Key Points
Question
How effective are scalable, relatively low-resource mindfulness-based interventions (MBIs) delivered via telehealth for veterans with chronic pain?
Findings
In this multisite randomized clinical trial, both group and self-paced MBIs improved pain-related function and biopsychosocial outcomes among veterans with chronic pain and high levels of psychiatric comorbidity. The likelihood of 30% improvement from baseline was greater for group MBI at 10 weeks and 6 months and for self-paced MBI at 10 weeks, 6 months, and 1 year.
Meaning
These scalable, relatively low-resource MBIs conducted virtually could help accelerate the implementation of nonpharmacological pain treatment in health care systems.
Abstract
Importance
Although mindfulness-based interventions (MBIs) are evidence-based treatments for chronic pain and comorbid conditions, implementing them at scale poses many challenges, such as the need for dedicated space and trained instructors.
Objective
To examine group and self-paced, scalable, telehealth MBIs, for veterans with chronic pain, compared to usual care.
Design, Setting, and Participants
This was a randomized clinical trial of veterans with moderate to severe chronic pain, recruited from 3 Veterans Affairs facilities from November 2020 to May 2022. Follow-up was completed in August 2023.
Interventions
Two 8-week telehealth MBIs (group and self-paced) were compared to usual care (control). The group MBI was done via videoconference with prerecorded mindfulness education and skill training videos by an experienced instructor, accompanied by facilitated discussions. The self-paced MBI was similar but completed asynchronously and supplemented by 3 individual facilitator calls.
Main Outcomes and Measures
The primary outcome was pain-related function using the Brief Pain Inventory interference scale at 3 time points: 10 weeks, 6 months, and 1 year. Secondary outcomes included biopsychosocial outcomes: pain intensity, physical function, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, patient ratings of improvement of pain, and posttraumatic stress disorder.
Results
Among 811 veterans randomized (mean [SD] age, 54.6 [12.9] years; 387 [47.7%] women), 694 participants (85.6%) completed the trial. Averaged across all 3 time points, pain interference scores were significantly lower for both MBIs compared to usual care (group MBI vs control difference: −0.4 [95% CI, −0.7 to −0.2]; self-paced vs control difference: −0.7 [95% CI, −1.0 to −0.4]). Additionally, both MBI arms had significantly better scores on the following secondary outcomes: pain intensity, patient global impression of change, physical function, fatigue, sleep disturbance, social roles and activities, depression, and posttraumatic stress disorder. Both group and self-paced MBIs did not significantly differ from one another. The probability of 30% improvement from baseline compared to control was greater for group MBI at 10 weeks and 6 months, and for self-paced MBI, at all 3 time points.
Conclusions and Relevance
In this randomized clinical trial, scalable telehealth MBIs improved pain-related function and biopsychosocial outcomes compared to usual care among veterans with chronic pain. Relatively low-resource telehealth-based MBIs could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems.
Trial Registration
ClinicalTrials.gov Identifier: NCT04526158
This randomized clinical trial examines the effectiveness of group and self-paced scalable, telehealth mindfulness-based interventions for veterans with chronic pain compared to usual care.
Introduction
Chronic pain, a prevalent, debilitating, and costly national problem,1,2 disproportionately affects veterans.3,4 Although guidelines now recommend evidence-based nonpharmacologic treatment approaches for chronic pain,5,6 they are underused due to myriad barriers at the patient, clinician, and organization levels.1,2,7,8,9,10 Mindfulness-based interventions (MBIs) can improve chronic pain5,11,12,13,14,15 and comorbid conditions, such as posttraumatic stress disorder (PTSD), sleep disorders, depression, and substance misuse,16,17 and are now recommended as a first-line treatment.5,18 However, many MBIs, including mindfulness-based stress reduction (MBSR), one of the most widely adopted MBIs,19 have features that pose barriers to implementation.12,20
This pragmatic randomized clinical trial (RCT) is part of the Pain Management Collaboratory.21,22 The study examined the effectiveness of 2 scalable telehealth approaches for delivering MBIs aimed at improving pain-related functioning and comorbid biopsychosocial conditions for veterans with chronic pain. Each MBI was adapted from MBSR, and they addressed previously identified implementation barriers at the organization level (eg, scalability) and patient level (eg, time required for sessions and practice and accessibility).12,20
Methods
Study Design and Participants
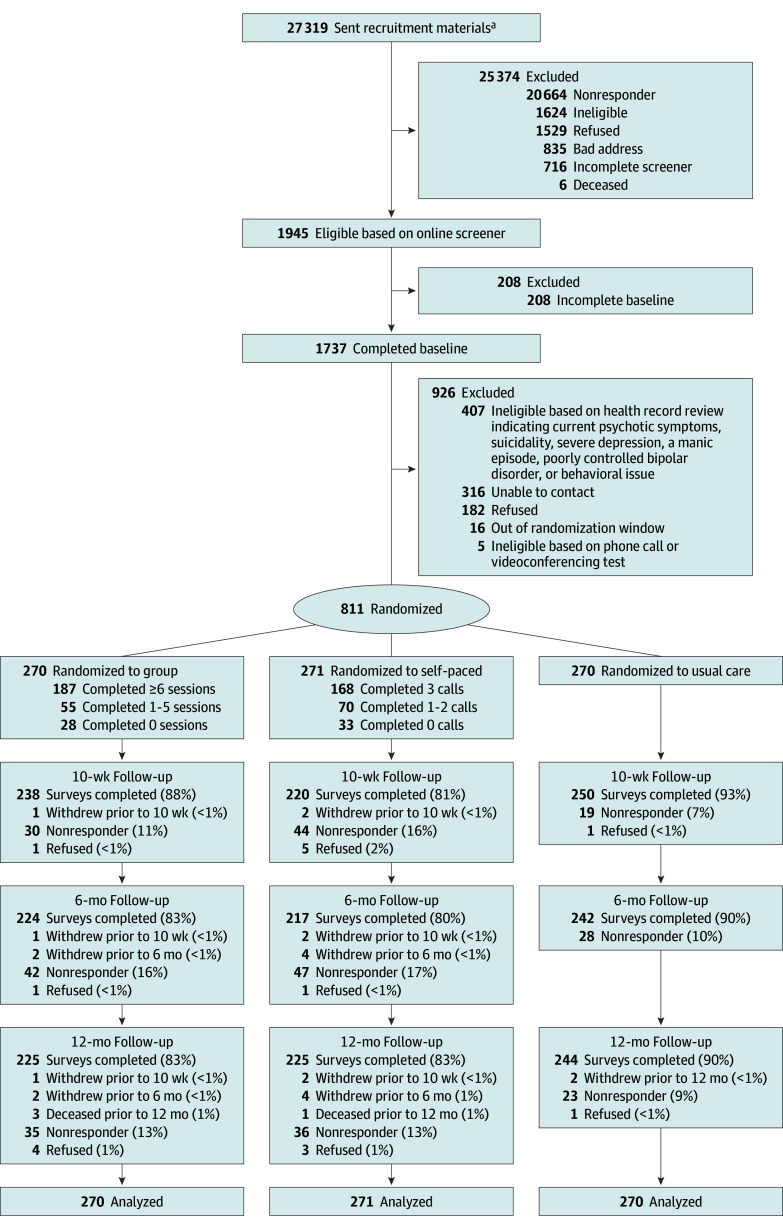
This RCT was approved by the VA Central Institutional Review Board before data collection (see Supplement 1 for the trial protocol). Oral informed consent was obtained from all participants. This study was an RCT comparing group MBI, self-paced MBI, and usual care (control). The study design was primarily pragmatic with explanatory features for intervention delivery and follow-up.21,23,24 A full description of the study can be found in prior publications.25,26 Adherence to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline was ensured. Study participants were recruited from November 2020 to May 2022 in 6 waves. Follow-up occurred from March 2021 to August 2023. Figure 1 delineates participant enrollment and follow-up.
