Abstract
Whether the human fetus and the prenatal intrauterine environment (amniotic fluid and placenta) are stably colonized by microbial communities in a healthy pregnancy remains a subject of debate. Here we evaluate recent studies that characterized microbial populations in human fetuses from the perspectives of reproductive biology, microbial ecology, bioinformatics, immunology, clinical microbiology and gnotobiology, and assess possible mechanisms by which the fetus might interact with microorganisms. Our analysis indicates that the detected microbial signals are likely the result of contamination during the clinical procedures to obtain fetal samples or during DNA extraction and DNA sequencing. Furthermore, the existence of live and replicating microbial populations in healthy fetal tissues is not compatible with fundamental concepts of immunology, clinical microbiology and the derivation of germ-free mammals. These conclusions are important to our understanding of human immune development and illustrate common pitfalls in the microbial analyses of many other low-biomass environments. The pursuit of a fetal microbiome serves as a cautionary example of the challenges of sequence-based microbiome studies when biomass is low or absent, and emphasizes the need for a trans-disciplinary approach that goes beyond contamination controls by also incorporating biological, ecological and mechanistic concepts.
Fetal immune development prepares the neonate for life in a microbial world and underpins lifelong health1-4. Neonates born at term are not immunologically naive and are specifically adapted to cope with abrupt exposure to microbial, dietary and environmental stimuli5,6. Several research groups have characterized immune cell development in human fetal tissues7-9. However, our mechanistic understanding of how and when immune priming by microorganisms occurs, and the factors that drive it, is incomplete. The long-held view that the prenatal intrauterine environment (placenta, amniotic fluid and fetus) is protected from live microorganisms10 has been recently challenged11-15, leading to the hypothesis that fetal immune development may be driven by the presence of live microorganisms at intrauterine sites16-20. Some groups have reported the presence of a microbiota13, defined as a community of microorganisms in a defined habitat, or a microbiome15, referring to a microbiota as well as their constituent genes and metabolites, which form a dynamic and interactive micro-ecosystem that is integrated within environments including eukaryotic hosts21. However, these interpretations have been debated22-28 because several concurrent studies29-35 suggest that contaminating microbial DNA in sequencing data from sites of low microbial biomass36-38 is likely to be the only source of microbial DNA detected in the intrauterine environment. Since 2020, four studies have characterized the microbiology of the human fetus directly, and these studies have come to opposing and irreconcilable conclusions. Two reports described viable low-density microbial populations in human fetal intestines39 and organs40, and linked these microorganisms to fetal immune development. By contrast, two other research groups, which include several of the authors of this perspective, reported no detectable microorganisms in the fetal meconium and intestines30,41.
Such disagreement over a fundamental aspect of human biology poses a challenge for scientific progress. The notion of a fetal microbiome, if proven correct, has implications for clinical medicine and would call for a comprehensive reappraisal of previous concepts and research. It would require a radical revision of our understanding of the development of the immune system and other systems in early life and the anatomical and immunological mechanisms that mediate host–microbe interactions within fetal tissues. Failure to resolve this issue risks diverting finite resources into research that results in no advancement for fetal and maternal health, and misguided attempts to therapeutically modify a non-existent fetal microbiome. The dilemma has further relevance for the characterization of the microbiota in other low-biomass samples, such as those derived from blood, the brain, other internal organs and cancer tissues. Therefore, we assembled a trans-disciplinary group of scientists and clinician scientists to examine experimental evidence relating to how and when the fetus becomes prepared for life with microorganisms, to identify research pitfalls and mitigation strategies, and to propose specific directions for future research.
Claims and counterclaims
Although disagreement over the presence of microorganisms in prenatal intrauterine locations (placenta and amniotic fluid) spans dozens of studies with contradictory findings12,14,15,23,29,31-34,37,42-44, we focused our analysis on four recent studies, because they provide a direct assessment of the fetus itself30,39-41. Collecting human fetal samples is difficult and can only occur after the termination of a pregnancy, or immediately before birth by C-section. Three of the studies used samples collected after vaginally delivered, elective, second-trimester pregnancy terminations39-41, and one collected samples from breech C-section deliveries immediately at birth30.
Rackaityte et al.39 reported that 18 bacterial taxa were enriched in the intestinal contents of vaginally delivered fetuses from second-trimester terminations compared to negative controls using 16S rRNA gene amplicon sequencing (V4 region). To account for contamination, the authors removed operational taxonomic units (OTUs) that were detected in more than 50% of procedural controls, and then identified remaining contaminants in silico (using the decontam R package). They found that most fetal samples were microbiologically similar to negative controls (labelled as ‘other meconium’; n = 25), but that some samples, dominated by Lactobacillus (six samples) or Micrococcaceae (nine samples), had distinct bacterial profiles. The authors also detected low amounts of total bacteria by quantitative PCR (qPCR), fluorescent in situ hybridization (FISH), scanning electron microscopy (SEM) and culture (as discussed below).
Several of the study’s conclusions have been challenged by de Goffau et al.45, who reanalysed the publicly available data and found no evidence for a distinct bacterial profile in the subset of samples with matched procedural controls, and concluded that the positive findings were caused by a sequencing batch effect (indicative of contamination) and further contamination during culture45. In addition, the suggestion that particles detected in SEM micrographs constitute micrococci39 was disputed, as their size exceeded that of known Micrococcaceae45. Furthermore, the 16S rRNA gene sequence of the Micrococcus luteus cultured from the fetal samples differed from that detected by sequencing, further supporting contamination during culture (M. luteus is a common contaminant of clean rooms and surgical instruments46,47).
Mishra et al.40 detected a low but consistent microbial signal across tissues of vaginally delivered fetuses from second-trimester terminations by 16S rRNA gene amplicon sequencing (V4–V5 region), with seven genera enriched in fetal samples (Lactobacillus, Staphylococcus, Pseudomonas, Flavobacterium, Afipia, Bradyrhizobium and Brevundimonas). The 16S rRNA gene-sequencing data were accompanied by SEM, RNA-in situ hybridization (RNA-ISH) and culture. In recognition of the high risk of contamination, all samples were processed in isolation with negative controls collected during sample processing. In contrast to Rackaityte et al., Mishra et al. found that Micrococcus was enriched in phosphate-buffered saline (PBS) reagent controls, and reported it as a contaminant, with the M. luteus cells detected by culture being consistent with the size and morphology of the coccoid structures that were found by SEM40.
Both Rackaityte et al. and Mishra et al. included assays of fetal immune development and concluded that the microorganisms detected could contribute to immune maturation. In Rackaityte et al.39, this conclusion was based on differences in T cell composition and epithelial transcription between fetal intestines in which Micrococcaceae were observed to be dominant and those in which this taxon was absent, leading to the suggestion that bacterial antigens contribute to T cell activation and immunological memory in utero. Mishra et al.40 used flow cytometry to expand on previous findings of effector (TNF- and IFNγ-producing) memory (CD45RO+) T cells in fetal tissues, including gut tissue and mesenteric lymph nodes. Bacterial isolates cultured from the fetal samples, including Staphylococcus and Lactobacillus strains, induced in vitro activation of memory T cells isolated from fetal mesenteric lymph nodes.
In contrast to these reports, Li et al.41, who also investigated fetal intestinal tissue from second-trimester terminations, did not detect bacterial DNA by PCR (V4 region of the 16S rRNA gene, 35 cycles) on the basis of a visual inspection of agarose gels in any of the 101 samples tested. The authors detected a diverse set of microbially derived metabolites that were present and enriched in the fetal intestinal samples, and hypothesized that these microbiota-derived metabolites are passed via the mother’s blood through the placenta to ‘educate’ the fetal immune system. This conclusion is supported by research in mice that showed that fetal immune education can be driven in the absence of direct microbial exposure by trans-placental passage of microbial metabolites originating from the maternal gut48,49.
Kennedy et al.30 used a different approach and collected samples using rectal swabs during elective C-section for breech presentation at term gestation30. Comparisons with environmental and reagentnegative controls from two independent sequencing runs were included to account for contamination and stochastic noise. No microbial signal distinct from negative controls was detected, and aerobic and anaerobic bacteria (Staphylococcus epidermidis and Cutibacterium acnes (formerly Propionibacterium acnes)) detected by culture of fetal samples were identified by the authors as skin contaminants.
