspective and had strong effectiveness evidence from long-term follow-up of a pragmatic randomized controlled trial. Furthermore, our model more accurately reflects very high treatment costs for advanced-stage melanomas which previous studies do not. Further interventional and economic research would be valuable in evaluating organizational policies or financial incentives to promote sun protection and policies to lower costs in sun-protection markets (e.g. sunscreen, sun shade materials) to improve access and uptake.
CONCLUSIONS
Compared with no program, investment in a WA SunSmart program is expected to prevent 13 728 KCs, 636 melanomas and 46 melanoma deaths per 100 000 population. The return on investment to the WA Government is $8.70 for every $1 invested in WA SunSmart.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the past and present Cancer Council WA staff involved in delivery of the SunSmart program in Western Australia.
Contributor Information
Louisa G Collins, Population Health Department, QIMR Berghofer Medical Research Institute, Brisbane, 300 Herston Rd, Herston QLD 4006, Queensland, Australia; Faculty of Medicine, The University of Queensland, Brisbane, Queensland, 20 Weightman St, Herston QLD 4006, Australia; School of Nursing and Cancer and Palliative Care Outcomes Centre, Queensland University of Technology, Brisbane, Level 1, 420 Bagot Rd, Subiaco WA 6008, Queensland, Australia.
Carolyn Minto, Cancer Council Western Australia, Perth, Level 1, 420 Bagot Rd, Subiaco WA 6008, Western Australia, Australia.
Melissa Ledger, Cancer Council Western Australia, Perth, Level 1, 420 Bagot Rd, Subiaco WA 6008, Western Australia, Australia.
Sally Blane, Cancer Council Western Australia, Perth, Level 1, 420 Bagot Rd, Subiaco WA 6008, Western Australia, Australia.
Delia Hendrie, Curtin School of Population Health, Curtin University, Perth, Kent St, Bentley WA 6102, Western Australia, Australia.
AUTHOR CONTRIBUTIONS
L.G.C. conceived the evaluation model structure, constructed the model, analysed the data and drafted the manuscript. D.H. co-wrote the first draft of the manuscript and provided health economic oversight and expertise. C.M., M.L. and S.B. provided information on the WA SunSmart program and contributed to writing and editing the manuscript and data inputs. All authors contributed to editing and proofing the final version of the manuscript.
FUNDING
This work was supported by the Cancer Council Western Australia. The Western Australian Health Promotion Foundation (Healthway) and the Western Australian Department of Health have funded the implementation of the SunSmart program in Western Australia since 1991.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. The authors were responsible for all content and editorial decisions related to the research and development of this publication.
ETHICAL APPROVAL
Ethical approval was not required as the analysis involved available secondary data sources.
DATA AVAILABILITY
The data for this modelling study are from published sources and are available publicly or through academic subscriptions. The authors’ will share these publications upon request if required.
