Key Points
Question
Is HSK16149, a selective ligand of the a2δ subunit of voltage-gated calcium channels, effective and safe for Chinese patients with diabetic peripheral neuropathic pain (DPNP)?
Findings
In this randomized, adaptive phase 2 to 3 clinical trial of 725 patients who were randomly assigned to receive HSK16149 in doses of 40 mg/d, 80 mg/d, 120 mg/d, or 160 mg/d, pregabalin in a dose of 300 mg, or placebo, the efficacy of HSK16149 (40 and 80 mg/d) was superior to placebo in all groups for relieving DPNP and appeared well tolerated.
Meaning
These findings suggest that 40- and 80-mg/d HSK16149 can be considered as new interventional therapies for Chinese patients with DPNP.
This randomized, adaptive phase 2 to 3 clinical trial assesses the efficacy and safety of HSK16149 capsules, an oral γ-aminobutyric acid (GABA) analogue, to treat patients with diabetic peripheral neuropathic pain.
Abstract
Importance
Many patients with diabetic peripheral neuropathic pain (DPNP) experience inadequate relief, despite best available medical treatments. There are no approved and effective therapies for patients with DPNP in China.
Objective
To evaluate the efficacy and safety of capsules containing γ-aminobutyric acid (GABA) analogue HSK16149 in the treatment of Chinese patients with DPNP.
Design, Setting, and Participants
This phase 2 to 3 adaptive randomized clinical trial was multicenter, double blind, and placebo and pregabalin controlled. The trial started on December 10, 2020, and concluded on July 8, 2022. In stage 1, various doses of HSK16149 were evaluated to determine safety and efficacy for stage 2. The second stage then validated the efficacy and safety of the recommended dose.
Intervention
In stage 1, enrolled patients (n = 363) were randomized 1:1:1:1:1:1 to 4 HSK16149 doses (40, 80, 120, or 160 mg/d), pregabalin (300 mg/d), or placebo. In stage 2, patients (n = 362) were randomized 1:1:1 to receive HSK16149, 40 or 80 mg/d, or placebo. The final efficacy and safety analysis pooled data from patients receiving the same treatment.
Main Outcomes and Measures
The primary efficacy end point in stage 1 was the change from baseline in average daily pain score (ADPS) at week 5. The primary efficacy end point in stage 2 was the change from baseline in ADPS at week 13. When the final statistical analysis was performed, the P values calculated from the independent data of each phase were combined using the weighted inverse normal method to make statistical inferences.
Results
Of 725 randomized patients in the full-analysis set (393 men [54.2%]; mean [SD] age, 58.80 [9.53] years; 700 [96.6%] of Han Chinese ethnicity), 177 received placebo; 178, HSK16149, 40 mg/d; 179, HSK16149, 80 mg/d; 66, HSK16149, 120 mg/d; 63, HSK16149, 160 mg/d; and 62, pregabalin, 300 mg/d. A total of 644 patients (88.8%) completed the study. The 40- and 80-mg/d doses of HSK16149 were recommended in stage 2. At week 13, the ADPS mean (SD) change from baseline was −2.24 (1.55) for the 40-mg/d and −2.16 (1.79) for 80-mg/d groups and −1.23 (1.68) for the placebo group, showing statistical significance for both HSK16149 doses vs placebo (both P < .001). In a safety set (n = 726), 545 patients (75.1%) had adverse events, which were generally mild to moderate, with dizziness and somnolence being the most common.
Conclusions and Relevance
Forty- and eighty-mg/d doses of HSK16149 were recommended for treating patients with DPNP in China. The efficacy of HSK16149 capsules was superior to placebo in all groups for relieving DPNP and appeared well tolerated.
Trial Registration
ClinicalTrials.gov Identifier: NCT04647773
Introduction
Diabetes has become a leading public health problem globally. The International Diabetes Federation Diabetes Atlas reports that the global crude prevalence of diabetes among people aged 20 to 79 years was 10.5% in 2021 and is projected to be 11.3% in 2030 and 12.2% in 2045.1 China has the most adult patients with diabetes aged 20 to 79 years, totaling 140.9 million.1 Diabetic peripheral neuropathic pain (DPNP) frequently manifests as symmetric peripheral neuropathic pain with a predominance of distal limb involvement.2 About 50% of individuals with diabetes and roughly 13% of people with impaired glucose tolerance experience pain, a typical clinical sign of DPNP.2,3 About 39% of patients with diabetes and DPNP are untreated.4 Given the vast number of patients with diabetes in China, it is anticipated that the prevalence of DPNP is substantial and negatively impacts the quality of life for most patients with diabetes.
The clinical treatment of DPNP is mainly based on the control of blood glucose level, and the pain can be relieved by the individualized use of analgesic drugs, combined treatment, and sufficient treatment course. Pregabalin is first-line systemic treatment for DPNP recommended by the American Academy of Neurology.4 In January 2019, the Pharmaceutical and Medical Devices Agency of Japan approved the new oral γ-aminobutyric acid (GABA) analogue substance mirogabalin for the treatment of DPNP. However, neither pregabalin nor mirogabalin has been approved for marketing in China.
The HSK16149 capsule is an oral GABA analogue developed by Haisco Pharmaceutical Group Co, Ltd, that binds to the calcium channel α2δ subreceptors α2δ-1 and α2δ-2 and reduces the inward flow of calcium ions from voltage-dependent calcium channels in the central nervous system, thereby reducing glutamate and norepinephrine levels, in addition to having antiepileptic, analgesic, and antianxiety properties. Compared with pregabalin, HSK16149 binds more strongly to α2δ subreceptors and is expected to have better analgesic efficacy. This double-blind, placebo- and pregabalin-controlled multicenter randomized clinical trial, designed with a phase 2 to 3 adaptive approach, assessed the efficacy and safety of HSK16149 in patients with DPNP in China.