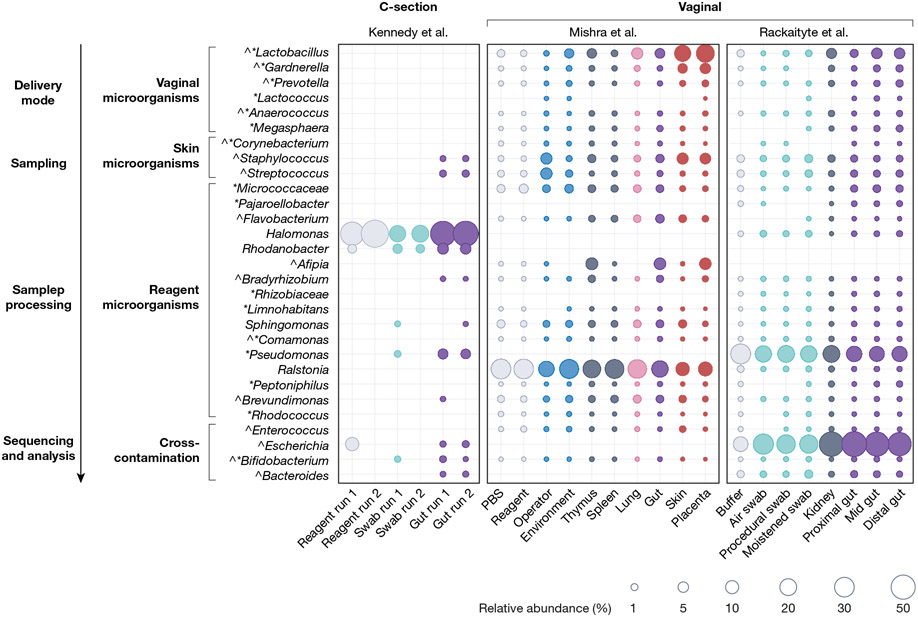
To compare these reports, we reanalysed the publicly available unfiltered microbial profiling data associated with the three publications that reported sequence data and determined the relative abundance of each detected genus. Although there was good agreement between the two studies using second-trimester vaginally delivered fetuses39,40, the bacterial taxa that were detected in fetuses from C-sections30 were significantly different (Fig. 1). The number of genera was much lower in C-section-derived fetuses, and entire groups of microorganisms–especially those usually found in the vagina–were absent. Most importantly, in the studies that claimed fetal microbial colonization39,40, every genus detected in fetal samples was also detected in most of the control samples.
Fig. 1 |. Relative abundance of bacterial taxa from three recent fetal studies.
Distribution and mean relative abundance (%) of taxa present In fetal samples from three recent studies30,39,40 investigating the fetal microbiome, and their corresponding abundance in control samples. Taxa were selected on the basis of the following criteria: genera that were cultured from or detected as enriched in fetal samples as described by Mishra et al.40 (indicated by ^) or by Rackaityte et al.39 (indicated by *, including the family Micrococcaceae); all genera detected in fetal samples from Kennedy et al.30 and the PBS-enriched genus Ralstonia40. Taxa were grouped by potential source of contamination in agreement with the likely origin of genera (for skin microorganisms) and previous studies that characterized sources of contamination36-38. Publicly available unfiltered relative abundance microbiota profiling data associated with each publication were merged into a single phyloseq object (RRID:SCR_01380). Amplicon sequence variants (ASVs) were grouped at the genus or family level (for Micrococcaceae). The mean relative abundance of each taxon was calculated for each sample type within each study and plotted in R (tidyverse, ggplot2; RRID:SCR_014601). Dot size corresponds to the mean relative abundance by sample type and study (mean relative abundances of less than 0.0001% were excluded). Dots are coloured by sample type: reagent controls in grey (Mishra: PBS n = 42, reagent n = 23; Rackaityte: buffer n = 11; Kennedy: reagent n = 2), sampling negatives in aqua (Kennedy: swab n = 1; Rackaityte: air swab n = 19; procedural swab n = 16; moistened swab n = 17) and environmental negatives in sky blue (Mishra: environment n = 47, operator n = 12), internal controls in indigo (Mishra: thymus n = 27, spleen n = 12; Rackaityte: kidney n = 16), fetal lung in pink (Mishra: n = 25), fetal gut in purple (Kennedy: n = 20; Mishra: n = 44; Rackaityte: proximal n = 41, mid n = 45, distal n = 42), and external tissues in red (Mishra: skin n = 35, placenta n = 16).
Reproductive biology and obstetrics perspectives
The embryo and fetus develop within the uterus but not in the uterine cavity per se. The early embryo invades the maternal decidua and is completely embedded by ten days after fertilization. The fetus grows within the amniotic cavity, which originates between the trophoblast and inner cell mass in the second week after fertilization, surrounded by two layers of reproductive membranes and bathed in amniotic fluid. Hence, even if microorganisms were present in the uterine cavity50, they would have to pass through to the amniotic cavity and enter the amniotic fluid to colonize the fetus. Amniotic fluid has antimicrobial properties, being enriched for example in lysozyme51, human β-defensin 2 (ref. 52) and GP340 (DMBT1)53, which binds and agglutinates diverse Gram-negative and Gram-positive bacteria.
The placenta mediates communication between the fetus and the mother and is a potent immune organ that protects the fetus. Historically, the placenta has been considered sterile (defined here as free from living microorganisms), but in 2014 a complex but low-biomass placental microbiome was detected by DNA sequencing. The proposed placental microbiome showed some similarity with sequencing data of microbial communities of the oral cavity15. Contamination controls were not included in this study, and subsequent evaluation of the work found that most of the genera detected were also common contaminants26,36,38,54. Several detected taxa, such as Gloeobacter, a genus of photosynthetic cyanobacteria, appeared biologically implausible as a component of a putative placental microbiome24,55. Since this early report, dozens of studies have conducted sequence-based microbial analyses of placental tissues, with opposing conclusions (as reviewed by Bolte et al.20).
Regardless of whether placental samples are collected by biopsy via the vagina, clinically by chorionic villus sampling or after delivery, it is always necessary to control for contamination, particularly from the tissues through which a placenta must pass before sampling. Accordingly, de Goffau et al.29 performed a comprehensive study of the possible placental microbiome, using samples from uncomplicated and complicated (pre-eclampsia and small for gestational age) pregnancies that were delivered both at term and preterm either vaginally or by C-section. Sampling was confined to the placental terminal villi (fetal tissue), as this represents the site of exchange (across the vasculosyncytial membrane) between the fetus and the mother’s blood and tissues. The authors detected a range of species that are known to dominate the vaginal microbiota56, such as Lactobacillus iners, Lactobacillus jensenii, Lactobacillus crispatus, Lactobacillus gasseri and Gardnerella vaginalis. When the presence of vaginal microorganisms and those in the laboratory reagents (the ‘kitome’) were accounted for, there was no evidence for a placental microbiome, which is in agreement with several additional recent studies23,29,31-34,37.
Pathogenic infection of the placenta by viral or bacterial pathogens is a well-recognized clinical phenomenon that contributes to preterm birth and neonatal sepsis57. de Goffau et al. detected Streptococcus agalactiae in around 5% of cases as the only verifiable bacterial signal in placentas obtained by C-section deliveries that were conducted before the rupture of the fetal membranes and the onset of labour29. The presence of this species is plausible as it colonizes the genital tract of about 20% of women and has invasive potential, being an important cause of maternal and neonatal sepsis58. However, the ability of specific pathogens to colonize and/or infect the placenta is distinct from the presence of an indigenous microbiota–that is, a prevalently stable, non-pathogenic, complex microbial community that is metabolically active21.
Research claiming that viable low-density microbial communities are present in the fetal intestine39 and fetal organs40 likewise calls for an evaluation of the sampling process. Mishra et al. obtained fetal tissues after medical termination of pregnancy in the second trimester with prostaglandins40. This procedure typically involves the individual going through hours of labour and often leads to the rupture of the fetal membranes hours before vaginal delivery. Even with a standardized approach, labour may be prolonged and may be accompanied by infection and fever, which are common with second-trimester terminations59,60. Both Li et al.41 and Rackaityte et al.39 also used second-trimester terminations but obtained the fetal tissues from core facilities. The tissues used by Li et al. were from surgical terminations (14–23 weeks) performed with mechanical dilation. Rackaityte et al.61 did not provide sufficient information to determine whether fetuses were obtained through surgical procedures or medical inductions. Although the latter increases the risk of the fetus being exposed to vaginal microorganisms during labour, both procedures involve vaginal delivery of the fetus. As outlined below, the reported microbiology of these fetuses mainly reflects the sources of microorganisms to which they were exposed during these procedures.