Figure 1. CONSORT Flow Diagram.
The flow diagram shows the movement of 811 participants through trial recruitment, randomization, and final data analysis.
aPatients were randomly selected from a pool of 121 441 individuals.
Recruitment took place among patients at 3 VA Health Care Systems in Minneapolis, Minnesota; Durham, North Carolina; and Los Angeles, California. To be deemed eligible, participants must have met the following criteria: (1) two qualifying pain diagnoses in their electronic health record (EHR) within the same diagnostic category at least 90 days apart during the previous 2 years27 (eTable 1 in Supplement 2), (2) a pain duration of at least 6 months, (3) pain intensity score of at least 4 during the past week on a numerical rating scale from 0 to 10,28 (4) access to a smartphone and the internet, (5) willingness to engage in intervention-specific procedures (eg, meeting online for sessions), and (6) not currently enrolled in another pain study or MBSR program. We excluded patients based on medical record review indicating current psychotic symptoms, suicidality, severe depression, a manic episode, poorly controlled bipolar disorder, or indications of serious behavioral issues (eTable 2 in Supplement 2).
A gender-stratified random sample of potentially eligible VA patients was recruited from the EHR. Women, who represented 11% of veterans meeting EHR criteria, were oversampled. Patients were sent introductory materials inviting them to complete a brief screener via the study website. Patients who met initial eligibility criteria were asked to complete a baseline survey, after which study staff conducted a medical record review. Study staff then called eligible participants (prioritizing women to ensure balanced gender representation) and verified their availability, commitment, and possession of necessary technology.
Study participants were randomized to one of 3 study arms, group MBI, self-paced MBI, or usual care, in a 1:1:1 ratio. Allocation concealment methods were designed to maximize internal validity within the constraints of the study design.29,30 The computer-generated randomization list was concealed from the research team conducting eligibility assessments and enrollment, using a centralized electronic randomization system. Outcome assessors were blinded to group assignment.
Interventions
Participants in all 3 groups continued receiving pain care as usual. The usual care (control) arm did not have access to the MBIs but was given access to self-paced MBI materials after the follow-up period was complete. Descriptions of the interventions are provided in eTable 3 in Supplement 2 using the Template for Intervention Description and Replication (TIDieR) checklist and guideline.31 The design of the 2 MBI arms was informed by the Behavior Change Wheel model,32 which incorporated behavioral change strategies to enhance participants’ capability, opportunity, and motivation to engage in helpful pain self-management behaviors.32 Five facilitators (4 authors, K.B., L.J.S.C., M.B., M.M., and 1 nonauthor, Sierra Hennessy) were trained to deliver the MBIs.
Group MBI Arm
The group MBI consisted of eight 90-minute weekly group sessions with between 6 and 16 participants. The sessions were delivered via secure videoconferencing and preceded by a 90-minute technical session. The program consisted of educational and instructional videos presented by a trained, certified mindfulness instructor (A.C.H) rated as advanced by the Mindfulness-Based Interventions: Teaching Assessment Criteria.33
Content focused on providing participants with opportunities and resources to develop their mindfulness and pain self-management capabilities and motivations.32 This included mindfulness-related knowledge and skills in regulating attention and emotions, establishing body awareness, and shifting self-perceptions, considered essential content of MBIs.33 In-session video viewing was interspersed with workbook reflections and group discussions, led by a trained facilitator. Participants were encouraged to practice on their own between sessions, using a workbook, mobile application, and study website, which included the same videos presented in the group sessions.
Self-Paced MBI Arm
The self-paced MBI consisted of eight 30- to 60-minute asynchronous weekly sessions of the same curriculum and materials as the group MBI but without group interaction. Participants received 3 phone calls from a facilitator at the beginning, middle, and end of the program (25-60 minutes each) to address technical/logistical issues and discuss progress, plans for practice, and strategies to address challenges.
Main Outcomes and Measures
Primary and secondary outcomes were assessed at baseline, 10 weeks, 6 months, and 12 months. We used modified Dillman survey procedures, described elsewhere.25 Follow-up outcome assessments were conducted mostly by online surveys; if necessary, surveys were completed by postal mail.
Primary Outcome
The primary outcome, change in pain-related function over time, was assessed using the Brief Pain Inventory (BPI) interference subscale score.34,35,36 This subscale assessed pain interference levels, ranging from 0 to 10, in the following 7 domains: general activity, mood, walking, work, relationships, sleep, and enjoyment of life. Responder analyses (reporting percentages of participants with no improvement or who were worsening, improving by 30% or more, 50% or more, and 75% or more) were assessed at all time points. Thresholds of 30% and 50% improvement from baseline were considered moderate and substantial improvement, respectively.24,37
Secondary Outcomes
Patient-Reported Outcomes Measurement Information System 29-item profile (PROMIS-29, version 2.0) measures were used to assess physical function, anxiety, fatigue, sleep disturbance, and participation in social roles and activities.38,39 Depression was assessed by the Patient Health Questionnaire depression scale (PHQ-8).40 The PTSD Checklist for the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (PCL-5) was used for assessing PTSD severity.41 We also assessed the following using self-report methods: pain intensity (using the BPI intensity score, which uses a 0 to 10 numeric rating scale for average pain, current pain, worst pain, and least pain with higher scores indicating worse pain), perceived change in pain (using the Pain Global Rating of Change, a single-item measure of global improvement with treatment on a 7-point scale),28 adverse events, and use of additional pain treatments.42
Reported participant characteristics included demographics, study site, pain diagnoses, mental and behavioral health diagnoses from the EHR, primary and secondary outcomes, and potential mediators. Race and ethnicity were assessed on the baseline survey using predefined response options to describe the sample's sociodemographic characteristics.