Methods
Study Design
This study used an adaptive design to seamlessly link phases 2 and 3 together.5,6 The study consisted of 2 stages, with the first stage designed to initially assess the efficacy and safety of different doses of HSK16149 and recommend the optimal dose of HSK16149 that was safe and effective to enter the second stage study. The second stage was designed to confirm the efficacy and safety of the recommended dose of HSK16149 for DPNP in the first stage. The comprehensive trial protocol and statistical analysis plan can be found in Supplement 1. Overall design is shown in Figure 1, and a detailed study design is included in eMethods 1 in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The study was approved by the ethics committees or institutional review boards of the leading unit (the First Hospital of Peking University, Beijing, China) and other subcenters. Written informed consent was obtained from individual participants. The trial started on December 10, 2020, and concluded on July 8, 2022.
Figure 1. Study Design.
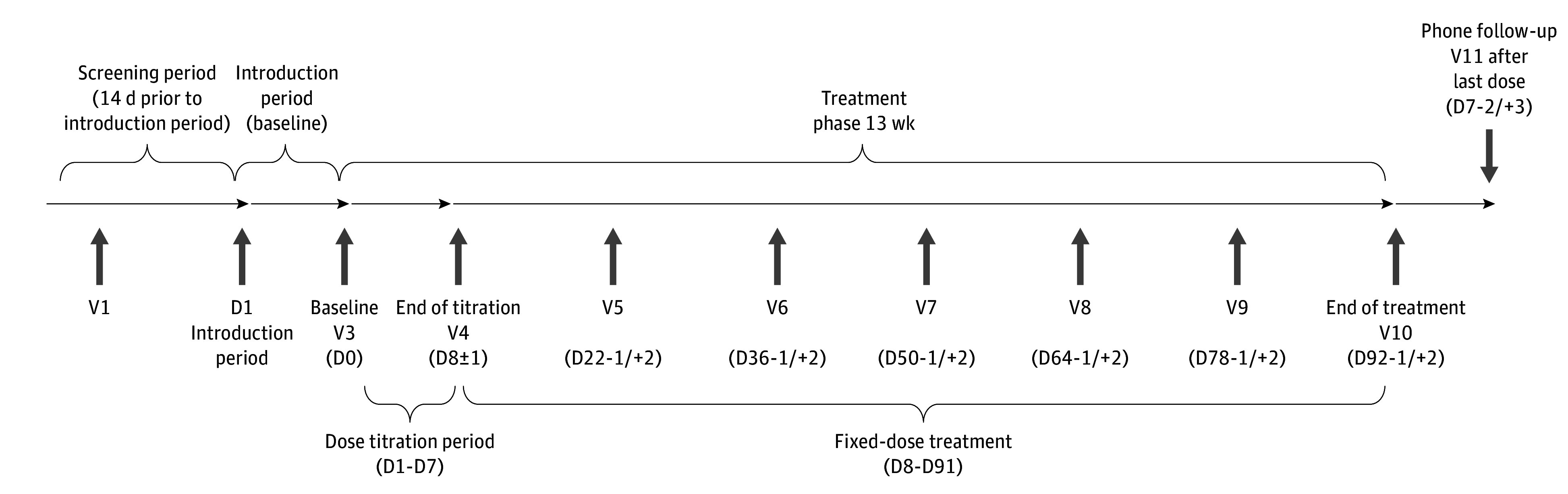
D indicates day; V, visit. Visit time windows refer to the setting of a visit period, within which the completion of the relevant test operations specified for this visit are all in accordance with the requirements of the study protocol, which contributes to the smooth and scientific conduct of the clinical study.
Randomization for this study was performed using the Interactive Response Technology system, which automatically randomly assigned treatment groups according to randomization numbers based on the principle of block group randomization. In the first stage, enrolled patients were randomized 1:1:1:1:1:1 to receive HSK16149, 40 mg/d (20 mg twice daily), 80 mg/d (40 mg twice daily), 120 mg/d (60 mg twice daily), or 160 mg/d (80 mg twice daily), placebo (twice daily), and pregabalin, 300 mg/d (150 mg twice daily). An interim analysis of safety and efficacy was conducted by an independent statistician after approximately 360 patients had completed 5 weeks of study treatment. The results were submitted to the independent data monitoring committee for further review, which then recommended the optimal dose of HSK16149 for stage 2. Enrollment for stage 1 of the study continued during the interim analysis, and patients who were enrolled continued to receive their designated treatment. Additionally, they underwent a comprehensive safety assessment 7 days after the final administration of the trial drugs. The sponsor, investigators, and patients remained blinded throughout the study period.
In the second stage, patients were randomized to receive 1 of the 2 recommended HSK16149 doses or placebo on a 1:1:1 basis. Simultaneously, enrollment in the stage 1 study was discontinued, and participants already enrolled continued to receive the assigned study treatment, completing a safety follow-up visit 7 days after the final administration of the trial drugs. At the end of the study, data from the same treatment of patients were consolidated for final efficacy and safety analysis.