Microbial ecology perspectives
Host–microbe relationships range from mutualism (a prolonged symbiotic association from which both benefit), to commensalism (the host is unaffected), to pathogenesis, in which the microorganism harms the host. Although claims for fetal microbial exposure39,40 have not established the nature of the host–microbe interaction, and the duration of exposure or colonization, they have suggested that live organisms have a beneficial role in fetal immune development, thereby implying a symbiosis. The microbiological approaches applied by Rackaityte et al.39 and Mishra et al.40 are, in large part, robust, and well suited to studying symbiotic microbial populations. The combination of 16S rRNA gene sequencing, qPCR, microscopy, FISH and culture is laudable, as the approaches are complementary. Next-generation sequencing of 16S rRNA gene amplicons provides a broad community overview and can detect microorganisms that escape cultivation, whereas qPCR, microscopy and bacterial cultures have a high dynamic range, low detection limits and reasonable specificity. The DNA-sequence-based microbiota composition data in both studies are quite consistent (Fig. 1), which suggests that several of the bacterial taxa detected were present in the samples and not artefacts derived from laboratory reagents or DNA-isolation-kit contamination. However, although the microbiological analyses of samples were sound, the sampling procedures allowed the introduction of contaminant species, and critical controls to determine whether contamination occurred were missing.
In agreement with the unavoidable vaginal exposure of fetuses obtained by second-trimester abortions (see above), both Rackaityte et al.39 and Mishra et al.40 found that the genera Lactobacillus and Gardnerella, which dominate the vaginal microbiota56, were among their most consistent findings (Fig. 1). The species cultured by Mishra et al.–G. vaginalis, L. iners and L.jensenii–are largely restricted to the human vagina62. Other microorganisms detected, such as Staphylococcus species and Cutibacterium acnes, are skin commensals. As shown in Fig. 1, the abundances of Lactobacillus, Gardnerella and Staphylococcus that were found by Mishra et al. showed gradients with high population levels in fetal samples exposed to sources of contaminants (placenta and skin) and lower levels in internal samples (gut, lung, spleen and thymus). The omission of vaginal controls by both Rackaityte et al. and Mishra et al. to determine the microbiota of vaginally delivered fetuses is a considerable limitation that casts doubt on the authors’ conclusion that the microorganisms originate from the womb. Indeed, Li et al.41 obtained samples from second-trimester surgical terminations using mechanical dilatation, which reduces the risk of bacterial exposure to the fetus during sampling. In this study, positive bacterial PCR results were not reported, which raises the possibility that sampling contamination may be a serious confounder in both of the other studies that claimed the presence of microorganisms at these sites.
Although vaginal controls were not included by Rackaityte et al.39 and Mishra et al.40, direct comparisons of their findings with those of Kennedy et al.30 also provide evidence for vaginal contamination of terminated fetuses (Fig. 1). The C-section-derived fetal samples in Kennedy et al., which were not exposed to the vagina, carried no Gardnerella or Lactobacillus, but instead contained skin and reagent contaminants30,54. Despite attempts to reduce contamination, C-section-derived fetal meconium had at least one positive culture30. Kennedy et al. did not consider these microorganisms to be of fetal origin, as they were skin commensals, and half of the samples, as well as many culture replicates, did not show growth. The authors concluded that such inconsistencies point to stochastic contamination and not colonization by a stable functional microbial community.
In addition to the potential detection of contaminants, the bacterial load found in terminated fetuses was extremely low39,40. Signals derived from qPCR experiments were only marginally higher than those of controls, with Mishra et al. reporting cycle thresholds (Ct) of more than 30 cycles, with Ct values for negative controls being around 31–32 cycles. Cell counts as detected by both microscopy and culture were also low. Mishra et al. reported fewer than 100 colonies on average per entire fetus, with high inconsistencies among individual fetuses and tissues (see Table S6 in the original publication40). Such findings are more likely to be a result of contamination than colonization.
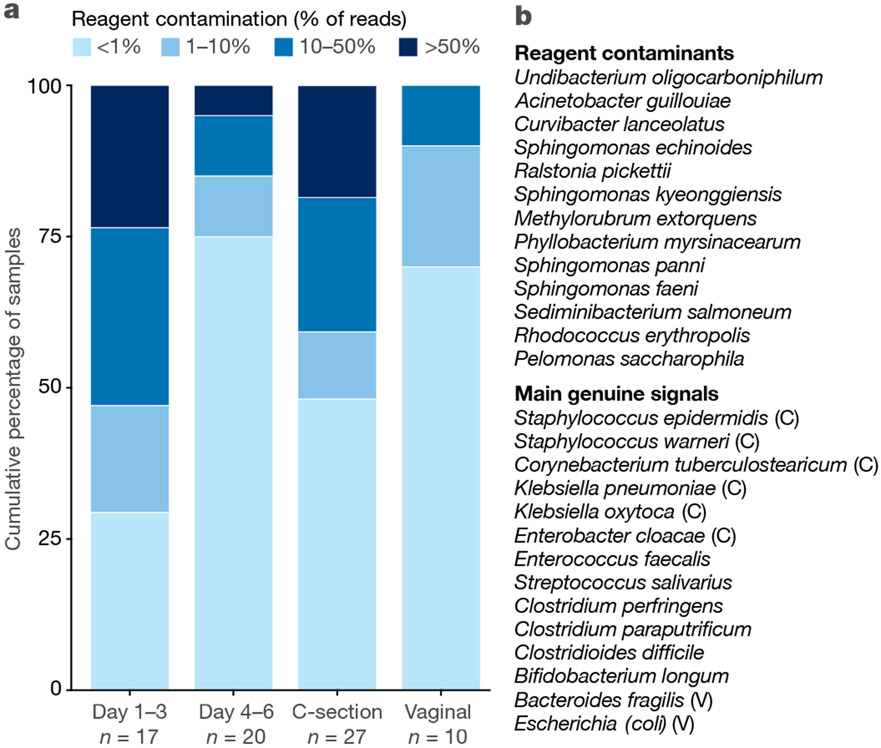
Neonatal meconium samples have been studied for a century by culture-based methods, and, more recently, by DNA sequencing. Evaluations of such samples are also associated with contradictory findings11,43,44,63, probably owing to contamination64 and because postnatal colonization may occur before the first passage of meconium26. However, when meconium is passed soon after birth, culturable bacteria are seldom detected (as reviewed by Perez-Muñoz et al.26). In agreement with this, an analysis of meconium samples collected from extremely premature infants65 showed that taxa regularly identified as contaminants36,38 make up a large proportion of sequences that are collected within the first three days after delivery and which drop to levels below 1% of the total microbiota profile in most samples at days 4–6 (Fig. 2). This indicated that bacterial sequences that cannot be assigned to contamination are initially rare in early meconium, which is consistent with a recent study that applied strict controls for sequencing and culture and did not detect a meconium microbiota64.
Fig. 2 |. Reagent contamination in meconium samples from extremely premature infants.
a, Representation of the percentage of reagent contamination (% of total sequence reads) in the first meconium of extremely premature infants collected in a previous study65 in relation to the day of procurement of said samples (day 1–3 or day 4–6) or in regard to the mode of delivery (C-section or vaginal). Colours indicate the percentage of sequence reads assigned to reagent contamination (legend on top). The day of procurement is significantly correlated with the percentage of reagent contamination reads (P = 0.005 by Mann–Whitney U test or P = 0.01 by Spearman rho test) and the mode of delivery shows a trend (P = 0.07 by Mann–Whitney U test). The number of samples (n) is noted below each category. b, Top, list of reagent contaminants shown together in a. Bottom, list of the most abundant sample-associated-signals and their association (or lack thereof owing to limited size of cohort) with vaginal (V) or C-section (C) delivery.
Members of an authentic fetal microbiota should be, in theory, detectable in early-life faecal samples independent of birth mode. There is, indeed, some overlap between the reported fetal microbial taxa39,40, for example, staphylococci, enterococci, lactobacilli and enterobacteria, and the microbiota detected in infant faecal samples in the first week of life66-68. However, there have been few attempts to track species and strains to confirm fetal origin. One study investigated gastric aspirates of newborn infants immediately after birth69; this should in theory detect in utero bacterial exposure as the fetus swallows amniotic fluid (as demonstrated by the detection of pathogenic Ureaplasma species70). However, aspirates from vaginally born infants contained the specific Lactobacillus species (L. iners and L. crispatus) that also dominate the microbiota of the vagina, whereas most samples from C-section deliveries contained low microbial loads near the detection limit and clustered with negative controls69. This finding is consistent with vaginal transfer of microorganisms to a sterile fetus during delivery. In addition, many of the genuine bacterial signals that were detected in early meconium65 were typical maternal skin representatives (Staphylococcus spp. and Corynebacterium spp.) and were strongly associated with C-section, or in the case of vaginal deliveries, species that are common in the maternal faecal microbiota (Escherichia coli and Bacteroides fragilis) (Fig. 2), indicating that these genuine signals were derived from microorganisms acquired ex utero.