REFERENCES
- Australian Bureau of Statistics (ABS) (2023a) Consumer Price Index, Australia, March 2022. ABS, Canberra, Australia. [Google Scholar]
- Australian Bureau of Statistics (ABS) (2023b) National, State and Territory Population, June 2023. ABS, Canberra, Australia. [Google Scholar]
- Australian Institute of Health and Welfare (AIHW) (2022) Disease Expenditure in Australia 2019–20. AIHW, Canberra, Australia. [Google Scholar]
- Cancer Council Western Australia (2022) 2022 Summer Sun Protection Survey (Life in Australia) Analytical Report. Cancer Council Western Australia, Perth, Western Australia, Australia. [Google Scholar]
- Cancer Council Victoria and Victorian Cancer Registry. (2023) Melanoma Fact Sheet. https://www.cancervic.org.au/research/vcr/cancer-fact-sheets/melanoma.html#melanoma-distribution-by-stage-at-diagnosis Cancer Council Victoria, Melbourne. [Google Scholar]
- Carter, H. E., Schofield, D. J. and Shrestha, R. (2016) The productivity costs of premature mortality due to cancer in Australia: Evidence from a microsimulation model. PLoS One, 11, e0167521, 10.1371/journal.pone.0167521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelak, K. and Chakole, S. (2023) The role of social determinants of health in promoting health equality: a narrative review. Cureus, 15, e33425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government Department of Health (2021) National Preventive Health Strategy 2021–2030. Australian Government, Canberra, Australia. [Google Scholar]
- Coupe, N., Cotterill, S. and Peters, S. (2018) Tailoring lifestyle interventions to low socio-economic populations: a qualitative study. BMC Public Health, 18, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington, S., Williams, G. and Neale, R. (2003). A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol, 139, 451–455. [DOI] [PubMed] [Google Scholar]
- Doran, C., Ling, R., Byrnes, J., Crane, M., Searles, A., Perez, D. et al. (2015) Estimating the economic costs of skin cancer in New South Wales, Australia. BMC Public Health, 16, 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran, C. M., Ling, R., Byrnes, J., Crane, M., Shakeshaft, A. P., Searles, A. et al. (2016) Benefit cost analysis of three skin cancer public education mass-media campaigns implemented in New South Wales, Australia. PLoS One, 11, e0147665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden, M., Hainsworth, R., Gordon, L. G., Epton, T., Lorigan, P., Rhodes, L. E. et al. (2022) Cost-effectiveness of a policy-based intervention to reduce melanoma and other skin cancers associated with indoor tanning. British Journal of Dermatology, 187, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, T. M., Gordon, L. G., Webb, A., Kift, R., Foeglein, A. and Neale, R. E. (2023) Making the sunshine vitamin—how much sun exposure is needed to maintain 25-hydroxy vitamin D concentration? Photochemistry and Photobiology, 100, 746–755. [DOI] [PubMed] [Google Scholar]
- Eskander, A., Marqueen, K. E., Edwards, H. A., Joshua, A. M., Petrella, T. M., de Almeida, J. R. et al. (2021) To ban or not to ban tanning bed use for minors: a cost-effectiveness analysis from multiple US perspectives for invasive melanoma. Cancer, 127, 2333–2341. [DOI] [PubMed] [Google Scholar]
- Garcia-Mochon, L., Joan Rovira, F. and Espin, J. (2021) Cost transferability problems in economic evaluation as a framework for an European health care and social costs database. Cost Effectiveness and Resource Allocation, 19, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin, C., Sebaratnam, D. and Fernandez-Penas, P. (2015) Quality of life in non-melanoma skin cancer. The Australasian Journal of Dermatology, 56, 70–76. [DOI] [PubMed] [Google Scholar]
- Gershenwald, J., Scolyer, R., Hess, K., Sondak, V., Long, G., Ross, M. et al. (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: A Cancer Journal for Clinicians, 67, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, L. G., Elliott, T., Olsen, C., Pandeya, N. and Whiteman, D. C. (2018) Out-of-pocket medical expenses for Queenslanders with a major cancer. Med J Aust. (Research letter), 208(11), 497, 10.5694/mja17.00815 [DOI] [PubMed] [Google Scholar]