Patients
Eligible patients were those aged 18 to 75 years with a diagnosis of diabetes who met the following criteria: a definite history of diabetes; neuropathy present at or after the time of the diagnosis of diabetes; clinical signs and symptoms consistent with the presentation of DPNP; stable glycemic control within 3 months prior to screening; and mean pain visual analog scale (VAS) score of at least 40 mm and less than 90 mm in the past 24 hours assessed at screening. A detailed study participant inclusion and exclusion criteria is included in the eMethods 2 in Supplement 2.
Sample Size and Randomization
Based on a comprehensive analysis of the results related to previous clinical studies of similar drugs, the difference in change from baseline in ADPS between the test group and the placebo group at 13 weeks of study treatment was conservatively estimated to be 0.6, with an SD taken as 1.8, and a 1-sided significance level of α = .025.7 Each dose group in stage 1 was referenced to 60 cases per group for the purpose of dose, for a total of 360 cases in the 6 groups. Stage 1 was analyzed at midterm when approximately 360 participants (6 dose groups, approximately 60 enrolled in each group) had completed 5 weeks of study treatment. Based on the results of the midterm analysis, the 2 HSK16149 dose groups and the placebo group were recommended for stage 2 trial. The sample size for stage 2 was estimated as the percentage of cases entering the stage 2 dose groups that reached the predicted stage 2 sample size (eg, 50% of the predicted sample size will be reached).
Outcomes
The primary efficacy end point in stage 1 was the change from baseline in ADPS at week 5 of treatment with the test drug. The primary efficacy end point in stage 2 was the change from baseline in ADPS at week 13 of treatment with the test drug. The change from baseline in weekly ADPS from weeks 1 to 13 was analyzed using a repeated-measures analysis of variance, a repeated-measures mixed-effects model based on the restricted maximum likelihood estimation method, with baseline ADPS (<6 and ≥6 points) as covariates and independent variables including treatment group, time, and interaction between time and treatment group. The difference between the test and placebo groups was evaluated by calculating the mean difference in the change in ADPS from baseline between groups and its 95% CI. As for sensitivity analysis, after P values from 2 stages were combined, 3 sensitivity analyses based on how the missing week 13 ADPS was calculated were performed, that is, based on the last observation carried forward method for filling in the analysis on the full-analysis set (FAS), and based on the unfilled data in the analysis on FAS.
Secondary efficacy end points in stage 1 were ADPS response rates at week 5 of treatment with the experimental drug (proportion of patients with ≥30% and ≥50% reduction in ADPS from baseline at week 5). Secondary efficacy end point in stage 2 included change from baseline in ADPS per week during the study treatment period; ADPS response rate at week 13; change from baseline in VAS scores at week 13; change from baseline in average daily sleep interference score (ADSIS) at week 13; change from baseline in the short form McGill Pain Questionnaire (SF-MPQ) scores at week 13; change from baseline in 5-level EQ-5D (EQ-5D-5L) scores at week 13; and change from baseline in the Patient Global Impression of Change (PGIC) scores at week 13. Safety indicators included adverse events (AE), laboratory tests, physical examination results, vital signs, 12-lead electrocardiogram, and neurological examination results during the study period. Subgroup analyses were performed for patients who had used acetaminophen during and 2 weeks before baseline and 2 weeks before the 13-week visit in the FAS.
Statistical Analysis
This trial used a seamless phase 2 to 3 design, primarily using the approach described by Friede and Kieser.8 In the midterm analysis, each test group was compared with the control group based on the short-term primary efficacy index data, and the test group with the largest number of statistics was selected for the confirmatory phase. When the final statistical analysis was performed, the P values calculated from the independent data of each phase were combined using the weighted inverse normal method to make statistical inferences. The method proposed by Friede and Kieser8 was used to test whether the HSK16149 dose selected was superior to placebo in terms of the primary efficacy index or key secondary efficacy end points. The method includes a many-to-one comparison test, blocked test, and combination test method.8 The FAS set was used as a sensitivity analysis for the change from baseline in ADPS at week 13. All secondary efficacy metrics were tested for differences in efficacy between the 2 selected trial groups and the placebo group using the Bonferroni correction (1-sided α = .0125 significance level). We used SAS, version 9.4 (SAS Institute, Inc), for the statistical analysis of this study. A detailed description of the statistical analysis is included in the eMethods 3 in Supplement 2.
Results
Patient Characteristics
As shown in Figure 2 and eTable 1 in Supplement 2, a total of 998 patients from 53 study centers were screened in this study; 770 were successfully enrolled; and 729 were successfully randomized and assigned to receive HSK16149, 40 mg/d (n = 179), HSK16149, 80 mg/d (n = 180), HSK16149, 120 mg/d (n = 66), HSK16149, 160 mg/d (n = 64), placebo (n = 178), or pregabalin, 300 mg/d (n = 62). A total of 644 patients (88.8%) completed the study, including 159 receiving HSK16149, 40 mg/d, 163 receiving HSK16149, 80 mg/d, 53 receiving HSK16149, 120 mg/d, 50 receiving HSK16149, 160 mg/d, 164 receiving placebo, and 55 receiving pregabalin.
Figure 2. Patient Disposition.