Research is beginning to determine the origin of post-partum neonatal microbial colonizers and has shown a delay in the appearance of bacterial species that are presumed to originate from the mother’s gut (for example, Bifidobacterium and Bacteroides species) in early faecal samples of infants born by C-section66,67,71-73. A substantial proportion of strains acquired by infants postnatally can be traced back to their mother’s faecal samples73-75, and faecal microbiota transplant from the mother restores the microbiome in infants delivered by C-section76. Thus, the published evidence, although incomplete, suggests that the early-life microbiota in humans is acquired through the vertical and horizontal transfer of microorganisms whose origin is faecal or environmental (from outside) rather than fetal (from inside).
Bioinformatic and data science perspectives
Characterizing low-biomass samples by 16S rRNA gene amplicon sequencing is challenging as DNA contamination can occur from the microbial DNA present in reagents, labware, tools, instruments and DNA-isolation kits36-38, and through cross-contamination between PCR tubes or wells, sequencing runs or sequencing lanes37. A common misconception in the field of low-microbial-biomass samples is that the use of negative controls is sufficient to account for all kinds of contaminants. Commonly, imperfect negative controls are used that account for only a limited number of the sample-processing steps or are not spread evenly amongst all batches (thus not accounting for processing days, reagent batches and different sequencing runs), leading to batch effects that may be mistaken for genuine signals45. Overreliance on or under-analysis of such negative controls, in combination with the misapplication of contamination-removal programs like decontam77, specifically by not having negative controls in all batches, frequently results in false positive signals owing to the detection of contaminants45. Even with appropriate controls, it is challenging to separate genuine signals from low abundance contaminants as signals may appear sporadically in samples and negative controls78. Thus, suboptimal processing of sequencing control samples may not reveal the full spectrum of contaminants because only the most abundant species of contaminants are consistently detected. On the other hand, potentially genuine sample-associated signals sometimes also erroneously appear in negative-control samples through cross-contamination during the PCR or sequencing steps (machine contamination)37.
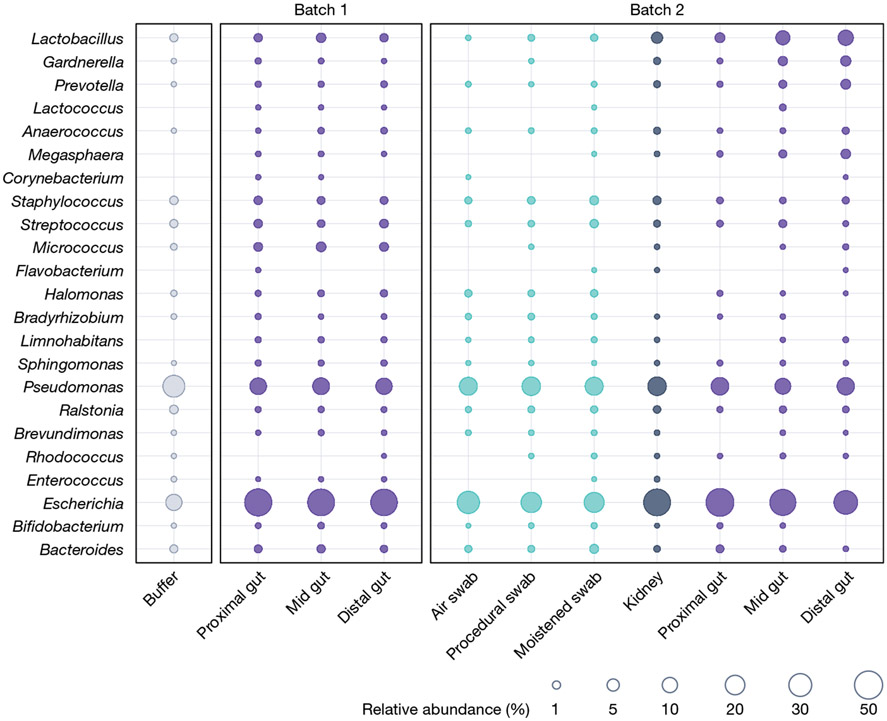
In the case of both Rackaityte et al.39. and Mishra et al.40, many of the taxa reported are common contaminants (Fig. 1). The most obvious case is Bradyrhizobium, which is one of the most dominant and consistent contaminants found in sequencing studies38,79. Rackaityte et al. interpreted the presence of Micrococcus and Lactobacillus as genuine fetal inhabitants, but a reanalysis of the data suggested that these findings were a result of batch effects (indicative of contamination45). Although the authors rejected this interpretation61, this batch effect is clearly visible if the findings of the different batches are plotted together(Fig. 3). Furthermore, in the study by Mishra et al., the authors concluded that Micrococus was likely to be a contaminant40, whereas the genera Afipia, Flavobacterium, Pseudomonas and Brevundimonas were reported as part of the fetal microbiota40, although these taxa are also commonly detected as kit or laboratory reagent contaminants36,38.
Fig. 3 |. Relative abundance of bacterial taxa in samples from Rackaityte et al.
Distribution and mean relative abundance (%) of taxa present In fetal and control samples from Rackaityte et al.39 by batch as defined by Rackaityte et al.61. Dominant taxa were selected as described in Fig. 1. Publicly available unfiltered relative abundance microbiota data associated with each publication were merged into a single phyloseq object (RRID:SCR_01380). ASVs were grouped at the genus or family (for Micrococcaceae) level. The mean relative abundance of each taxon was calculated for each sample type within each batch and plotted in R (tidyverse, ggplot2; RRID:SCR_014601). Dot size corresponds to the mean relative abundance by sample type and batch. Dots are coloured by sample type: reagent controls in grey (buffer), sampling negative controls in aqua, internal controls in indigo (kidney) and fetal gut samples in purple.
Mishra et al. and Rackaityte et al. also reported a marginally higher total bacterial load in fetal samples, as compared to controls, using qPCR39,40. However, nucleic acids (DNA, RNA and tRNA) in tissue samples (which are absent in negative controls) might have a DNA carrier effect80, leading to a more efficient DNA precipitation of prokaryotic material. In addition, bacterial PCR primers that target the 16S rRNA gene can also amplify mitochondrial DNA81, which is evolutionarily of bacterial origin. Together, these factors offer alternative explanations for a higher microbial burden in samples from low-biomass sites compared to controls. Rackaityte et al. removed human mitochondrial DNA (mtDNA) from their 16S rRNA gene-sequence-based results that co-amplified in the PCR, but neither study accounted for mtDNA in their qPCR analysis, although their qPCR primers targeted the 16S rRNA gene and were therefore potentially susceptible to cross-reactivity39,40.
Immunological perspective
The enteric microbiota is a potent driver of adaptive mucosal immune maturation and priming in the adult host82-85. Besides their intrinsic immunogenic nature, microorganisms also generate metabolites that promote and shape immune maturation and priming86-88. Although the early fetal immune system is immature, recent research shows the migration of fetal dendritic cells (DCs) to the mesenteric lymph nodes; somatic hypermutation in fetal B cells; and an expansion of T cell receptor repertoire diversity, evenness and activation during late fetal development7,89,90.
The existence of metabolically active microorganisms in the fetus could, in principle, provide one possible explanation for these findings. Mishra et al.40 used an autologous T cell expansion assay to show that fetal DCs loaded with antigen from bacteria that had been isolated from fetal tissues stimulated the proliferation of CD45RO+ and CD69+ T cells. T cell proliferation was reduced but still detectable in the absence of DC-derived cytokine release, suggesting an activated memory response40. Evidence that the fetal T cell memory response is specific for the bacteria present in one individual fetus would be necessary to strengthen the interpretation that specific immune responses are routinely driven by fetal bacterial colonization.
There are alternative explanations for fetal immune responses apart from bona fide microbial colonization. Maternal antigen–IgG complexes have been detected in cord blood, and trans-placental immune priming of the fetal immune system in early gestation has been demonstrated91,92 Cross-reactivity, as observed for microbiota reactive enteric secretory immunoglobulin A, would support fetal priming by maternal microbial antigens87. Similarly, maternal-microbiota-derived molecules partly bound to IgG stimulated innate immune maturation of the fetal gut in mice48, and maternal intestinal carriage of Prevotella has been reported to protect the offspring from food allergy in humans93. Thus, antigens and metabolites derived from the maternal microbiota can pass the placental filter directly or bound to IgG, and offer an alternative explanation for the observed fetal immune responses94.