Statistical Analysis
Sample size was based on the ability to detect an effect size of 0.33 between groups in pain-related function over the entire 1-year follow-up period with 80% power and a 2-sided α level of .0167 to account for the 3 group comparisons.25 The initial target sample size of 750 was increased to 811 to increase the number of women enrolled. Analyses were based on the intention-to-treat method for all randomized participants. All model-based analyses incorporated design factors of the gender-stratified sampling frame, survey wave, and site. For analyses of the primary outcome, we regressed BPI interference score on randomized group assignment using a linear mixed model for repeated measures with random intercepts for participants. In addition to study design factors, we adjusted for baseline values of BPI interference score and baseline pain self-efficacy, which varied among groups. Because the intraclass correlation coefficient for the group MBI was very small (0.005) and had no impact on results, it was not accounted for in the primary analysis. Between-group differences over the entire follow-up period were the primary test of treatment-group differences (for all 3 comparisons of the 3 arms). Between-group differences were estimated for each of the time points (ie, 10 weeks, 6 months, and 1 year) using a 2-way interaction between group and categorical time. Potential effect modification due to gender for the primary outcome was assessed using a 3-way interaction between gender, group, and categorical time. Secondary outcomes were similarly analyzed using linear mixed-effect models. All secondary group comparisons were exploratory and no adjustments for multiplicity were applied. Mediational and additional gender subgroup analyses will be presented in a separate report.
We addressed missing data using multiple imputation with chained equations stratified by intervention to generate 200 imputed datasets. The imputation models included all variables specified for the primary analysis and additional auxiliary variables based on results from stepwise regression hypothesis testing with a significance level of P < .10. All analyses were performed using Stata statistical software, version 18 (StataCorp LLC). Regression estimates and variances from imputed datasets were generated using the mimrgns Stata program, which combines results using Rubin rules.43
Results
Study Participants
Among 811 veterans randomized (mean [SD] age, 54.6 [12.9] years; 387 [47.7%] women) (Table 1), 694 participants (85.6%) completed the trial(Figure 1). The racial and ethnic breakdown of the sample was 10 American Indian/Alaska Native (1.2%); 6 Asian American (0.7%); 204 Black or African American (25.2%); 51 (6.3%) Hispanic; 1 Native Hawaiian/Pacific Islander (0.1%); and 536 White (66.1%) participants. A total of 404 participants (49.8%) attained a bachelor’s degree or higher, and only 248 (30.6%) reported a comfortable household financial situation (Table 1). Extremity pain/arthritis was the most common diagnosis, affecting 562 participants (69.3%), followed by 387 participants (48%) with back pain. Overall, 509 participants (62.8%) had at least 1 mental illness diagnosis in the EHR (eTable 4 in Supplement 2). Baseline characteristics between the randomized groups were similar, including pain treatment use; however, pain self-efficacy scores were higher among participants in the self-paced group (Table 1).
Table 1. Baseline Sociodemographic and Pain-Related Characteristics by Randomization Arm.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Group | Self-paced | Usual care | Total | |
| Participants, No. | 270 | 271 | 270 | 811 |
| Demographics | ||||
| Gender | ||||
| Man | 137 (50.7) | 143 (52.8) | 144 (53.3) | 424 (52.3) |
| Woman | 133 (49.3) | 128 (47.2) | 126 (46.7) | 387 (47.7) |
| Age, mean (SD), y | 54.6 (12.9) | 55.7 (13.0) | 55.0 (12.2) | 54.6 (12.9) |
| Race and ethnicity | ||||
| American Indian/Alaska Native | 2 (0.8) | 2 (0.8) | 6 (2.3) | 10 (1.2) |
| Asian American | 1 (0.4) | 3 (1.1) | 2 (0.8) | 6 (0.8) |
| Black or African American | 72 (27.3) | 66 (24.9) | 66 (25.0) | 204 (25.7) |
| Hispanic | 15 (5.6) | 16 (5.9) | 20 (7.4) | 51 (6.3) |
| Native Hawaiian/Pacific Islander | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| White | 179 (67.8) | 180 (67.9) | 177 (67.1) | 536 (66.1%) |
| Multiracial | 9 (3.4) | 14 (5.3) | 13 (4.9) | 36 (4.5) |
| Household financial situation | ||||
| Live comfortably | 83 (30.7) | 77 (28.4) | 88 (32.6) | 248 (30.6) |
| Meet your basic expenses with a little left over for extras | 127 (47.0) | 115 (42.4) | 102 (37.8) | 344 (42.4) |
| Just meet your basic expenses | 53 (19.6) | 68 (25.1) | 68 (25.2) | 189 (23.3) |
| Do not even have enough to meet basic expenses | 7 (2.6) | 11 (4.1) | 12 (4.4) | 30 (3.7) |
| Marital status | ||||
| Married | 139 (51.5) | 134 (49.5) | 155 (57.4) | 428 (52.8) |