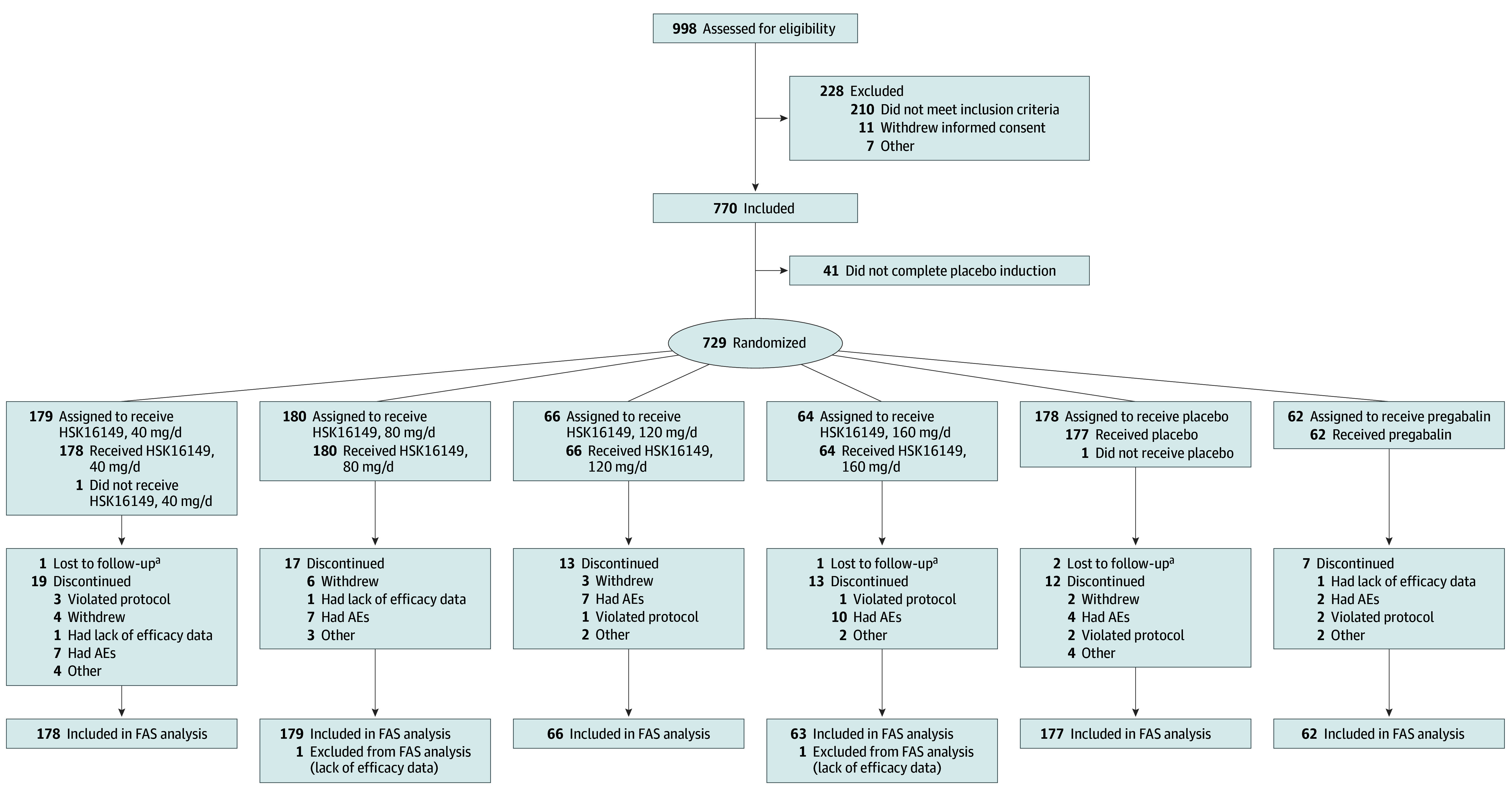
AE indicates adverse event; FAS, full analysis set.
aParticipant could not be reached during multiple telephone calls.
Based on the FAS (n = 725), demographic characteristics of the participants are summarized in Table 1. A total of 393 patients were men (54.2%) and 332 were women (45.8%). The mean (SD) age of the 725 patients was 58.80 (9.53) years. The mean (SD) height of the 725 patients was 164.72 (8.28) cm. The mean weight (SD) of the 724 patients with available data was 66.66 (11.76) kg. The mean (SD) body mass index (calculated as the weight in kilograms divided by the height in meters squared) of the 724 patients with available data was 24.49 (3.39). In the FAS, the demographic variables were largely consistent across groups. A total of 713 patients had medical history and current diagnoses. The baseline characteristics of patients’ medical history and present diagnoses were largely comparable between groups (eTable 2 in Supplement 2).
Table 1. Demographic Characteristics Summary in Full Analysis Set.
| Characteristic | Treatment group | All (n = 725) | |||||
|---|---|---|---|---|---|---|---|
| HSK16149, 40 mg/d (n = 178) | HSK16149, 80 mg/d (n = 179) | HSK16149, 120 mg/d (n = 66) | HSK16149, 160 mg/d (n = 63) | Placebo (n = 177) | Pregabalin, 300 mg/d (n = 62) | ||
| Age, mean (SD), y | 58.7 (9.9) | 58.6 (9.4) | 60.5 (6.9) | 58.3 (8.7) | 59.3 (9.7) | 56.7 (11.2) | 58.8 (9.5) |
| Sex, No. (%) | |||||||
| Men | 94 (52.8) | 100 (55.9) | 31 (47.0) | 32 (18.1) | 101 (57.1) | 35 (56.5) | 393 (54.2) |
| Women | 84 (47.2) | 79 (44.1) | 35 (53.0) | 31 (49.2) | 76 (42.9) | 27 (43.5) | 332 (45.8) |
| Weight, mean (SD), kg | 66.81 (13.28) | 67.14 (11.21) | 65.08 (11.48) | 65.73 (10.33) | 67.42 (11.13) | 65.29 (12.14) | 66.66 (11.76) |
| Height, mean (SD), cm | 164.87 (8.91) | 165.39 (7.84) | 164.61 (8.36) | 163.05 (7.31) | 164.67 (8.51) | 164.25 (7.84) | 164.72 (8.28) |
| BMI, mean (SD) | 24.46 (3.74) | 24.47 (3.34) | 23.90 (2.96) | 24.65 (2.89) | 24.80 (3.12) | 24.19 (4.08) | 24.49 (3.39) |
Abbreviation: BMI, body mass index (calculated as the weight in kilograms divided by the height in meters squared).