The hypothesis of a low-biomass fetal microbiome requires the identification of host mechanisms that control and tolerate bacterial populations and prevent overt inflammation and tissue destruction in the presence of viable microorganisms, many of which are opportunistic pathogens (see below). Alongside this, mechanisms by which the commensal or symbiotic microorganisms survive the immune response and antimicrobial effector molecules would also have to be identified, and it is unclear how the fetal immune system would differentiate between pathogens and symbionts once protective barriers are breached57. Given that such immunological and anatomical mechanisms have not been identified or even proposed28, the observed immune maturation and priming during fetal development is probably not induced through colonization of the fetus with live microorganisms. Instead, fetal immune development might be driven through maternal immune components or microbial fragments and metabolites crossing the placenta, which protects the sterile fetus from live microorganisms through multiple layers of immunological defence57.
Clinical microbiology perspective
No part of the human body is impregnable to bacterial invasion. Transient bloodstream bacteraemia can result from innocuous activities such as brushing the teeth95, and most host tissues can tolerate occasional ingress by microorganisms. However, to avoid serious pathology, bacteraemia must be rapidly cleared by innate immune mechanisms and inflammation. Some pathogens establish persistent infections that may be asymptomatic either by evading the immune system or by forming persister cells in response to antibiotic treatment96. The claims for non-pathogenic fetal microbial exposure39,40 have not established whether host–microbe interactions reflect small-scale translocation, asymptomatic infection, persistent symbiosis or mutualism.
The ‘fetal-enriched taxa’ reported include Micrococcus, Lactobacillus, Flavobacterium, Staphylococcus, Escherichia, Enterococcus, Afipia, Pseudomonas,Bradyrhizobium and Brevundimonas39,40. Mishra et al. also report successful culturing of lactobacilli and staphylococci from fetal tissue40, but the lack of unambiguous species-level taxonomic identification of the cultured organisms is a major technical limitation. Bacteria such as Micrococcus, which were detected in fetal intestines by Rackaityte et al.61, rarely cause invasive infection in humans. Their prolonged presence within healthy tissues and transmission through the placenta would require bacterial mechanisms of resistance against antimicrobial effector molecules of the host innate immune system57. Such mechanisms have not been described for the genus Micrococcus, which is an environmental organism found in water, dust and soil, and is also a common contaminant46,47. Lactobacilli are usually of low pathogenic potential; they inhabit external mucosal surfaces of healthy humans, including the nose97 and the vagina56, and are often used as probiotics98. However, some strains and species of lactobacilli do express potential virulence factors99-101, resist oxidative stress102 and grow in the absence of iron103, which allows them to cause serious infections such as endocarditis when provided with the opportunity to access the bloodstream104,105. This raises potential problems with the interpretation of lactobacilli as asymptomatic colonizers of fetal tissue rather than contaminants that are picked up during vaginal delivery.
An even greater challenge arises when species of the genus Staphylococcus are considered, particularly strains that were cultured from fetal tissue and that exhibit high-level 16S rRNA gene-sequence identity (99–100%) to Staphylococcus aureus and several closely related coagulase-negative Staphylococcus species (CoNS)40. These organisms can be long-term colonizers of external mucosal surfaces of humans106,107 and do not typically cause disease unless the mucosal barrier is breached. However, once they bypass mucosal barriers, they can deploy a more extensive repertoire of virulence factors to invade tissues by degrading connective tissues and, in the case of S. aureus, a repertoire of over a dozen cytolytic toxins that kill human cells108,109. CoNS, on the other hand, are ubiquitous skin colonizers. Their detection in clinical diagnostic laboratories is so common that it is considered a major diagnostic challenge110,111 and is usually assumed to reflect contamination from the patient and occasionally the healthcare worker, in the absence of other reasons to suspect a CoNS infection77-79. There are, however, distinct clinical scenarios in which the presence of CoNS and their pathogenic capacity are considered critical: for example, in patients with indwelling medical devices and in preterm neonates; they are the most common cause of late-onset neonatal sepsis112. Therefore, given that they are either contaminants or overt pathogens, the detection of staphylococci, no matter whether S. aureus or CoNS, is difficult to reconcile with in utero colonization of a healthy fetus.
Other bacteria identified as part of a notional ‘fetal microbiome’, such as Enterococcus faecalis and Klebsiella pneumoniae, are equally problematic. These belong to a group known as ‘ESKAPE pathogens’, which include Enterococcus faecium, S. aureus, K. pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species. The lethality of tissue colonization with ESKAPE pathogens is well documented, and these microorganisms are leading causes of healthcare-acquired infections worldwide, with considerable mortality and morbidity, even when treated with antibiotics113. Several ESKAPE pathogens readily survive in adverse conditions outside of vertebrate hosts, including drying, oxidative stress and exposure to heat or sanitation chemicals114. They are likely to persist on inanimate surfaces including utensils or clinical fabrics115,116, thereby increasing their likelihood of being contaminants. Although these microorganisms were not reported at the species level40, it is noteworthy that closely related organisms can also cause neonatal sepsis117-119, which makes them unlikely to be colonizers of a healthy fetus.
A consideration prompted by a notional fetal microbiome is the possibility that the fetus might cope better with nosocomial pathogens than neonates or even adults. However, there is ample evidence to show that amniotic fluid, the placenta and fetal tissues are highly susceptible to bacterial infection, and the outcomes of infections with S. agalactiae or Listeria monocytogenes are often catastrophic120,121. Notably, in L. monocytogenes infections that occur during the third trimester of pregnancy, fetal infection progresses, whereas the mother’s infection can be cleared, indicating that the fetus does not have greater resistance to infection than an adult human. Therefore, from a clinical perspective, most interpretations brought forward in recent publications39,40 with regard to the presence of microorganisms in fetuses seem to be biologically difficult to reconcile, as it is highly plausible that they would result in harm to or death of the fetus. In agreement with this conclusion, in a series of well-controlled studies in various clinical settings, DiGiulio and co-workers found no evidence for microorganisms in amniotic fluid except when associated with neonatal morbidity and mortality122-125.
Gnotobiology perspective
The traditional assumption that the human fetus is free from other life forms in utero is based mainly on the observation that, with few exceptions, bacterial and viral pathogens that infect the mother are incapable of crossing the placental barrier to infect the fetus126-128. In addition, the amnio-chorionic membranes that enclose the fetus in the uterine cavity, as well as the cervical mucus plug, protect the fetus from external microorganisms. Sterility of the fetus is the basis for the derivation by hysterectomy of germ-free mammals (mainly mice and rats, but also pigs and other species26), which have long been used to study the biochemical, metabolic and immunological influences of microorganisms on their mammalian hosts129-131. The primary consideration is whether germ-free animals are truly ‘free of all demonstrable forms of microbial life’132. If they lack microbial associates, there cannot be a fetal microbiome. Testing germ-free animals for contaminating microorganisms uses microscopic observation of stained faecal smears, culture of faeces in nutrient media under various conditions of temperature and gaseous atmosphere127,132-134, PCR using ‘universal bacterial’ primers133,135, and serological assays for viral infections136. These tests consistently demonstrate an absence of microbial associates. Therefore, gnotobiology provides strong evidence that the fetus in utero is sterile.
A healthy human fetus is sterile
Through multiple angles of explanatory considerations, we conclude that the evidence is strongly in favour of the ‘sterile womb’ hypothesis. Although it is impossible to disprove the occasional presence of live microorganisms in a healthy human fetus, the available data do not support stable, abundant colonizers under normal, non-pathogenic circumstances. We are aware that our position conflicts with dozens of publications that claim evidence for in utero microbial populations20, but we are confident in the validity of our multi-layered approach.
The processes by which the fetus matures and becomes immunologically equipped for life in a microbial world have lifelong implications. Aside from the caution and safeguards recommended in this perspective article (Box 1), our aim here is not to dissuade scientists from investigating the microbial drivers of fetal immune development. We agree with proposals that there is a need to better understand microbial interactions at the maternal–fetal interface20, but do not think that symbiotic microbial populations in the placenta or fetus play a role in this. Paradoxically, we contend that sterile tissues are both immunologically and microbiologically fascinating, but require an adjustment of the methodological approaches used. How does the fetus mature and become immunologically equipped for life in a microbial world in the absence of direct exposure to live microorganisms? Are maternal-derived microbial metabolites sufficient for fetal immune education? Future research could include explorations of how maternal microbial-derived metabolites and small molecules, as well as maternal immune components, prepare the fetus for the microbial challenges of postnatal life94.
