Abstract
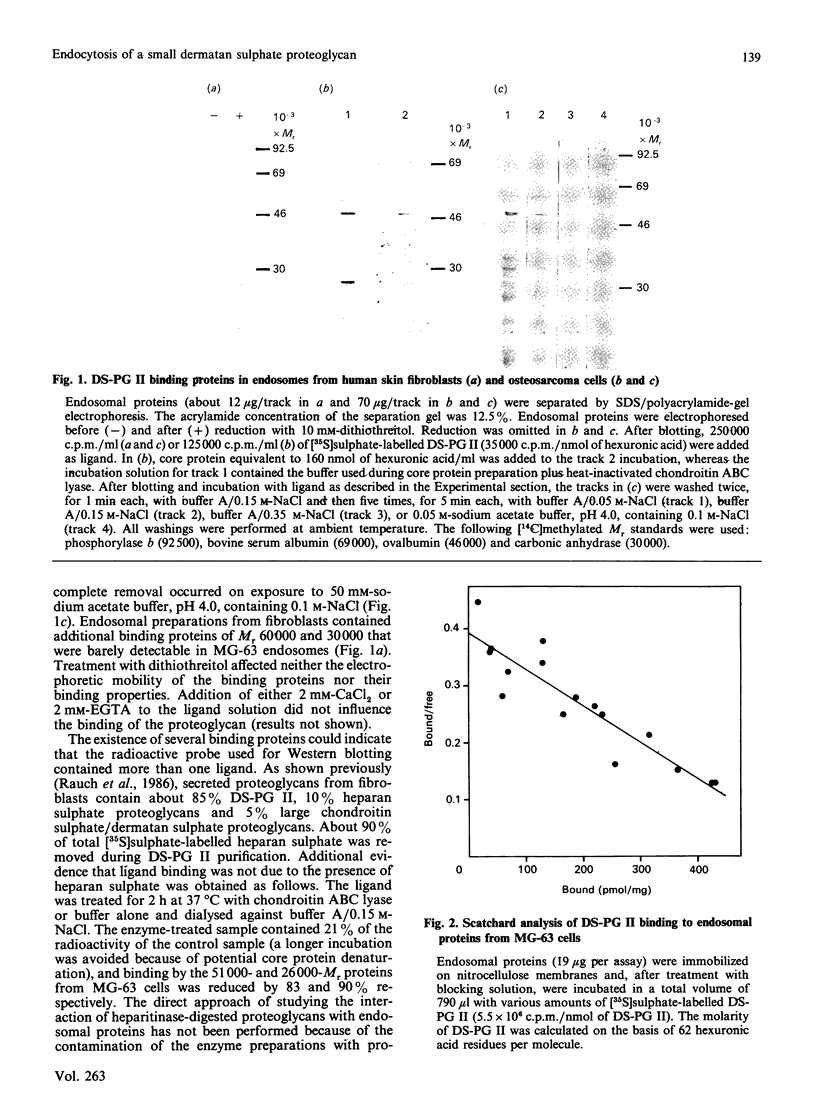
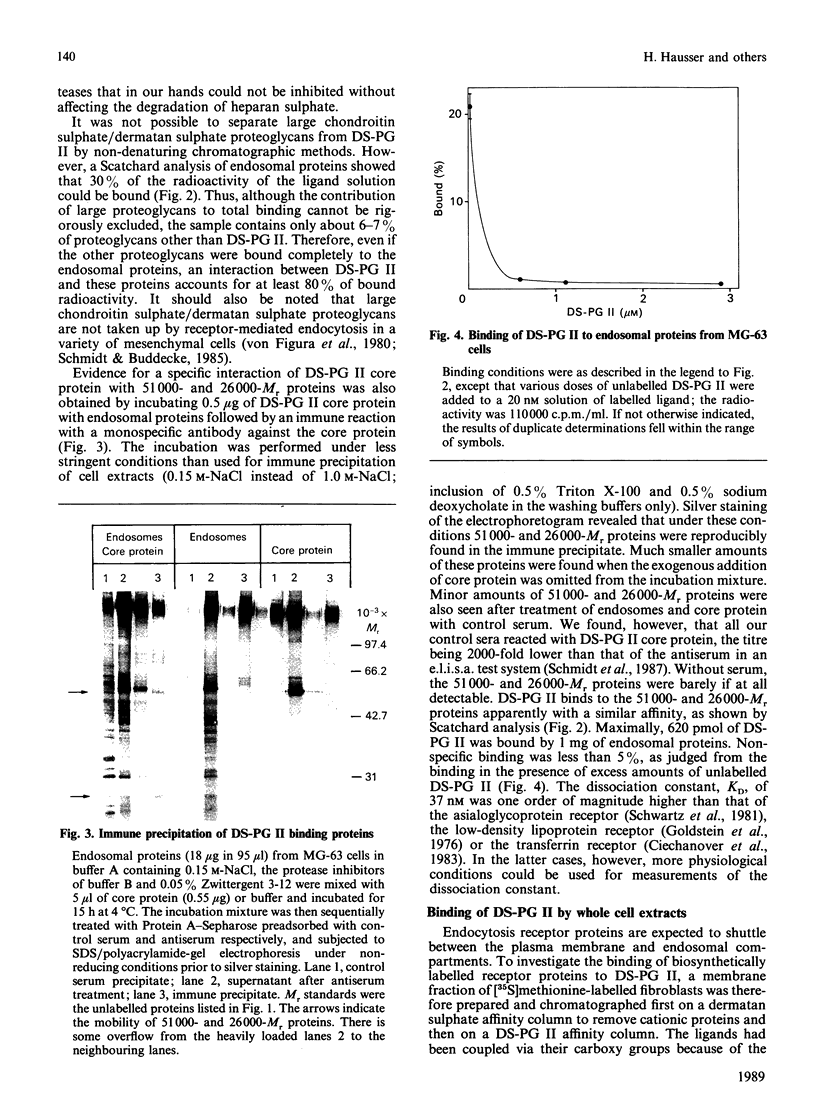
Endosomal preparations from human osteosarcoma cells and from fibroblasts contain 51,000- and 26,000-Mr proteins which bind a small dermatan sulphate proteoglycan after SDS/polyacrylamide-gel electrophoresis and Western blotting. Binding can be inhibited by unlabelled proteoglycan core protein. The proteins co-precipitate with a proteoglycan core protein-antibody complex. Scatchard analysis of immobilized endosomal proteins yielded a KD of about 37 nM for the proteoglycan. In intact cells proteins of the same size can be found. They are sensitive to trypsinization. A 51,000-Mr protein is the predominant membrane protein with strong binding to immobilized dermatan sulphate proteoglycan. There are additional proteoglycan-binding proteins with Mr values of around 30,000 and 14,000 which are insensitive to trypsin treatment. In contrast with the 51,000- and 26,000-Mr proteins, they resist deoxycholate/Triton X-100 extraction several days after subcultivation.
Full text
PDF

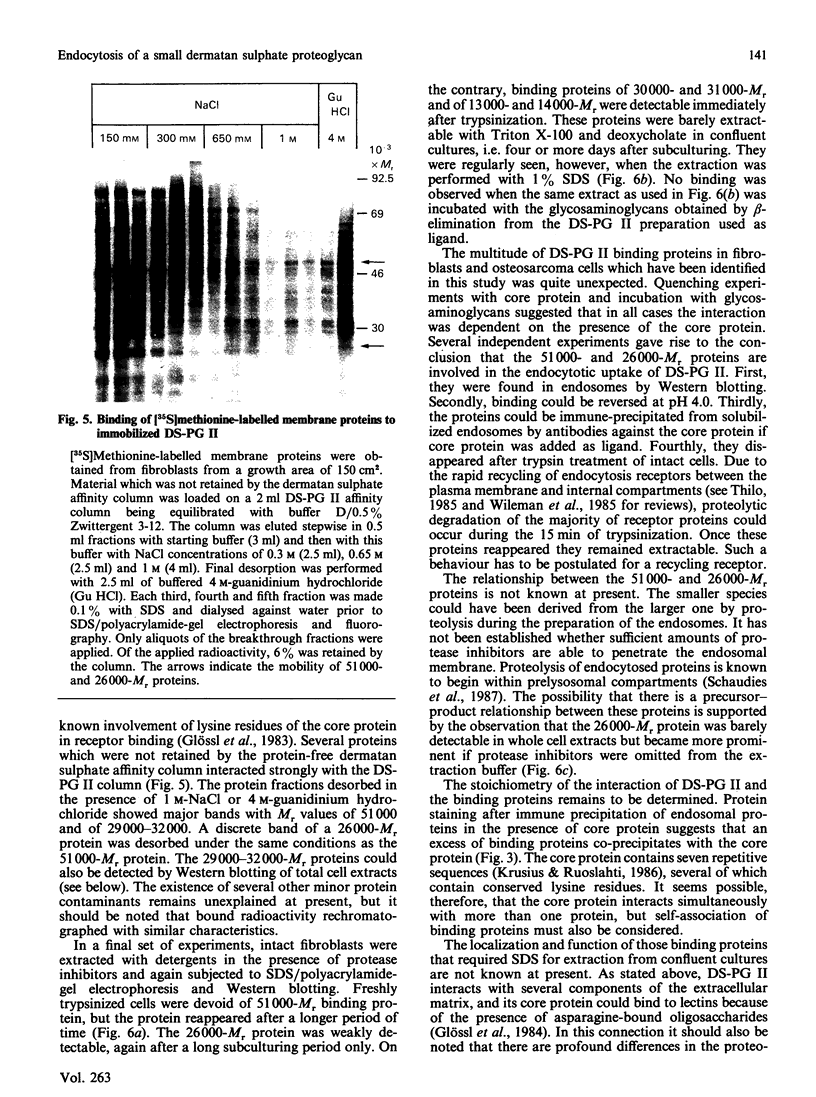
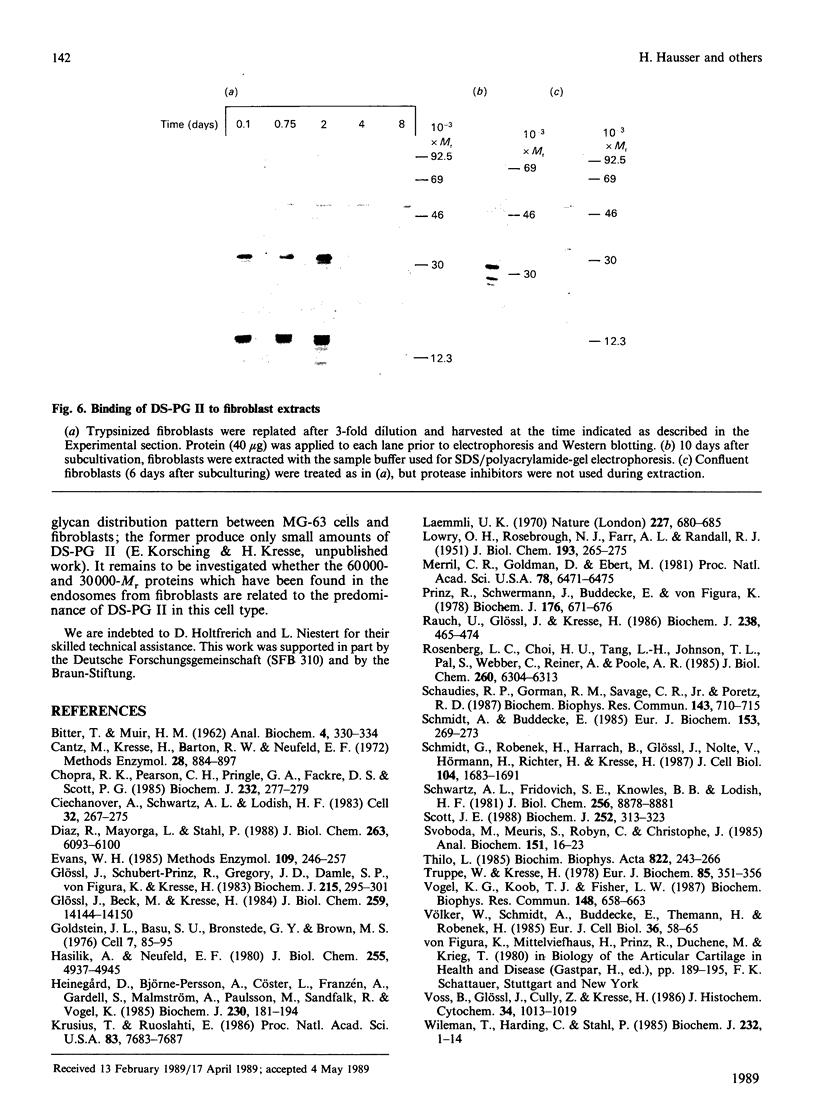