Primary Efficacy End Points
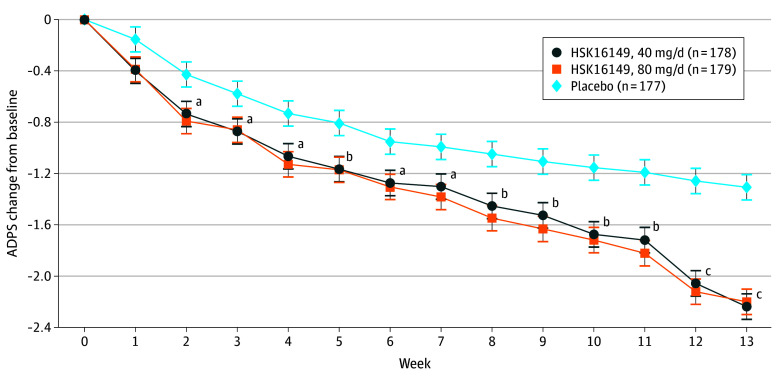
The primary efficacy end point in stage 1 was the change from baseline in ADPS at week 5 of treatment with the test drug. In FAS, at week 5, mean (SD) change from baseline was −1.12 (1.19) in the 40-mg/d group (n = 166), −1.13 (1.32) in the 80-mg/d group (n = 167), −1.03 (1.28) in the 120-mg/d group (n = 59), and −1.13 (1.16) in the 160-mg/d group (n = 51) for those receiving HSK16149, −0.75 (1.15) in the placebo group (n = 171), and −1.02 (1.12) in the pregabalin group (n = 59). The primary efficacy end point in stage 2 was the change from baseline in ADPS at week 13 of treatment with the test drug. At week 13, the mean (SD) ADPS change from baseline were −2.24 (1.55) in the 40-mg/d group (n = 157), −2.16 (1.79) in the 80-mg/d group (n = 161), −2.03 (1.69) in the 120-mg/d group (n = 53), and −2.15 (1.79) in the 160-mg/d group (n = 50) among those receiving HSK16149, −1.23 (1.68) in the placebo group (n = 165), and −2.09 (1.57) in the pregabalin group (n = 54) (eTable 3 in Supplement 2). All combined P values associated with the 40- and 80-mg/d doses of HSK16149 were less than .025; therefore the 40- and 80-mg/d doses were statistically significantly superior to placebo (Figure 3 and Table 2).
Figure 3. Average Daily Pain Score (ADPS) Change From Baseline, Weeks 1 to 13.
Baseline ADPS (<6 and ≥6) were used as covariables, and independent variables included treatment group, time, and interaction between time and treatment group. A repeated-measure mixed-effects model based on restricted maximum likelihood estimation was constructed to obtain the difference between groups and 95% CI (error bars).
aP < .05 compared with placebo.
bP < .01 compared with placebo.
cP < .001 compared with placebo.
Table 2. Mean Change in ADPS From Baseline at Week 13 (Full Analysis Set)a.
| Stage 1 hypothesis | P value | w1 | Stage 2 hypothesis | P value | w2 | Combined P value |
|---|---|---|---|---|---|---|
| HSK16149 study dose, 40 mg/d | ||||||
| H1234: p1234,1 | .60 | 0.58 | H1234: p12,2 | <.001 | 0.81 | <.001 |
| H123: p123,1 | .54 | 0.58 | H123: p12,2 | <.001 | 0.81 | <.001 |
| H124: p124,1 | .54 | 0.58 | H124: p12,2 | <.001 | 0.81 | <.001 |
| H134: p134,1 | .54 | 0.58 | H134: p1,2 | <.001 | 0.81 | <.001 |
| H12: p12,1 | .45 | 0.58 | H12: p12,2 | <.001 | 0.81 | <.001 |
| H13: p13,1 | .45 | 0.58 | H13: p1,2 | <.001 | 0.81 | <.001 |
| H14: p14,1 | .45 | 0.58 | H14: p1,2 | <.001 | 0.81 | <.001 |
| H1: p1,1 | .31 | 0.58 | H1: p1,2 | <.001 | 0.81 | <.001 |
| HSK16149 study dose, 80 mg/d | ||||||
| H1234: p1234,1 | .60 | 0.58 | H1234: p12,2 | <.001 | 0.81 | <.001 |
| H123: p123,1 | .54 | 0.58 | H123: p12,2 | <.001 | 0.81 | <.001 |
| H124: p124,1 | .54 | 0.58 | H124: p12,2 | <.001 | 0.81 | <.001 |
| H234: p234,1 | .70 | 0.58 | H234: p2,2 | <.001 | 0.81 | <.001 |
| H12: p12,1 | .45 | 0.58 | H12: p12,2 | <.001 | 0.81 | <.001 |
| H23: p23,1 | .62 | 0.58 | H23: p2,2 | <.001 | 0.81 | <.001 |
| H24: p24,1 | .61 | 0.58 | H24: p2,2 | <.001 | 0.81 | <.001 |
| H2: p2,1 | .51 | 0.58 | H2: p2,2 | <.001 | 0.81 | <.001 |
Abbreviation: ADPS, average daily pain score.