| Divorced/separated/widowed | 73 (27.0) | 81 (29.9) | 71 (26.3) | 225 (27.7) |
| Never married/single | 50 (18.5) | 53 (19.6) | 43 (15.9) | 146 (18.0) |
| Unknown | 8 (3.0) | 3 (1.1) | 1 (0.4) | 12 (1.5) |
| Employment status | ||||
| Employed | 118 (43.7) | 106 (39.1) | 109 (40.4) | 333 (41.1) |
| Disabled | 57 (21.1) | 55 (20.3) | 65 (24.1) | 177 (21.8) |
| Retired | 68 (25.2) | 73 (26.9) | 63 (23.3) | 204 (25.2) |
| Other | 27 (10.0) | 37 (13.7) | 33 (12.2) | 97 (12.0) |
| Education | ||||
| ≤High school | 21 (7.8) | 16 (5.9) | 16 (5.9) | 53 (6.5) |
| Some college | 119 (44.1) | 116 (42.8) | 119 (44.1) | 354 (43.7) |
| Bachelor’s degree | 69 (25.6) | 77 (28.4) | 75 (27.8) | 221 (27.3) |
| ≥Master’s degree | 61 (22.6) | 62 (22.9) | 60 (22.2) | 183 (22.6) |
| Rurality | ||||
| Urban | 167 (61.9) | 158 (58.3) | 173 (64.1) | 498 (61.4) |
| Rural | 87 (32.2) | 96 (35.4) | 82 (30.4) | 265 (32.7) |
| Highly rural | 3 (1.1) | 3 (1.1) | 3 (1.1) | 9 (1.1) |
| Recruitment site | ||||
| Durham, North Carolina | 111 (41.1) | 125 (46.1) | 106 (39.3) | 342 (42.2) |
| Minneapolis, Minnesota | 116 (43.0) | 104 (38.4) | 125 (46.3) | 345 (42.5) |
| Los Angeles, California | 43 (15.9) | 42 (15.5) | 39 (14.4) | 124 (15.3) |
| Primary and secondary outcomes | ||||
| Pain functioning (BPI Pain Interference) | 5.8 (1.9) | 5.4 (2.0) | 5.6 (1.9) | 5.6 (2.0) |
| Pain intensity (BPI Pain Intensity) | 5.6 (1.6) | 5.4 (1.5) | 5.6 (1.6) | 5.5 (1.6) |
| Physical function (PROMIS) | 12.3 (3.2) | 12.4 (3.4) | 12.3 (3.5) | 12.3 (3.4) |
| Anxiety (PROMIS) | 9.8 (3.9) | 9.7 (3.8) | 9.9 (4.0) | 9.8 (3.9) |
| Fatigue (PROMIS) | 13.9 (4.0) | 13.6 (4.1) | 13.6 (4.1) | 13.7 (4.1) |
| Sleep disturbance (PROMIS) | 14.2 (3.5) | 14.0 (3.8) | 14.3 (3.7) | 14.2 (3.7) |
| Participation in social roles and activities (PROMIS) | 10.0 (2.9) | 10.6 (3.2) | 10.4 (3.3) | 10.3 (3.1) |
| Depression (PHQ-8) | 9.8 (5.8) | 9.4 (5.7) | 9.5 (5.7) | 9.6 (5.8) |
| PTSD (PCL-5) | 26.8 (18.6) | 27.2 (19.7) | 27.8 (20.1) | 27.2 (19.5) |
| Mediators | ||||
| Pain catastrophizing (PCS) | 23.5 (11.4) | 21.4 (10.8) | 22.3 (11.6) | 22.4 (11.3) |
| Pain self-efficacy (PSEQ) | 30.1 (11.9) | 33.0 (12.7) | 31.4 (11.7) | 31.5 (12.1) |
| Perceived stress (PSFF) | 31.5 (4.1) | 31.4 (4.1) | 31.0 (4.0) | 31.3 (4.1) |
| Mindfulness (AMPS) | 26.8 (14.2) | 26.2 (13.9) | 25.8 (13.7) | 26.3 (13.9) |
Abbreviations: AMPS, Applied Mindfulness Process Scale; BPI, Brief Pain Inventory; PCL-5, 5-Item PTSD Checklist; PCS, Pain Catastrophizing Scale; PHQ-8, 8-Item Patient Health Questionnaire; PROMIS, Patient-Reported Outcomes Measurement Information System; PSEQ, Pain Self-Efficacy Questionnaire; PSFF, Perceived Stress Fixed Form; PTSD, posttraumatic stress disorder.
Relative to baseline, all 3 groups continued to use multiple pain treatments at similar frequencies over the 1-year follow-up. The only exceptions were the increased use of relaxation techniques and meditation/mindfulness, with much larger increases, as expected, in the 2 MBI groups (eTable 5 in Supplement 2).
Intervention Engagement
Adherence rates were 187 of 270 participants (69%) in the group MBI (defined as completing 6 or more of the 9 sessions) and 207 of 271 (76%) in the self-paced MBI (defined as completing 2 or more of the 3 facilitator calls). At 10 weeks for both MBI arms, 425 of 541 participants (78.6%) reported weekly engagement in mindful mini practices; 360 of 541 (66.5%) continued to engage in the mini practices at 12 months (eTable 6 in Supplement 2).
Primary Outcomes
No significant effect modification by gender was found for the primary outcome; thus, gender was included as a covariate in the primary model with no interaction term. Missing data imputation models included all variables specified for the primary analysis and 4 auxiliary variables significantly associated with missingness (age at index date, employment status, an indicator for any mental health diagnosis, and an indicator for an attention deficit and hyperactivity disorder diagnosis).
Averaged across the 3 follow-up time points, the group MBI arm had significantly lower BPI interference scores compared to usual care (group MBI vs usual care difference: −0.4 [95% CI, −0.7 to −0.2]; P = .002; effect size: −0.2 [95% CI, −0.4 to −0.1]). Similarly, the self-paced MBI arm had significantly lower BPI interference scores compared to usual care (self-paced vs control score difference: −0.7 [95% CI, −1.0 to −0.4]; P < .001; effect size: −0.4 [95% CI, −0.5 to −0.2]). Comparing the MBI arms with each other, no significant difference was found averaged across 3 time points, using a threshold of .0167 to adjust for multiple comparisons (group vs self-paced MBI score difference: 0.3 [95% CI, 0.0-0.6]; P = .03; effect size: 0.2 [95% CI, 0.0-0.3]) (Table 2).
Table 2. Patient-Reported Outcomes Among Group Mindfulness-Based Intervention (MBI) Arm, Self-Paced MBI Arm, or Usual Carea.