- Gordon, L. G., Leung, W., Johns, R., McNoe, B., Lindsay, D., Merollini, K. M. D., Elliott, T. M., Neale, R. E, Olsen, C. M., Pandeya, N. and Whiteman, D. C. (2022b). Estimated healthcare costs of melanoma and keratinocyte skin cancers in Australia and Aotearoa New Zealand in 2021. Intl J Environ Res Public Health, 2022b;19, 3178, 10.3390/ijerph19063178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, L., Olsen, C., Whiteman, D., Elliott, T., Janda, M. and Green, A. (2020a) Prevention versus early detection for long-term control of melanoma and keratinocyte carcinomas: a cost-effectiveness modelling study. BMJ Open, 10, e034388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, L., Shih, S., Watts, C., Goldsbury, D. and Green, A. (2022a) The economics of skin cancer prevention with implications for Australia and New Zealand. Public Health Research and Practice, 32, e31502119. [DOI] [PubMed] [Google Scholar]
- Gordon, L. G., Lindsay, D., Olsen, C. and Whiteman, D. W. (2023) Health utilities and health-related quality of life of patients with keratinocyte skin cancers. JEADV Clinical Practice, 2, 983–993. [Google Scholar]
- Gordon, L. G. and Rowell, D. (2015) Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. European Journal of Cancer Prevention, 24, 141–149. [DOI] [PubMed] [Google Scholar]
- Gordon, L. G., Sinclair, C., Cleaves, N., Makin, J. K., Rodriguez-Acevedo, A. J. and Green, A. C. (2020b) Consequences of banning commercial solaria in 2016 in Australia. Health Policy, 124, 665–670. [DOI] [PubMed] [Google Scholar]
- Green, A., Baade, P., Coory, M., Aitken, J. and Smithers, M. (2012) Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. Journal of Clinical Oncology, 30, 1462–1467. [DOI] [PubMed] [Google Scholar]
- Green, A. C., Hughes, M. C. B., Williams, G. M., Malt, M., Beesley, V. L., Khosrotehrani, K. and Smithers, B. M. (2022b) Increased melanoma recurrence in patients with multiple primary invasive melanomas. J Am Acad Dermatol, 86, 1366–1369, 10.1016/j.jaad.2021.05.025 [DOI] [PubMed] [Google Scholar]
- Green, A., Williams, G., Logan, V. and Strutton, G. (2011) Reduced melanoma after regular sunscreen use: randomised trial follow-up. Journal of Clinical Oncology, 29, 257–263. [DOI] [PubMed] [Google Scholar]
- Green, A., Williams, G., Neale, R., Hart, V., Leslie, D., Parsons, P. et al. (1999) Daily sunscreen application and beta carotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet, 354, 723–729. [DOI] [PubMed] [Google Scholar]
- Greinnert, R., Breitbart, E., Mohr, P. and Volkmer, B. (2014) Health initiatives for the prevention of skin cancer. In Reichrath, J. (ed.), Sunlight, Vitamin D and Skin Cancer, Springer, New York, NY, pp. 485–499. [DOI] [PubMed] [Google Scholar]
- Guy, G. P., Jr, Zhang, Y., Ekwueme, D. U., Rim, S. H. and Watson, M. (2017) The potential impact of reducing indoor tanning on melanoma prevention and treatment costs in the United States: an economic analysis. Journal of the American Academy of Dermatology, 76, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A., Hill, S., Chin, G., Li, J. and Walkom, E. (2008) The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994-2004. Medical Decision Making, 28, 713–722. [DOI] [PubMed] [Google Scholar]
- Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A., Carswell, C. et al. (2022) Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. British Medical Journal, 376, e067975.35017145 [Google Scholar]
- Independent Hospital Pricing Authority (2020) National Hospital Cost Data Collection Report, Public Sector, Round 22 (Financial Year 2017–18). Sydney, New South Wales, Australia, Australian Government. [Google Scholar]
- Janda, M., Youl, P., Neale, R., Aitken, J., Whiteman, D., Gordon, L. and Baade, P. (2014) Clinical skin examination outcomes following a video delivered behavioural intervention: analysis from the randomised controlled skin awareness trial. JAMA Dermatol, 150, 372–379, 10.1001/jamadermatol.2013.9313 [DOI] [PubMed] [Google Scholar]