Missing week 13 ADPSs were calculated based on multiple fill (multiple imputation) for filling. H is statistical hypothesis test; p is the P value of test H; wj (j = 1 or 2) is the weight of the first and second stages given in advance, satisfying 0 ≤ wj ≤ 1 and w12 + w22 = 1 (w12 + w22 = 1: w12 plus w22 is equal to 1).
In the subgroup analyses, all combined P values associated with the 40- and 80-mg/d doses of HSK16149 were less than .025, supporting the statistically significant superiority of the 40- or 80-mg/d dose to placebo (eTable 4 in Supplement 2). In addition, stratified analyses according to age and DPNP disease course further showed that the mean (SD) ADPS change was significantly improved in the 40- and 80-mg/d HSK16149 dose groups (−2.38 [1.58 (P < .001)] and −1.89 [1.75 (P = .01)], respectively) vs the placebo group (−1.25 [1.69]) in subgroup analyses excluding participants who had a DPNP duration of less than 1 year. In addition, stratified analyses according to age and DPNP disease course further showed that the mean ADPS changes were significantly improved in the 40- and 80-mg/d HSK16149 dose groups vs the placebo group in each subgroup (eTables 5 and 6 in Supplement 2), especially in patients who were elderly (aged ≥65 years) or had a longer duration of DPNP (>1 year) and were treated with HSK16149, 80 mg/d.
The results of sensitivity analysis for ADPS change from baseline at week 13 were similar to the results of the primary analysis. The HSK16149 40- and 80-mg/d doses were statistically significantly superior to placebo in these sensitivity analyses (eTables 7 and 8 in Supplement 2).
Key Secondary Efficacy End Points
A summary of the weekly change from baseline in patients’ ADPS based on FAS is shown in eTable 9 in Supplement 2. Starting from week 8, there was a significant difference in the change from baseline between the HSK16149 40- and 80-mg/d groups and the placebo group in each week. As for ADPS response rate, at week 13, the proportion of responders with at least a 30% or at least a 50% decrease were significantly higher in the HSK16149 40-mg/d group (57.3% or 32.0%, respectively) and 80-mg/d group (51.4% or 36.3%, respectively) relative to the placebo group (31.6% or 18.1%, respectively) (eFigure in Supplement 2). The mean (SD) score changes from baseline at week 13 in the HSK16149 40-mg/d or 80-mg/d groups were significantly superior to placebo for ADSIS (−1.73 [1.48] and −1.73 [1.60], respectively)) and for VAS (−22.9 [16.83] and −23.0 [18.32], respectively) (P < .001 for both) (eTables 10 and 11 in Supplement 2). The mean (SD) changes in SF-MPQ scores (−3.50 [4.41]; P = .009) and the EQ-5D-5L scores (8.20 [17.27]; P = .008) were statistically improved in the HSK16149 40-mg/d group but not in the HSK16149 80-mg/d group (−3.10 [4.21 (P = .10)] and 6.70 [16.38 (P = .07)], respectively), compared with placebo (eTables 12 and 13 in Supplement 2). As for the rates of improvement in PGIC scores (slightly improved and above) and the improvement rate of SF-MPQ (PPI grade), the HSK16149 40-mg/d group showed improvement compared with placebo (eTables 14 and 15 in Supplement 2).
Safety Assessment
In the safety set (n = 726), 545 patients (75.1%) had 1391 AEs, and 536 (73.8%) had 1310 treatment-emergent AEs (TEAEs); 251 (34.6%) had 416 drug-related TEAEs; 51 (7.0%) had 63 grade 3 or higher TEAEs; and 6 (0.8%) had 6 grade 3 or higher drug-related TEAEs. Forty patients (5.5%) had 51 serious AEs (SAEs), of whom 36 (5.0%) had 47 treatment-emergent SAEs (TESAEs), and only 2 (0.3%) had 2 drug-related TESAEs, both of which were dizziness in patients in the HSK16149 80-mg/d and 160-mg/d groups (eTables 16 and 17 in Supplement 2).
The type and incidence of drug-related TEAEs did not differ significantly between groups, except for the incidence of dizziness and somnolence, which showed a dose correlation (eTable 18 in Supplement 2). Most TEAEs were not treated, and a few were treated with combined drug therapy or nondrug therapy. Most TEAEs were cured or improved, and a few TEAEs were persistent or unknown. No patients had TEAEs that resulted in death.
Discussion
In China, patients with DPNP face a lack of approved and effective therapies. This randomized phase 2 to 3 adaptive trial has shed light on the potential of HSK16149 (40 and 80 mg/d) for treating DPNP in Chinese patients. The primary efficacy assessment based on FAS revealed that HSK16149 (40 and 80 mg/d) exhibited statistically significant superiority over the placebo in mean change from baseline in ADPS at week 13 of treatment. Even after accounting for missing data through remedial measures, the study consistently demonstrated that HSK16149 (40 and 80 mg/d) maintained statistical superiority over the placebo.
Subgroup analysis, considering age and DPNP disease course, further highlighted the effectiveness of HSK16149 across all patient categories. Notably, the 80-mg/d treatment showed enhanced efficacy in elderly individuals (aged ≥65 years) or those with a prolonged DPNP duration (>1 year). Additionally, various secondary efficacy end points, including ADPS response rate, VAS score, ADSIS score, PGIC improvement rate, and SF-MPQ PPI grade improvement, affirmed the significant superiority of HSK16149 (40 and 80 mg/d) over the placebo in this study.