Box 1. Experimental considerations for low-biomass research.
High-biomass samples
Examples:
Faeces, dental plaque, wastewater, soil.
Impact of contamination:
Very low:
The high microbial biomass derived from the sample dominates the signal from background contamination, meaning that most observations are robust.
Mitigations:
Experimental design seldom needs to be substantially adjusted to account for contamination. Inclusion of ‘blank’ negative sequencing controls and removing samples with substantial levels of contamination using basic post-sequencing analysis is nevertheless prudent.
Low-biomass samples
Examples:
Skin swabs, nasal tract swabs, breast milk, most respiratory tract samples, tissue biopsies and mucosal samples, including intestinal crypts.
Impact of contamination:
Low to high:
Contaminated samples are progressively affected with reducing input microbial biomass38.
Mitigations:
Inclusion of multiple controls for recognition of contamination. Ideally, samples should be concentrated before processing to increase input biomass. Consideration of potential sources of contamination during the sample acquisition stage is always recommended. After sample collection, processing should be carried out in a clean-room environment, preferably with all surfaces bleached and UV-treated. DNA extraction may benefit from the use of non-kit-based methods (for example, phenol-chloroform extractions) in which plastic-ware and reagents can be UV-treated before use. Contamination from DNA-isolation and PCR kits is usually identifiable, particularly if well-defined batches are created64 and controlled using different lot numbers of kits. Regardless of the method of DNA extraction, the presence of contaminants should be monitored by including ‘blank’ negative controls. Inclusion of controls generated by serial dilution of DNA of known composition (for example, mock community) will indicate the biomass level at which contamination becomes a dominant feature of sequencing results. Contamination may also be estimated before sequencing by qPCR using serially diluted known quantities of spiked input DNA. Post-sequencing analyses, using programs like decontam, and analysis steps described by de Goffau et al.36 and used by Heida et al.65, will usually identify contaminants.
Samples in which the existence of microorganisms is not established (potential ‘no (zero) biomass’ samples)
Examples:
Placental and fetal tissues, amniotic fluid, meconium, brain tissue and cerebrospinal fluid, blood, bone and internal cancer tissues, healthy middle ear samples.
Impact of contamination:
High and potentially up to 100% unless infection or injury is present.
Mitigations:
Experimental design should be directed specifically against contamination. Initial assessment using quantitative methods (for example, qPCR) with low detection limit and microscopic visualization (for example, Gram staining or labelling by FISH) is required to determine whether microorganisms are present, before embarking on sequencing approaches. Such techniques are still susceptible to sample contamination and other artefacts (for example, non-specific staining or auto-fluorescence from mucins can sometimes appear ‘microbe-like’ in size and shape)45,144. All mitigations outlined for ‘low biomass’ samples above should be adopted. Repeating sample processing with different DNA extraction kits or methods32 and/or at different days can be informative145. These will track the presence of species in sequencing profiles associated with specific kits, reagents or environment. Species that are repeatedly detected regardless of the technical approach are more likely to be genuine signals, unless they were introduced during sample collection. Binary statistics (absence–presence) are recommended. The presence of microorganisms identified by sequencing should be verified with a different technique such as cultivation, another sequencing technique with sufficient taxonomic resolution, and/or species-specific qPCR or FISH using high magnification to visualize the size and morphology of individual microbial cells.
Lessons for low-biomass research
Contamination is always a potential confounder in microbiology but is of particular concern for those studying low- or no-biomass samples36,38. The issue has been highlighted by recent reports of human tissues, such as blood, brain and cancers (Box 1), which were previously thought to contain no, or very little, bacterial biomass, but apparently contain diverse microbial communities. As with the intrauterine studies described above, these microbial populations are often discussed considering their perceived importance for human diseases and health.
In studies on low-biomass samples, it is challenging to identify relevant signals from among contaminating noise. In instances of contamination, a tissue may be misjudged as non-sterile, whereas in others, a real microbiological signal may be obfuscated by contamination. The removal of all sequences present in negative-control samples, or that have been previously identified as contaminants in the literature, may result in a loss of authentic signals. Post-sequencing contamination removal using software packages such as decontam77 or other statistical approaches36,137 have been developed to remove the more abundant contaminants, leading to microbiome profiles that are more likely to reflect the real community. Practical examples of contamination removal in 16S rRNA gene-sequence data are provided by Heida et al.65, Saffarian et al.138, and Jorissen et al.139, and we expand on these examples in Box 1.
We draw attention to the distinction between ‘low biomass’ and ‘no (zero) biomass’ samples. This has practical significance; true ‘low (microbial) biomass’ samples are amenable to contamination-removal approaches but ‘no (microbial) biomass’ samples require a different approach (Box 1). For credible assertions of the presence of microorganisms, multiple layers of evidence are required. Potentially genuine signals found with contamination-sensitive sequencing approaches, even with strict controls included, should be verified using a quantitative, sensitive (lower detection limit), and less contamination-prone approach such as a species-specific qPCR. Because contamination removal will provide data regardless of whether microorganisms are present or absent, the starting proposition should be the null hypothesis to avoid confirmation bias28, particularly when results are inconsistent and at the outer technical limits for detection, or if results defy mechanistic plausibility.
Given the limitation of sequencing approaches, confirmation by alternative methods, such as FISH and culture, is required. However, as shown by recent studies of fetal samples, even a combination of approaches has the potential to produce false findings, because contamination during sampling is a considerable challenge. We posit that studies on all low-biomass samples could benefit from a similar trans-disciplinary assessment to that applied above for fetal samples, to interpret findings considering biological and mechanistic explanations28. When obligately photosynthetic, psychrophilic, thermophilic, halophilic or chemolithoautotrophic bacteria are found in human tissues that do not provide the growth conditions for such organisms24,140, or if the detected genera are known contaminants of laboratory kits or reagents (such as readily culturable Proteobacteria like Pseudomonas and E. coli, for example)141-143, the authenticity of such signals should be questioned.
Acknowledgements
T.B. receives funding from the Deutsche Forschungsgemeinschaft (German Research Foundation no. BR2925 10-1 & PL241 16-1). F.D.B. is funded by AI045008, AI120489, R33HL137063, CA219871, AI139240, and the PennCHOP Microbiome Program. J.D. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement ERC-2017-AdG no. 788191—Homo.symbiosus). W.M.d.V. is supported by the Gravitation grant 024.002.002 of the Netherlands Organization for Scientific Research. W.M.d.V. and A.S. are supported by the Academy of Finland (grants 1308255 and 1325103). A.M.E. is funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services, under grant number U19AI110818 to the Broad Institute. M.A.E. is funded through grants R01HD102318, R01HD098867 and R01NR014784. S.C.G.-V. was funded through a Peter Hans Hofschneider Professorship provided by the Stiftung Molekulare Biomedizin. M.G.G. and D.M.S are funded by the Canada Research Chairs Program. L.J.H. is supported by Wellcome Trust Investigator Awards 100974/C/13/Z and 220876/Z/20/Z and by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Gut Microbes and Health BB/R012490/1 and its constituent projects BBS/E/F/000PR10353 and BBS/E/F/000PR10356. M.W.H. has received funding from the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101019157). S.L. has received funding from the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement 852600 Lacto-Be). A.J.M. receives funding from ERCAd HHMM-Neonates and Swiss National Science Sinergia. O.K. is supported by the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement ERC-2020-COG no. 101001355). Work in the laboratories of P.W.O., L.O. and J.W. is supported by Science Foundation Ireland (SFI) through a Centre award (APC/SFI/12/RC/2273_P2)to APC Microbiome Ireland. J.W. acknowledges support through an SFI Professorship (19/RP/6853) and thanks V. McMahon for coordination of this review and R. O’Callaghan for encouragement. J.R. acknowledges funding from the Interuniversity Special Research Fund (iBOF) Flanders (FLEXIGUT R-11423), the Rega Institute, VIB and KU Leuven. N.S. receives funding from the ERC (ERC-STG project MetaPG-716575 and ERC-CoG microTOUCH-101045015)and from the European H2020 program (ONCOBIOME-825410 project, MASTER-818368 project and IHMCSA-964590). F.S. is supported in part by Science Foundation Ireland. G.C.S.S. acknowledges funding from the Medical Research Council (UK; MR/K021133/1) and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (Women’s Health theme). D.M.S. is funded by the Canadian Institute for Health Research and the Canada Research Chairs Program. A.W.W. receives core funding support from the Scottish Government’s Rural and Environment Science and Analytical Services (RESAS). M.Y. is supported by the Azrieli Faculty Fellowship.