- Køster, B., Meyer, M. K. H., Søgaard, J. and Dalum, P. (2020) Benefit-cost analysis of the Danish Sun Safety Campaign 2007-2015: cost savings from sunburn and sunbed use reduction and derived skin cancer reductions 2007-2040 in the Danish population. PharmacoEconomics – Open, 4, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, C. K., Cust, A. E., Smit, A. K., Trevena, L., Fernandez-Penas, P., Nieweg, O. E. et al.; Managing Your Risk Study Group. (2023) Long-term cost-effectiveness of a melanoma prevention program using genomic risk information compared with standard prevention advice in Australia. Genetics in Medicine, 25, 100970. [DOI] [PubMed] [Google Scholar]
- Mofidi, A., Tompa, E., Song, C., Tenkate, T., Arrandale, V., Jardine, K. J. et al. (2021) Economic evaluation of interventions to reduce solar ultraviolet radiation (UVR) exposure among construction workers. Journal of Occupational and Environmental Hygiene, 18, 250–264. [DOI] [PubMed] [Google Scholar]
- Montague, M., Borland, R. and Sinclair, C. (2001) Slip! Slop! Slap! and SunSmart, 1980–2000: skin cancer control and 20 years of population-based campaigning. Health Education and Behavior, 28, 290–305. [DOI] [PubMed] [Google Scholar]
- Olsen, C. M., Pandeya, N., Green, A. C., Ragaini, B. S., Venn, A. J. and Whiteman, D. C. (2022) Keratinocyte cancer incidence in Australia: a review of population-based incidence trends and estimates of lifetime risk. Public Health Research and Practice, 32, 3212203. [DOI] [PubMed] [Google Scholar]
- Pandeya, N., Olsen, C. and Whiteman, D. (2017) The incidence and multiplicity rates of keratinocyte cancers in Australia. Medical Journal of Australia, 207, 339–343. [DOI] [PubMed] [Google Scholar]
- Pandeya, N., Purdie, D., Green, A. and Williams, G. (2005) Repeated occurrence of basal cell carcinoma of the skin and multifailure survival analysis: follow-up data from the Nambour Skin Cancer Prevention Trial. American Journal of Epidemiology, 161, 748–754. [DOI] [PubMed] [Google Scholar]
- Peraral, E. (2014) Characteristics of the SunSmart program with reference to health education and health promotion concepts. Global Dermatology, 1, 24–26. [Google Scholar]
- Pil, L., Hoorens, I., Vossaert, K., Kruse, V., Tromme, I., Speybroeck, N. et al. (2016) Burden of skin cancer in Belgium and cost-effectiveness of primary prevention by reducing ultraviolet exposure. Preventive Medicine, 93, 177–182. [DOI] [PubMed] [Google Scholar]
- Sanders, G., Newmann, P., Bau, A., Brock, D., Feeny, D., Krahn, M. et al. (2016) Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Journal of the American Medical Association, 316, 1093–1103. [DOI] [PubMed] [Google Scholar]
- Shih, S., Carter, R., Heward, S. and Sinclair, C. (2017) Economic evaluation of future skin cancer prevention in Australia. Preventive Medicine, 99, 7–12. [DOI] [PubMed] [Google Scholar]
- Tran, A., Fogarty, G., Nowak, A., Espinoza, D., Rowbotham, N., Stockler, M. et al. (2018) A systematic review and meta-analysis of utility estimates in melanoma. British Journal of Dermatology, 178, 384–393. [DOI] [PubMed] [Google Scholar]
- Turner, H., Archer, R., Downey, L., Isaranuwatchai, W., Chalkidou, K., Jit, M. et al. (2021) An introduction to the main types of economic evaluations used for informing priority setting and resource allocation in healthcare: key features, uses, and limitations. Frontiers in Public Health, 9, 722927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, K., Mehrmal, S., Uppal, P., Giesey, R. L. and Delost, G. R. (2021) The global burden of skin cancer: a longitudinal analysis from the Global Burden of Disease Study, 1990-2017. JAAD International, 2, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pols, J., Williams, G., Neale, R., Clavarino, A. and Green, A. (2006a) Long-term increase in sunscreen use in an Australian community after a skin cancer prevention trial. Preventive Medicine, 42, 171–176. [DOI] [PubMed] [Google Scholar]
- van der Pols, J., Williams, G., Pandeya, N., Logan, V. and Green, A. (2006b) Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiology, Biomarkers & Prevention, 15, 2546–2548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this modelling study are from published sources and are available publicly or through academic subscriptions. The authors’ will share these publications upon request if required.