To our knowledge, this trial was the longest of its kind and the first large-scale randomized, double-blind investigation on DPNP conducted in China. Our study design was a seamless phase 2 to 3,9 with the first phase being a dose exploratory study, with a pregabalin positive control set up to inform dose selection, and the second phase of the confirmatory study being, according to the guidelines, a superiority design vs placebo. Despite pregabalin being recommended as the initial analgesic treatment for DPNP by most guidelines, limited evidence exists to certify the efficacy of pregabalin in Chinese patients with DPNP through large and double-blind randomized clinical trials. This study provides an initial demonstration of the effectiveness of pregabalin in treating patients with DPNP in China.
Another strength of this trial is its distinction as, to our knowledge, the only trial involving drugs for DPNP that does not use titration. In contrast to all previous trials with oral GABA analogues, including gabapentin, pregabalin, and mirogabalin, which involved titration, our trial with HSK16149 stands out for its unique approach. The decision to forgo titration in our trial was based on preclinical and phase 1 studies evaluating the safety, tolerability, and pharmacokinetics of HSK16149 in healthy patients in China.10 The data from these studies indicated that HSK16149 exhibited better safety in the central nervous system compared with pregabalin and was well tolerated in healthy Chinese patients. Consequently, in this study, we recommended target doses of 20 and 40 mg twice daily without titration. An exciting revelation from our study was that the efficacy of HSK16149 at 40 and 80 mg/d without titration surpassed that of the placebo in all groups, and the drug appeared to be well-tolerated. This novel method of drug administration is anticipated to bring significant convenience to both patients with DPNP and physicians involved in the treatment process.
HSK16149 is an oral GABA analogue, similar to pregabalin. In in vitro binding experiments,10 HSK16149 exhibited greater potency, binding to the α2δ subunit 23 times more effectively than pregabalin. The minimum effective dose of HSK16149 (10 mg/kg) was comparable in efficacy to 30 mg/kg of pregabalin.10 These findings collectively suggest that HSK16149 may act more rapidly and efficiently than pregabalin. Comparison of the change from baseline in ADPS at weeks 5 and 13 of treatment with HSK16149 and pregabalin revealed a continuous downward trend in ADPS. This suggests that HSK16149 may offer potential long-term benefits for patients with DPNP. However, further validation is needed through more extensive long-term clinical trials. A 52-week, open-label, multicenter trial assessing the efficacy and safety of HSK16149 in treating Chinese patients with DPNP has been completed,11 and the results of this long-term clinical trial may provide conclusive evidence that HSK16149 is a more potent and safer analgesic for patients with DPNP.
Our study also revealed significant superiority of HSK16149 (40 and 80 mg/d) over placebo across multiple secondary efficacy end points. However, a significant number of patients who did not respond positively to monotherapy may need to switch to different monotherapies or to combination treatments. Recent findings from a double-blind, randomized crossover trial in patients with DPNP12 suggested combination therapies, particularly pregabalin and amitriptyline, may provide better pain relief for patients who had inadequate pain control with monotherapy. Considering that HSK16149 shares the same target as pregabalin and offers certain advantages, it is conceivable that combining HSK16149 with other agents could enhance pain relief for patients unresponsive to monotherapy. However, confirming this hypothesis would necessitate multiple future clinical trials.
As for safety, most of the AEs in this trial were mild to moderate and resolved spontaneously without pharmacological or nonpharmacological treatment, and a few AEs required pharmacological or nonpharmacological treatment for improvement or recovery. Dizziness, sleepiness, peripheral edema, weight gain, nausea and vomiting, and malaise were the most frequent adverse responses to HSK16149. The most frequent adverse reaction types and their frequency were comparable to those of other comparable medications.13 HSK16149 (40 and 80 mg/d) had good overall safety that was tolerable. It is noteworthy that, based on this study, HSK16149 was approved in China this year for the treatment of DPNP. Research on HSK16149 for the treatment of postherpetic neuralgia has yielded promising results (Daying Zhang, PhD, unpublished data, 2024).
Limitations
This study has some limitations. We took advantage of an adaptive seamless phase 2 to 3 design to integrate the dose selection and confirmatory phases of drug development. To overcome potential bias of such an adaptive design, an independent statistician conducted an interim analysis of safety and efficacy in phase 2, and the results were submitted to an independent data monitoring committee for review and recommendation for dose selection in phase 3. The sponsor, investigators, and patients remained blinded throughout the study period. In addition, multiple statistical methods were used to control overall type I error rate.8
Conclusions
In this randomized clinical trial, HSK16149, 40 and 80 mg/d, were recommended doses for treating patients in China with DPNP. The efficacy of HSK16149 capsules was superior to placebo in all groups for relieving DPNP and appeared well tolerated.