| Outcome | Mean (SE) | Difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Group MBI | Self-paced MBI | Usual care arm | Group MBI vs self-paced MBI | Group MBI vs usual care | Self-paced MBI vs usual care | |||
| Pain-related function (primary outcome) | ||||||||
| BPI interference subscale (range, 0-10; higher score = worse) | ||||||||
| 10 wk | 4.8 (0.1) | 4.4 (0.1) | 5.3 (0.1) | 0.4 (0.1 to 0.8) | −0.5 (−0.9 to −0.2) | −1.0 (−1.3 to −0.6) | ||
| 6 mo | 4.7 (0.1) | 4.5 (0.1) | 5.2 (0.1) | 0.2 (−0.2 to 0.5) | −0.5 (−0.8 to −0.1) | −0.6 (−1.0 to −0.3) | ||
| 1 y | 4.8 (0.1) | 4.5 (0.1) | 5.0 (0.1) | 0.3 (−0.1 to 0.6) | −0.3 (−0.6 to 0.1) | −0.6 (−0.9 to −0.2) | ||
| Averaged over 3 time points | 4.8 (0.1) | 4.5 (0.1) | 5.2 (0.1) | 0.3 (0.0 to 0.6) | −0.4 (−0.7 to −0.2) | −0.7 (−1.0 to −0.4) | ||
| Overall P valueb | NA | NA | NA | .03 | .002 | <.001 | ||
| Pain intensity (secondary outcome) | ||||||||
| BPI pain intensity subscale (range, 0-10; higher score = worse) | ||||||||
| 10 wk | 4.5 (0.1) | 4.6 (0.1) | 5.3 (0.1) | −0.0 (−0.3 to 0.3) | −0.7 (−1.0 to −0.4) | −0.7 (−1.0 to −0.4) | ||
| 6 mo | 4.8 (0.1) | 4.7 (0.1) | 5.0 (0.1) | 0.1 (−0.2 to 0.4) | −0.2 (−0.5 to 0.0) | −0.4 (−0.7 to −0.1) | ||
| 1 y | 4.7 (0.1) | 4.6 (0.1) | 4.9 (0.1) | 0.0 (−0.3 to 0.3) | −0.2 (−0.5 to 0.1) | −0.3 (−0.6 to 0.0) | ||
| Averaged over 3 time points | 4.7 (0.1) | 4.6 (0.1) | 5.1 (0.1) | 0.0 (−0.2 to 0.3) | −0.4 (−0.6 to −0.2) | −0.4 (−0.7 to −0.2) | ||
| Overall P valueb | NA | NA | NA | .74 | <.001 | <.001 | ||
| Perceived change in pain (secondary outcome) | ||||||||
| Patient Pain Global Rating of Change (range, 1-7; higher score = worse) | ||||||||
| 10 wk | 3.1 (0.1) | 3.2 (0.1) | 4.2 (0.1) | −0.1 (−0.3 to 0.2) | −1.1 (−1.3 to −0.8) | −1.0 (−1.3 to −0.7) | ||
| 6 mo | 3.6 (0.1) | 3.4 (0.1) | 4.1 (0.1) | 0.2 (−0.1 to 0.4) | −0.5 (−0.8 to −0.3) | −0.7 (−1.0 to −0.4) | ||
| 1 y | 3.6 (0.1) | 3.4 (0.1) | 4.0 (0.1) | 0.2 (−0.1 to 0.4) | −0.5 (−0.8 to −0.2) | −0.7 (−0.9 to −0.4) | ||
| Averaged over 3 time points | 3.4 (0.1) | 3.3 (0.1) | 4.1 (0.1) | 0.1 (−0.1 to 0.3) | −0.7 (−0.9 to −0.5) | −0.8 (−1.0 to −0.6) | ||
| Overall P valueb | NA | NA | NA | .44 | <.001 | <.001 | ||
| Physical function (secondary outcome) | ||||||||
| PROMIS physical function subscale (range, 4-20; lower score = worse) | ||||||||
| 10 wk | 13.3 (0.2) | 13.4 (0.2) | 12.5 (0.2) | −0.0 (−0.5 to 0.5) | 0.8 (0.4 to 1.3) | 0.9 (0.4 to 1.3) | ||
| 6 mo | 13.3 (0.2) | 13.2 (0.2) | 12.8 (0.2) | 0.1 (−0.4 to 0.6) | 0.5 (−0.0 to 0.9) | 0.4 (−0.1 to 0.9) | ||
| 1 y | 13.2 (0.2) | 13.4 (0.2) | 12.9 (0.2) | −0.2 (−0.7 to 0.3) | 0.3 (−0.2 to 0.8) | 0.5 (0.0 to 1.0) | ||
| Averaged over 3 time points | 13.3 (0.1) | 13.3 (0.1) | 12.7 (0.1) | −0.0 (−0.4 to 0.4) | 0.5 (0.2 to 0.9) | 0.6 (0.2 to 1.0) | ||
| Overall P valueb | NA | NA | NA | .83 | .01 | .004 | ||
| Fatigue (secondary outcome) | ||||||||
| PROMIS fatigue subscale (range, 4-20; higher score = worse) | ||||||||
| 10 wk | 12.9 (0.2) | 12.6 (0.2) | 13.5 (0.2) | 0.2 (−0.4 to 0.9) | −0.7 (−1.3 to −0.1) | −0.9 (−1.5 to −0.3) | ||
| 6 mo | 13.0 (0.2) | 12.8 (0.2) | 13.3 (0.2) | 0.1 (−0.5 to 0.8) | −0.4 (−1.0 to 0.3) | −0.5 (−1.1 to 0.1) | ||
| 1 y | 12.9 (0.2) | 12.6 (0.2) | 13.5 (0.2) | 0.2 (−0.5 to 0.9) | −0.7 (−1.3 to −0.1) | −0.9 (−1.5 to −0.3) | ||
| Averaged over 3 time points | 12.9 (0.2) | 12.7 (0.2) | 13.5 (0.2) | 0.2 (−0.3 to 0.7) | −0.6 (−1.1 to −0.1) | −0.8 (−1.3 to −0.3) | ||
| Overall P valueb | NA | NA | NA | .46 | .02 | .002 | ||
| Sleep disturbance (secondary outcome) | ||||||||
| PROMIS sleep disturbance subscale (range, 4-20; higher score = worse) | ||||||||
| 10 wk | 13.1 (0.2) | 13.2 (0.2) | 14.0 (0.2) | −0.2 (−0.8 to 0.4) | −1.0 (−1.5 to −0.4) | −0.8 (−1.3 to −0.2) | ||
| 6 mo | 13.5 (0.2) | 13.2 (0.2) | 13.9 (0.2) | 0.3 (−0.3 to 0.9) | −0.3 (−0.9 to 0.2) | −0.6 (−1.2 to −0.0) | ||
| 1 y | 13.2 (0.2) | 13.3 (0.2) | 13.9 (0.2) | −0.2 (−0.8 to 0.4) | −0.7 (−1.3 to −0.1) | −0.5 (−1.1 to 0.1) | ||
| Averaged over 3 time points | 13.3 (0.2) | 13.3 (0.2) | 13.9 (0.2) | −0.0 (−0.5 to 0.4) | −0.7 (−1.1 to −0.2) | −0.6 (−1.1 to −0.2) | ||
| Overall P valueb | NA | NA | NA | .89 | .004 | .01 | ||
| Social roles and responsibilities (secondary outcome) | ||||||||
| PROMIS participation in social roles and activities subscale (range, 4-20; lower score = worse) | ||||||||
| 10 wk | 11.9 (0.2) | 11.7 (0.2) | 10.7 (0.2) | 0.1 (−0.4 to 0.7) | 1.2 (0.7 to 1.7) | 1.0 (0.5 to 1.5) | ||
| 6 mo | 12.1 (0.2) | 11.9 (0.2) | 11.2 (0.2) | 0.2 (−0.4 to 0.7) | 0.9 (0.4 to 1.4) | 0.7 (0.2 to 1.2) | ||