Footnotes
Competing interests The authors declare no competing interests.
References
- 1.Macpherson AJ, de Aguero MG & Ganal-Vonarburg SC How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol 17, 508–517 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Kalbermatter C, Fernandez Trigo N, Christensen S & Ganal-Vonarburg SC Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol 12, 683022 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gensollen T, Iyer SS, Kasper DL & Blumberg RS How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain N The early life education of the immune system: moms, microbes and (missed) opportunities. Gut Microbes 12, 1824564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornef MW & Torow N ‘Layered immunity’ and the ‘neonatal window of opportunity’ — timed succession of non-redundant phases to establish mucosal host–microbial homeostasis after birth. Immunology 159, 15–25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torow N, Marsland BJ, Hornef MW & Gollwitzer ES Neonatal mucosal immunology. Mucosal Immunol. 10, 5–17 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Schreurs R et al. Human fetal TNF-α-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50, 462–476 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Stras SF et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell 51, 357–373 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Zhang X et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci. Transl. Med 6, 238ra272 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Tissier H Recherches sur la flore intestinale des nourrissons: (etat normal et pathologique). Doctoral dissertation, BIU Santé (1900). [Google Scholar]
- 11.He Q et al. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 12, 1794266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinson L et al. Comparison of bacterial DNA profiles in mid-trimester amniotic fluid samples from preterm and term deliveries. Front. Microbiol 11, 415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younge N et al. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 4, e127806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinson LF, Boyce MC, Payne MS & Keelan JA The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol 10, 1124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aagaard K et al. The placenta harbors a unique microbiome. Sci. Transl. Med 6, 237ra265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Argenio V The prenatal microbiome: a new player for human health. High Throughput 7, 38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funkhouser LJ & Bordenstein SR Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 11, e1001631 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stinson LF, Payne MS & Keelan JA Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Rev. Microbiol 43, 352–369 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Walker RW, Clemente JC, Peter I & Loos RJF The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr. Obes 12 (Suppl. 1), 3–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolte EE, Moorshead D & Aagaard KM Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 14, 4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg G et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blaser MJ et al. Lessons learned from the prenatal microbiome controversy. Microbiome 9, 8 (2021). Discussion about the prenatal microbiome controversy by several experts in the microbiome field.
- 23.Bushman FD De-discovery of the placenta microbiome. Am. J. Obstet. Gynecol 220, 213–214 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Editorial. Microbiome studies and “blue whales in the Himalayas”. Lancet Infect. Dis 18, 925 10.1016/S1473-3099(18)30503-6 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Hornef M & Penders J Does a prenatal bacterial microbiota exist? Mucosal Immunol. 10, 598–601 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE & Walter J A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segata N No bacteria found in healthy placentas. Nature 572, 317–318 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Walter J & Hornef MW A philosophical perspective on the prenatal in utero microbiome debate. Microbiome 9, 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Goffau MC et al. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 (2019). Sequencing study using robust controls, concluding that there is no evidence for a placental microbiome.
- 30. Kennedy KM et al. Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol 6, 865–873 (2021). The only sequencing study so far that characterized the microbial populations in human fetuses using meconium samples obtained after C-section, concluding that there is no evidence for a microbiota.
- 31.Kuperman AA et al. Deep microbial analysis of multiple placentas shows no evidence for a placental microbiome. BJOG 127, 159–169 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Lauder AP et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leiby JS et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theis KR et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am. J. Obstet. Gynecol 220, 267.e1–267.e39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterpu I et al. No evidence for a placental microbiome in human pregnancies at term. Am. J. Obstet. Gynecol 224, 296.e1–296.e23 (2021). [DOI] [PubMed] [Google Scholar]
- 36.de Goffau MC et al. Recognizing the reagent microbiome. Nat. Microbiol 3, 851–853 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Olomu IN et al. Elimination of “kitome” and “splashome” contamination results in lack of detection of a unique placental microbiome. BMC Microbiol. 20, 157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salter SJ et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rackaityte E et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med 26, 599–607 (2020). Microbial characterization of fetal samples obtained after vaginal delivery, reporting highly limited bacterial colonization.
- 40. Mishra A et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409 (2021). Analysis of fetal tissues obtained after medical termination of pregnancy in the second trimester and vaginal delivery, reporting microbial colonization of the fetus and bacterial priming of fetal immune cells.
- 41. Li Y et al. In utero human intestine harbors unique metabolomic features including bacterial metabolites. JCI Insight 5, e138751 (2020). Characterization of the microbiota in fetuses obtained by vaginal delivery, reporting no evidence for bacterial colonization.
- 42.Lim ES, Rodriguez C & Holtz LR Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 6, 87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y et al. Midtrimester amniotic fluid from healthy pregnancies has no microorganisms using multiple methods of microbiologic inquiry. Am. J. Obstet. Gynecol 223, 248.e1–248.e21 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Rehbinder EM et al. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am. J. Obstet. Gynecol 219, 289.e1–289.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 45.de Goffau MC, Charnock-Jones DS, Smith GCS & Parkhill J Batch effects account for the main findings of an in utero human intestinal bacterial colonization study. Microbiome 9, 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell S, Perry J & Meikle D Microbial contamination of non-disposable instruments in otolaryngology out-patients. J. Laryngol. Otol 117, 122–125 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Wistrand C, Soderquist B & Sundqvist AS Time-dependent bacterial air contamination of sterile fields in a controlled operating room environment: an experimental intervention study. J. Hosp. Infect 110, 97–102 (2021). [DOI] [PubMed] [Google Scholar]
- 48. Gomez de Aguero M et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016). Study demonstrating that aspects of prenatal immune development induced by maternal microbial compounds can occur in the absence of live microorganisms in the fetus.