Trial Protocol
eMethods 1. Study Design
eMethods 2. Selection of Study Population
eMethods 3. Statistical Analysis Plan
eFigure. The Responder Rates
eTable 1. All Participant Disposition
eTable 2. Summary of Medical History and Present Diagnoses by SOC and PT Classification With a Combined Incidence of ≥5.0%
eTable 3. Weekly ADPS Change From Baseline: FAS
eTable 4. Subgroup Analyses Performed for Participants Who Had Used Acetaminophen During and 2 Weeks Before Baseline and 2 Weeks Before the Week 13 Visit in the FAS
eTable 5. Week 13 ADPS Score Change From Baseline: FAS by Age
eTable 6. Week 13 ADPS Score Change From Baseline: FAS by DPNP Period
eTable 7. Week 13 ADPS Change From Baseline in Sensitivity Analysis: FAS
eTable 8. Week 13 ADPS Change From Baseline in Sensitivity Analysis: PPS
eTable 9. Key Secondary Efficacy End Points Weekly ADPS Change From Baseline: FAS
eTable 10. Week 13 ADSIS Score Change From Baseline: FAS
eTable 11. Week 13 VAS Score Change From Baseline: FAS
eTable 12. Week 13 SP-MPQ Score Change From Baseline: FAS
eTable 13. Week 13 EQ-5D-5L Score Change From Baseline: FAS
eTable 14. Week 13 PGIC Score: FAS
eTable 15. Week 13 SP-MPQ (PPI): FAS
eTable 16. Summary of All Adverse Events and Treatment-Emergent Adverse Events
eTable 17. Summary of Treatment-Period Adverse Events With a Combined Incidence of ≥1.0% by SOC and PT-SS
eTable 18. Summary of Treatment-Phase Adverse Events With a Combined Incidence of ≥ 1.0% by SOC and PT and Associated With the Investigational Drug-SS
Data Sharing Statement
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 10th ed. 2021. Accessed July 16, 2024. https://diabetesatlas.org/
- 2.Elafros MA, Andersen H, Bennett DL, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21(10):922-936. doi: 10.1016/S1474-4422(22)00188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40(6):828-849. doi: 10.1016/j.clinthera.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Bril V, England JD, Franklin GM, et al. ; American Academy of Neurology; American Asociation of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Evidence-based guideline: treatment of painful diabetic neuropathy–report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve. 2011;43(6):910-917. doi: 10.1002/mus.22092 [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375(1):65-74. doi: 10.1056/NEJMra1510061 [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Chow SC. Criteria for dose-finding in two-stage seamless adaptive design. J Biopharm Stat. 2019;29(5):908-919. doi: 10.1080/10543406.2019.1657130 [DOI] [PubMed] [Google Scholar]
- 7.Baba M, Matsui N, Kuroha M, Wasaki Y, Ohwada S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10(5):1299-1306. doi: 10.1111/jdi.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friede T, Kieser M. Blinded sample size recalculation for clinical trials with normal data and baseline adjusted analysis. Pharm Stat. 2011;10(1):8-13. doi: 10.1002/pst.398 [DOI] [PubMed] [Google Scholar]
- 9.Jiang L, Yuan Y. Seamless phase II/III design: a useful strategy to reduce the sample size for dose optimization. J Natl Cancer Inst. 2023;115(9):1092-1098. doi: 10.1093/jnci/djad103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gou X, Yu X, Bai D, et al. Pharmacology and mechanism of action of HSK16149, a selective ligand of α2δ subunit of voltage-gated calcium channel with analgesic activity in animal models of chronic pain. J Pharmacol Exp Ther. 2021;376(3):330-337. doi: 10.1124/jpet.120.000315 [DOI] [PubMed] [Google Scholar]
- 11.To Evaluate the Long-term Safety and Efficacy of HSK16149 in Chinese Patients With Peripheral Neuralgia. ClinicalTrials.gov identifier: NCT05890053. Updated May 24, 2023. Accessed July 16, 2024. https://clinicaltrials.gov/study/NCT05890053
- 12.Tesfaye S, Sloan G, Petrie J, et al. ; OPTION-DM trial group . Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680-690. doi: 10.1016/S0140-6736(22)01472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato J, Baba M, Kuroha M, et al. Safety and efficacy of mirogabalin for peripheral neuropathic pain: pooled analysis of two pivotal phase III studies. Clin Ther. 2021;43(5):822-835. doi: 10.1016/j.clinthera.2021.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Study Design
eMethods 2. Selection of Study Population
eMethods 3. Statistical Analysis Plan
eFigure. The Responder Rates
eTable 1. All Participant Disposition
eTable 2. Summary of Medical History and Present Diagnoses by SOC and PT Classification With a Combined Incidence of ≥5.0%
eTable 3. Weekly ADPS Change From Baseline: FAS
eTable 4. Subgroup Analyses Performed for Participants Who Had Used Acetaminophen During and 2 Weeks Before Baseline and 2 Weeks Before the Week 13 Visit in the FAS
eTable 5. Week 13 ADPS Score Change From Baseline: FAS by Age
eTable 6. Week 13 ADPS Score Change From Baseline: FAS by DPNP Period
eTable 7. Week 13 ADPS Change From Baseline in Sensitivity Analysis: FAS
eTable 8. Week 13 ADPS Change From Baseline in Sensitivity Analysis: PPS
eTable 9. Key Secondary Efficacy End Points Weekly ADPS Change From Baseline: FAS
eTable 10. Week 13 ADSIS Score Change From Baseline: FAS
eTable 11. Week 13 VAS Score Change From Baseline: FAS
eTable 12. Week 13 SP-MPQ Score Change From Baseline: FAS
eTable 13. Week 13 EQ-5D-5L Score Change From Baseline: FAS
eTable 14. Week 13 PGIC Score: FAS
eTable 15. Week 13 SP-MPQ (PPI): FAS
eTable 16. Summary of All Adverse Events and Treatment-Emergent Adverse Events
eTable 17. Summary of Treatment-Period Adverse Events With a Combined Incidence of ≥1.0% by SOC and PT-SS
eTable 18. Summary of Treatment-Phase Adverse Events With a Combined Incidence of ≥ 1.0% by SOC and PT and Associated With the Investigational Drug-SS
Data Sharing Statement