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Chopra R. K., Pearson C. H., Pringle G. A., Fackre D. S., Scott P. G. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem J. 1985 Nov 15;232(1):277–279. doi: 10.1042/bj2320277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. The asialoglycoprotein receptor internalizes and recycles independently of the transferrin and insulin receptors. Cell. 1983 Jan;32(1):267–275. doi: 10.1016/0092-8674(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Diaz R., Mayorga L., Stahl P. In vitro fusion of endosomes following receptor-mediated endocytosis. J Biol Chem. 1988 May 5;263(13):6093–6100. [PubMed] [Google Scholar]
- Evans W. H. Preparation of low-density "endosome" and "endosome"-depleted Golgi fractions from rat liver. Methods Enzymol. 1985;109:246–257. doi: 10.1016/0076-6879(85)09090-5. [DOI] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Glössl J., Schubert-Prinz R., Gregory J. D., Damle S. P., von Figura K., Kresse H. Receptor-mediated endocytosis of proteoglycans by human fibroblasts involves recognition of the protein core. Biochem J. 1983 Nov 1;215(2):295–301. doi: 10.1042/bj2150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Ebert M. Protein variations associated with Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6471–6475. doi: 10.1073/pnas.78.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz R., Schwermann J., Buddecke E., von Figura K. Endocytosis of sulphated proteoglycans by cultured skin fibroblasts. Biochem J. 1978 Dec 15;176(3):671–676. doi: 10.1042/bj1760671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U., Glössl J., Kresse H. Comparison of small proteoglycans from skin fibroblasts and vascular smooth-muscle cells. Biochem J. 1986 Sep 1;238(2):465–474. doi: 10.1042/bj2380465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Schaudies R. P., Gorman R. M., Savage C. R., Jr, Poretz R. D. Proteolytic processing of epidermal growth factor within endosomes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):710–715. doi: 10.1016/0006-291x(87)91412-4. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Buddecke E. High-uptake and low-uptake forms of proteoglycans secreted by arterial smooth muscle cells. Eur J Biochem. 1985 Dec 2;153(2):269–273. doi: 10.1111/j.1432-1033.1985.tb09297.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M., Meuris S., Robyn C., Christophe J. Rapid electrotransfer of proteins from polyacrylamide gel to nitrocellulose membrane using surface-conductive glass as anode. Anal Biochem. 1985 Nov 15;151(1):16–23. doi: 10.1016/0003-2697(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Thilo L. Quantification of endocytosis-derived membrane traffic. Biochim Biophys Acta. 1985 Sep 9;822(2):243–266. doi: 10.1016/0304-4157(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Truppe W., Kresse H. Uptake of proteoglycans and sulfated glycosaminoglycans by cultured skin fibroblasts. Eur J Biochem. 1978 Apr 17;85(2):351–356. doi: 10.1111/j.1432-1033.1978.tb12246.x. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J., Fisher L. W. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987 Oct 29;148(2):658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- Voss B., Glössl J., Cully Z., Kresse H. Immunocytochemical investigation on the distribution of small chondroitin sulfate-dermatan sulfate proteoglycan in the human. J Histochem Cytochem. 1986 Aug;34(8):1013–1019. doi: 10.1177/34.8.2426331. [DOI] [PubMed] [Google Scholar]
- Völker W., Schmidt A., Buddecke E., Themann H., Robenek H. Binding and degradation of proteoglycans by cultured arterial smooth muscle cells. II. Binding sites of proteoglycans on the cell surface. Eur J Cell Biol. 1985 Jan;36(1):58–65. [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]