| 1 y | 12.1 (0.2) | 12.2 (0.2) | 11.2 (0.2) | −0.1 (−0.7 to 0.4) | 0.9 (0.4 to 1.4) | 1.0 (0.5 to 1.5) | ||
| Averaged over 3 time points | 12.0 (0.2) | 11.9 (0.2) | 11.0 (0.1) | 0.1 (−0.4 to 0.5) | 1.0 (0.6 to 1.4) | 0.9 (0.5 to 1.3) | ||
| Overall P valueb | NA | NA | NA | .76 | <.001 | <.001 | ||
| Depression (secondary outcome) | ||||||||
| PHQ-8 (range, 0-24; higher score = worse) | ||||||||
| 10 wk | 8.3 (0.3) | 8.3 (0.3) | 9.0 (0.3) | 0.0 (−0.8 to 0.8) | −0.8 (−1.5 to 0.0) | −0.8 (−1.6 to −0.0) | ||
| 6 mo | 8.1 (0.3) | 8.4 (0.3) | 9.1 (0.3) | −0.3 (−1.1 to 0.5) | −1.0 (−1.8 to −0.2) | −0.7 (−1.5 to 0.1) | ||
| 1 y | 8.1 (0.3) | 8.0 (0.3) | 9.1 (0.3) | 0.1 (−0.7 to 0.9) | −1.0 (−1.8 to −0.2) | −1.1 (−1.9 to −0.3) | ||
| Averaged over 3 time points | 8.2 (0.2) | 8.2 (0.2) | 9.1 (0.2) | −0.0 (−0.7 to 0.6) | −0.9 (−1.5 to −0.3) | −0.9 (−1.5 to −0.2) | ||
| Overall P valueb | NA | NA | NA | .89 | .003 | .01 | ||
| Anxiety (secondary outcome) | ||||||||
| PROMIS anxiety subscale (range, 4-20; higher scores = worse) | ||||||||
| 10 wk | 9.1 (0.2) | 8.8 (0.2) | 9.3 (0.2) | 0.3 (−0.2 to 0.8) | −0.2 (−0.7 to 0.3) | −0.5 (−1.0 to 0.0) | ||
| 6 mo | 9.3 (0.2) | 8.7 (0.2) | 9.5 (0.2) | 0.5 (0.0 to 1.1) | −0.2 (−0.7 to 0.3) | −0.8 (−1.3 to −0.2) | ||
| 1 y | 9.3 (0.2) | 9.0 (0.2) | 9.5 (0.2) | 0.3 (−0.3 to 0.8) | −0.2 (−0.8 to 0.3) | −0.5 (−1.1 to 0.0) | ||
| Averaged over 3 time points | 9.2 (0.2) | 8.8 (0.2) | 9.4 (0.2) | 0.4 (−0.0 to 0.8) | −0.2 (−0.6 to 0.2) | −0.6 (−1.0 to −0.2) | ||
| Overall P valueb | NA | NA | NA | 0.08 | 0.3 | 0.005 | ||
| PTSD (secondary outcome) | ||||||||
| PCL-5 (range, 0-80; higher score = worse) | ||||||||
| 10 wk | 23.4 (0.8) | 22.8 (0.8) | 25.4 (0.8) | 0.6 (−1.6 to 2.8) | −2.0 (−4.1 to 0.1) | −2.6 (−4.7 to −0.4) | ||
| 6 mo | 22.5 (0.8) | 22.6 (0.8) | 24.8 (0.8) | −0.1 (−2.4 to 2.1) | −2.3 (−4.4 to −0.2) | −2.2 (−4.3 to 0.0) | ||
| 1 y | 22.5 (0.8) | 22.6 (0.8) | 24.4 (0.8) | −0.0 (−2.3 to 2.3) | −1.9 (−4.1 to 0.3) | −1.8 (−4.1 to 0.4) | ||
| Averaged over 3 time points | 22.8 (0.6) | 22.7 (0.6) | 24.9 (0.6) | 0.1 (−1.6 to 1.9) | −2.0 (−3.7 to −0.4) | −2.2 (−3.9 to −0.5) | ||
| Overall P valueb | NA | NA | NA | .89 | .02 | .01 | ||
Abbreviations: BPI, Brief Pain Inventory; NA, not applicable; PCL-5, 5-Item Posttraumatic Stress Disorder Checklist; PHQ-8, 8-Item Patient Health Questionnaire; PROMIS, Patient-Reported Outcomes Measurement Information System; PTSD, posttraumatic stress disorder; SE, standard error.
Estimates and SEs are calculated from linear mixed models with a random intercept for each participant, fit to multiply imputed datasets. Models were adjusted for participant cohort wave, Veterans Affairs recruitment site, gender, baseline pain self-efficacy score, and baseline value of the given model outcome (except for perceived change in pain, for which there was no baseline measurement).
The P value threshold for the primary outcome was adjusted for multiple comparisons to .0167 rather than .05. Thus, the difference between group MBI and self-paced MBI for pain interference was not statistically significant at this updated threshold.
Compared to usual care, both MBIs had significantly lower (improved) mean BPI interference scores at 10 weeks and 6 months. Participants in the self-paced MBI arm also reported lower BPI interference scores at 1 year (Figure 2).
Figure 2. Mean Brief Pain Inventory (BPI) Interference Score by Mindfulness-Based Intervention (MBI) Arm vs Usual Care.
Estimates are calculated from linear mixed models with a random intercept for each participant, fit to multiply imputed datasets. Models were adjusted for participant cohort wave, Veterans Affairs recruitment site, gender, and baseline pain self-efficacy score. Models at each time point (10 weeks, 6 months, and 1 year) were adjusted for baseline mean BPI Interference score.
Responder Analyses
The probability of 30% improvement from baseline on BPI interference scores compared to control was significantly greater for the group MBI arm at 10 weeks (control: 15.9%; group MBI: 33.6%; self-paced MBI: 40.3%) and 6 months (control: 22.2%; group MBI: 34.4%; self-paced MBI: 38.2%), and at all time points for the self-paced MBI, including 1 year (control: 24.1%; group MBI: 30.3%; self-paced MBI: 42.2%). The probability of 50% improvement relative to usual care for the group MBI arm was also greater at 10 weeks (control: 6.6%; group MBI: 14.0%; self-paced MBI: 21.3%), and at all time points for the self-paced MBI, including 6 months (control: 10.4%; group MBI: 14.7%; self-paced MBI: 19.2%) and 12 months (control: 13.3%; group MBI: 16.6%; self-paced MBI: 20.8%) (Table 3).