- 49.Vuong HE et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586, 281–286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker JM, Chase DM & Herbst-Kralovetz MM Uterine microbiota: residents, tourists, or invaders? Front. Immunol 9, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherry SH, Filler M & Harvey H Lysozyme content of amniotic fluid. Am. J. Obstet. Gynecol 116, 639–642 (1973). [DOI] [PubMed] [Google Scholar]
- 52.Soto E et al. Human β-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J. Matern. Fetal Neonatal Med 20, 15–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichhardt MP et al. The salivary scavenger and agglutinin in early life: diverse roles in amniotic fluid and in the infant intestine. J. Immunol 193, 5240–5248 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Sinha R et al. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat. Biotechnol 35, 1077–1086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grettenberger CL Novel Gloeobacterales spp. from diverse environments across the globe. mSphere 6, e0006121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravel J et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Megli CJ & Coyne CB Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat. Rev. Microbiol 20, 67–82 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armistead B, Oler E, Adams Waldorf K & Rajagopal L The double life of group B Streptococcus: asymptomatic colonizer and potent pathogen. J. Mol. Biol 431, 2914–2931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodd JM & Crowther CA Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database Syst. Rev 2010, CD004901 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nijman TA et al. Association between infection and fever in terminations of pregnancy using misoprostol: a retrospective cohort study. BMC Pregnancy Childbirth 17, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rackaityte E et al. Corroborating evidence refutes batch effect as explanation for fetal bacteria. Microbiome 9, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duar RM et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev 41, S27–S48 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Dominguez-Bello MG et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dos Santos SJ et al. Early neonatal meconium does not have a demonstrable microbiota determined through use of robust negative controls with cpn60-based microbiome profiling. Microbiol. Spectr 9, e0006721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heida FH et al. Weight shapes the intestinal microbiome in preterm infants: results of a prospective observational study. BMC Microbiol. 21, 219 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Backhed F et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Shao Y et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Podlesny D & Fricke WF Strain inheritance and neonatal gut microbiota development: a meta-analysis. Int. J. Med. Microbiol 311, 151483 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Bajorek S et al. Initial microbial community of the neonatal stomach immediately after birth. Gut Microbes 10, 289–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim SM et al. Gastric fluid versus amniotic fluid analysis for the identification of intra-amniotic infection due to Ureaplasma species. J. Matern. Fetal Neonatal Med 29, 2579–2587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin R et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 11, e0158498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yassour M et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med 8, 343ra381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell CM et al. Delivery mode affects stability of early infant gut microbiota. Cell Rep. Med 1, 100156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferretti P et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yassour M et al. Strain-Level analysis of mother-to-child bacterial transmission during the first few months of Life. Cell Host Microbe 24, 146–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Korpela K et al. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell 183, 324–334 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Davis NM, Proctor DM, Holmes SP, Relman DA & Callahan BJ Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dyrhovden R et al. Managing contamination and diverse bacterial loads in 16S rRNA deep sequencing of clinical samples: implications of the Law of small numbers. mBio 12, e0059821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laurence M, Hatzis C & Brash DE Common contaminants in next-generation sequencing that hinder discovery of Low-abundance microbes. PLoS One 9, e97876 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Read SJ Recovery efficiences on nucleic acid extraction kits as measured by quantitative LightCycler PCR. Mol. Pathol 54, 86–90 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker SP et al. Non-specific amplification of human DNA is a major challenge for 16S rRNA gene sequence analysis. Sci. Rep 10, 16356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cebra JJ, Periwal SB, Lee G, Lee F & Shroff KE Development and maintenance of the gut-associated Lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol 6, 13–18 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaboriau-Routhiau V et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009). [DOI] [PubMed] [Google Scholar]
- 84.Wesemann DR et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501, 112–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 584, 274–278 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Bacher P et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Kabbert J et al. High microbiota reactivity of adult human intestinal IgA requires somatic mutations. J. Exp. Med 217, e20200275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arpaia N et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGovern N et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rechavi E et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci. Transl. Med 7, 276ra225 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Casas R & Bjorksten B Detection of Fel d 1-immunoglobulin G immune complexes in cord blood and sera from allergic and non-allergic mothers. Pediatr. Allergy Immunol 12, 59–64 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Szepfalusi Z et al. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J. Allergy Clin. Immunol 106, 530–536 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Vuillermin PJ et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun 11, 1452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganal-Vonarburg SC, Hornef MW & Macpherson AJ Microbial–host molecular exchange and its functional consequences in early mammalian life. Science 368, 604–607 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Lockhart PB et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 117, 3118–3125 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher RA, Gollan B & Helaine S Persistent bacterial infections and persister cells. Nat. Rev. Microbiol 15, 453–464 (2017). [DOI] [PubMed] [Google Scholar]
- 97.De Boeck I et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 31, 107674 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Lebeer S, Vanderleyden J & De Keersmaecker SC Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol 8, 171–184 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Collins J et al. Fibrinogen-binding and platelet-aggregation activities of a Lactobacillus salivarius septicaemia isolate are mediated by a novel fibrinogen-binding protein. Mol. Microbiol 85, 862–877 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Kankainen M et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl Acad. Sci. USA 106, 17193–17198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rampersaud R et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol 193, 1034–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wuyts S et al. Large-scale phylogenomics of the Lactobacillus casei group highlights taxonomic inconsistencies and reveals novel clade-associated features. mSystems 2, e00061–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weinberg ED The Lactobacillus anomaly: total iron abstinence. Perspect. Biol. Med 40, 578–583 (1997). [DOI] [PubMed] [Google Scholar]
- 104.Hazards E Po. B et al. Update of the List of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 7: suitability of taxonomic units notified to EFSA until September 2017. EFSA J. 16, e05131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cannon JP, Lee TA, Bolanos JT & Danziger LH Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur. J. Clin. Microbiol. Infect. Dis 24, 31–40 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Richardson EJ et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol 2, 1468–1478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordon RJ & Lowy FD Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis 46 (Suppl. 5), S350–359 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Otto M Staphylococcus aureus toxins. Curr. Opin. Microbiol 17, 32–37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Powers ME & Bubeck Wardenburg J Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog. 10, e1003871 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Healy CM, Baker CJ, Palazzi DL, Campbell JR & Edwards MS Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit. J. Perinatol 33, 52–58 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Michels R, Last K, Becker SL & Papan C Update on coagulase-negative staphylococci–what the clinician should know. Microorganisms 9, 830 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marchant EA, Boyce GK, Sadarangani M & Lavoie PM Neonatal sepsis due to coagulase-negative staphylococci. Clin. Dev. Immunol 2013, 586076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhen X, Lundborg CS, Sun X, Hu X & Dong H Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob. Resist. Infect. Control 8, 137 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kamal SM, Simpson DJ, Wang Z, Ganzle M & Romling U Horizontal transmission of stress resistance genes shape the ecology of beta- and gamma-proteobacteria. Front. Microbiol 12, 696522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kramer A, Schwebke I & Kampf G How Long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis 6, 130 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neely AN & Maley MP Survival of enterococci and staphylococci on hospital fabrics and plastic. J. Clin. Microbiol 38, 724–726 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bizzarro MJ et al. Neonatal sepsis 2004–2013: the rise and fall of coagulase-negative staphylococci. J. Pediatr 166, 1193–1199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong Y, Speer CP & Glaser K Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 9, 621–633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glaser MA, Hughes LM, Jnah A & Newberry D Neonatal sepsis: a review of pathophysiology and current management strategies. Adv. Neonatal Care 21, 49–60 (2021). [DOI] [PubMed] [Google Scholar]
- 120.Nan C et al. Maternal group B Streptococcus-related stillbirth: a systematic review. BJOG 122, 1437–1445 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Vazquez-Boland JA, Krypotou E & Scortti M Listeria placental infection. mBio 8, e00949–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DiGiulio DB et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J. Perinat. Med 38, 503–513 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. DiGiulio DB et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 3, e3056 (2008). Sequencing study of amniotic fluid of 166 women in preterm labour with PCR and culture that showed near-complete positive correlation of bacterial detection with neonatal morbidity and mortality.
- 124.DiGiulio DB et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol 64, 38–57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DiGiulio DB et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J. Perinat. Med 38, 495–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Enders G, Daiminger A, Bader U, Exler S & Enders M Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol 52, 244–246 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Luckey TD Germfree Life and Gnotobiology (Academic Press, 1963). [Google Scholar]
- 128.Rasmussen SA, Jamieson DJ, Honein MA & Petersen LR Zika virus and birth defects–reviewing the evidence for causality. N. Engl. J. Med 374, 1981–1987 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Falk PG, Hooper LV, Midtvedt T & Gordon JI Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev 62, 1157–1170 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gordon HA & Pesti L The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol. Rev 35, 390–429 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hooper LV et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001). [DOI] [PubMed] [Google Scholar]
- 132.Wostman BS Germfree and Gnotobiotic Animal Models. Background and Applications (CRC Press, 1996). [Google Scholar]
- 133.Arvidsson C, Hallen A & Backhed F Generating and analyzing germ-free mice. Curr. Protoc. Mouse Biol 2, 307–316 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Carter PB, Norin E & Swennes AG Gnotobiotics and the microbiome. In The Laboratory Rat 3rd edn (eds Suckow MA et al.) Ch. 21, 827–848 (2020). [Google Scholar]
- 135.Qv L et al. Methods for establishment and maintenance of germ-free rat models. Front. Microbiol 11, 1148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schoeb TR & Eaton KA Gnotobiotics (Academic Press, 2017). [Google Scholar]
- 137.Jervis-Bardy J et al. Deriving accurate microbiota profiles from human samples with low bacterial content through post-sequencing processing of Illumina MiSeq data. Microbiome 3, 19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saffarian A et al. Crypt- and mucosa-associated core microbiotas in humans and their alteration in colon cancer patients. mBio 10, e01315–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jorissen J et al. Case–control microbiome study of chronic otitis media with effusion in children points at Streptococcus salivarius as a pathobiont-inhibiting species. mSystems 6, e00056–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salzberg S Does the placenta have a bacterial microbiome? Forbes (1 June 2020); https://www.forbes.com/sites/stevensalzberg/2020/06/01/does-the-placenta-have-a-bacterial-microbiome/?sh=7ae092ea250b. [Google Scholar]
- 141.Jost T, Lacroix C, Braegger C & Chassard C Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr 110, 1253–1262 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Treven P et al. Evaluation of human milk microbiota by 16S rRNA gene next-generation sequencing (NGS) and cultivation/MALDI-TOF mass spectrometry identification. Front. Microbiol 10, 2612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bihl S et al. When to suspect contamination rather than colonization—lessons from a putative fetal sheep microbiome. Gut Microbes 14, 2005751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kennedy KM et al. Over-celling fetal microbial exposure. Cell 184, 5839–5841 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Eisenhofer R et al. Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol 27, 105–117 (2019). [DOI] [PubMed] [Google Scholar]