Table 3. Probability Estimates for Changes in the Primary Outcome by Mindfulness-Based Intervention (MBI) Arm and Usual Care at Follow-Up Time Pointsa.
| Pain interference reduction | Improvement probability, % | Probability difference (95% CI), pp | ||||
|---|---|---|---|---|---|---|
| Group MBI | Self-paced MBI | Usual care | Group MBI minus self-paced MBI | Group MBI minus usual care | Self-paced MBI minus usual care | |
| 10 wk | ||||||
| No reduction or worsening | 33.8b | 30.7b | 43.7 | 3.2 (−5.2 to 11.5) | −9.8 (−18.1 to −1.6) | −13.0 (−21.3 to −4.7) |
| ≥30% | 33.6b | 40.3b | 15.9 | −6.7 (−15.4 to 1.9) | 17.6 (10.2 to 25.0) | 24.4 (16.6 to 32.1) |
| ≥50% | 14.0b | 21.3b,c | 6.6 | −7.3 (−14.2 to −0.5) | 7.4 (2.1 to 12.7) | 14.7 (8.6 to 20.8) |
| ≥75% | 2.2 | 7.1b,c | 1.7 | −5.0 (−8.9 to −1.0) | 0.5 (−2.1 to 3.0) | 5.4 (1.6 to 9.2) |
| 6 mo | ||||||
| No reduction or worsening | 32.7 | 30b | 41.7 | 2.7 (−5.6 to 11.0) | −9.0 (−17.4 to −0.1) | −11.7 (−20.1 to −3.4) |
| ≥30% | 34.4b | 38.2b | 22.2 | −3.8 (−12.5 to 4.8) | 12.2 (4.3 to 20.1) | 16.0 (7.8 to 24.2) |
| ≥50% | 14.7 | 19.2b | 10.4 | −4.5 (−11.4 to 2.4) | 4.2 (−1.8 to 10.2) | 8.7 (2.3 to 15.2) |
| ≥75% | 4.0 | 6.6a | 2.2 | −2.6 (−6.9 to 1.7) | 1.8 (−1.6 to 5.1) | 4.4 (0.5 to 8.2) |
| 1 y | ||||||
| No reduction or worsening | 33.5 | 29.2 | 37.4 | 4.2 (−4.0 to 12.4) | −4.0 (−12.3 to 4.3) | −8.2 (−16.4 to 0.0) |
| ≥30% | 30.3b | 42.2b,c | 24.1 | −12.0 (−20.5 to −3.4) | 6.2 (−1.7 to 14.0) | 18.1 (9.9 to 26.3) |
| ≥50% | 16.6 | 20.8b | 13.3 | −4.2 (−11.2 to 2.8) | 3.3 (−3.0 to 9.6) | 7.5 (0.9 to 14.1) |
| ≥75% | 7.6 | 7.9 | 4.7 | −0.3 (−5.1 to 4.6) | 2.9 (−1.5 to 7.3) | 3.2 (−1.1 to 7.5) |
Abbreviation: pp, percentage point.
Estimates and 95% CIs are calculated from generalized linear mixed models with a random intercept for each participant, fit to multiply imputed datasets. The model was adjusted for participant cohort wave, Veterans Affairs recruitment site, gender, baseline pain self-efficacy score, and baseline pain interference score.
Signifies an estimate in either the group MBI or self-paced MBI arm that is statistically significantly different from the estimate in the usual care arm at a significance level of P < .05 within the same follow-up time point.
Signifies an estimate in the self-paced MBI arm that is statistically significantly different from the estimate in the group MBI arm at a significance level of P < .05 within the same follow-up time point.
Secondary Outcomes
Both MBIs had significantly better scores compared to usual care for almost all secondary outcomes (pain intensity, physical function, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, perceived change in pain, and PTSD). Differences between group MBI and self-paced MBI arms were not statistically significant.
Adverse Events
No serious adverse events were reported in any of the 3 arms. At 10 weeks, 63 of 237 participants in the group arm (27%) and 49 of 215 participants in the self-paced arm (23%) reported an increase in psychological or physical symptoms on the 10-week assessment compared to 130 of 245 participants (53%) in the usual care arm (eTable 7 in Supplement 2).44
Discussion
In this multisite pragmatic RCT of scalable MBIs for chronic pain, both group and self-paced MBIs significantly improved pain functioning (the primary outcome) and biopsychosocial outcomes among veterans with chronic pain compared to usual care during the 1-year study. However, mean group differences for the primary outcome were small. The group and self-paced MBIs performed similarly. Several factors beyond the magnitude of group differences should be considered when interpreting the clinical importance of these findings. This includes consideration of the broader context of the condition studied, consistency between primary and secondary outcomes, results of responder analyses, durability of treatment effect over time, overall risk-benefit ratios, and access to treatment.37 Responder analyses are most appropriate for assessing clinically important changes at the individual patient level.37 In this RCT, the advantages of MBIs over usual care were sustained during the 1-year study, consistent across outcomes, and similar to other guideline-recommended chronic pain treatments including MBSR.11,30 These findings are noteworthy given the high levels of psychiatric comorbidities in the trial population. Further, significantly more participants in both MBIs reported 30% and 50% improvement in pain-related function at 10 weeks, considered moderate and substantial improvement, respectively.24,37 This effect was sustained for the self-paced MBI at 1 year. Engagement with the MBIs was high, and they were safely administered, with fewer physical and mental health symptoms reported relative to usual care, suggesting the MBIs may have protective effects.44 Overall, we consider the advantage of both scalable, low-resource MBIs (group and self-paced) compared to usual care clinically important.
The similar performance of the self-paced and group MBIs is somewhat surprising given the importance placed on group social support in other MBIs.45,46 Veterans in the self-paced arm did, however, receive 3 calls from a facilitator, providing individualized social support, problem-solving, and other behavior change techniques to encourage and support their engagement. Individualization is important to patients and an important aspect of building effective therapeutic alliances, especially in trauma-informed care.47,48 More individualized support may account for some of the performance of the self-paced MBI, in addition to the flexibility of participating in facilitator calls and engaging with mindfulness content at times convenient for the individual.
Strengths and Limitations
This RCT had several strengths that addressed existing gaps in the MBI and pain self-management literature.33 This includes clear articulation of intervention elements using the TIDieR checklist,31 application of robust behavioral models,31,33 larger sample sizes,49 a focus on veterans,50 a large sample of women veterans, and socioeconomically disadvantaged participants.49 The trial had many pragmatic design features (a focus of the Pain Management Collaboratory),21,22 aimed at facilitating future implementation. This includes recruitment from a real-world setting with broad inclusion and narrow exclusion criteria, resulting in a sample of patients with heterogeneous chronic pain conditions and high levels of psychiatric comorbidities receiving pain care at VA health centers. The MBIs were intentionally designed to meet the needs of veterans and the VA health system by addressing barriers to delivering and participating in more intensive MBIs. In addition to being shorter, the MBIs did not require certified mindfulness instructors, providing additional benefits of systematic content delivery (enhancing fidelity) and availability of content for veterans to review between sessions.
One limitation of this trial is that it was not designed to answer questions of how less resource-intensive MBIs perform compared to more intensive MBSR programs, or how remotely delivered programs compared to in-person MBIs. However, the magnitude of group differences between MBIs and usual care is similar to what has been observed in another high-quality study of in-person MBSR for pain conditions using 2-hour sessions and an optional retreat.14 Our study also did not address cost-effectiveness. Furthermore, this trial was not designed to control for time, attention, and other contextual factors because of its pragmatic design; patient expectations were also not measured. Therefore, nonspecific aspects of the MBIs may have contributed to improvement in outcomes.49,51 Qualitative results exploring veterans’ perceptions of the MBIs will provide additional insight into contextual factors and will be addressed in a separate publication. Blinding participants and facilitators to the interventions was also not possible. Roughly half of participants in the usual care arm also used meditation/mindfulness throughout the study; to what extent this may have reduced the advantage of MBI over usual care is unknown. Because many participants used concomitant treatment for their chronic pain, independent MBI performance is unknown; however, multimodal treatment is the standard of care for chronic pain management, and MBIs are typically used as adjunct interventions. Additionally, approximately 81% of veterans did not respond or declined to participate in the study in response to the initial recruitment material, which may affect the generalizability of these findings.
Conclusions
Two relatively low-resource, scalable MBIs (offered in group or self-paced formats), improved pain-related function and other biopsychosocial outcomes over 1 year compared to usual care among veterans with chronic pain and a high level of psychiatric comorbidities. The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems.
Trial Protocol
eTable 1. Pain Categories
eTable 2. Operational Definition for Exclusion Based on Chart Review
eTable 3. Intervention Description (based on Template for Intervention Description and Replication (TIDieR)1
eTable 4. Baseline Mental Health Diagnoses, Pain Diagnoses, and Prior Pain Treatment in the Past 3 Months by Randomization Group
eTable 5. Prior Pain Treatment at 10 weeks, 6 months and 12 months by Treatment Arm
eTable 6. Completion of Intervention Activities in a Typical Week, at 10 Weeks, 6 Months, and 12 Months by MBI Group
eTable 7. Side Effect Probabilities (and CIs) by Intervention Arm
Data Sharing Statement
References
- 1.Relieving Pain in America . A Blueprint for Transforming Prevention, Care, Education, and Research. National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 2.National Pain Strategy . A Comprehensive Population Health-Level Strategy for Pain. Department of Health and Human Services; 2016. [Google Scholar]
- 3.Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi: 10.1016/j.jpain.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qaseem A, Wilt TJ, McLean RM, et al. ; Clinical Guidelines Committee of the American College of Physicians . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514-530. doi: 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 6.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 7.National Academies of Sciences, Engineering, and Medicine . The role of nonpharmacological approaches to pain management: proceedings of a workshop. National Academies Press; 2019. [PubMed] [Google Scholar]
- 8.Gatchel RJ, Reuben DB, Dagenais S, et al. Research agenda for the prevention of pain and its impact: report of the work group on the prevention of acute and chronic pain of the federal pain research strategy. J Pain. 2018;19(8):837-851. doi: 10.1016/j.jpain.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 9.Taylor SL, Bolton R, Huynh A, et al. What should health care systems consider when implementing complementary and integrative health: lessons from Veterans Health Administration. J Altern Complement Med. 2019;25(S1):S52-S60. doi: 10.1089/acm.2018.0445 [DOI] [PubMed] [Google Scholar]
- 10.Heyward J, Jones CM, Compton WM, et al. Coverage of nonpharmacologic treatments for low back pain among US public and private insurers. JAMA Netw Open. 2018;1(6):e183044. doi: 10.1001/jamanetworkopen.2018.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anheyer D, Haller H, Barth J, Lauche R, Dobos G, Cramer H. Mindfulness-based stress reduction for treating low back pain: a systematic review and meta-analysis. Ann Intern Med. 2017;166(11):799-807. doi: 10.7326/M16-1997 [DOI] [PubMed] [Google Scholar]
- 12.Chiesa A, Serretti A. Mindfulness-based interventions for chronic pain: a systematic review of the evidence. J Altern Complement Med. 2011;17(1):83-93. doi: 10.1089/acm.2009.0546 [DOI] [PubMed] [Google Scholar]
- 13.Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2016;51(2):199-213. doi: 10.1007/s12160-016-9844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240-1249. doi: 10.1001/jama.2016.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016;176(3):329-337. doi: 10.1001/jamainternmed.2015.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465. doi: 10.1001/jama.2015.8361 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SL, Hoggatt KJ, Kligler B. Complementary and integrated health approaches: what do veterans use and want. J Gen Intern Med. 2019;34(7):1192-1199. doi: 10.1007/s11606-019-04862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Lee EKP, Mak ECW, Ho CY, Wong SYS. Mindfulness-based interventions: an overall review. Br Med Bull. 2021;138(1):41-57. doi: 10.1093/bmb/ldab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez ME, Kearney DJ, Simpson T, Felleman BI, Bernardi N, Sayre G. Challenges to enrollment and participation in mindfulness-based stress reduction among veterans: a qualitative study. J Altern Complement Med. 2015;21(7):409-421. doi: 10.1089/acm.2014.0324 [DOI] [PubMed] [Google Scholar]
- 21.Kerns RD, Brandt CA. NIH-DOD-VA Pain Management Collaboratory. Oxford University Press; 2020:S1-S4. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi J. Robert Kerns, PhD: researching nondrug approaches to pain management. JAMA. 2018;319(15):1535-1537. doi: 10.1001/jama.2018.0247 [DOI] [PubMed] [Google Scholar]
- 23.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Dworkin RH, Turk DC, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446-2461. doi: 10.1097/j.pain.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess DJ, Evans R, Allen KD, et al. Learning to Apply Mindfulness to Pain (LAMP): design for a pragmatic clinical trial of two mindfulness-based interventions for chronic pain. Pain Med. 2020;21(suppl 2):S29-S36. doi: 10.1093/pm/pnaa337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess DJ, Hagel Campbell EM, Branson M, et al. Exploring gender differences in veterans in a secondary analysis of a randomized controlled trial of mindfulness for chronic pain. Womens Health Rep (New Rochelle). 2024;5(1):82-92. doi: 10.1089/whr.2023.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi: 10.1097/j.pain.0000000000000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi: 10.1007/s11606-007-0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark L, Fairhurst C, Torgerson DJ. Allocation concealment in randomised controlled trials: are we getting better. BMJ. 2016;355:i5663. doi: 10.1136/bmj.i5663 [DOI] [PubMed] [Google Scholar]
- 30.Savović J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med. 2012;157(6):429-438. doi: 10.7326/0003-4819-157-6-201209180-00537 [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 32.Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81-95. doi: 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 33.Crane RS, Hecht FM. Intervention integrity in mindfulness-based research. Mindfulness (N Y). 2018;9(5):1370-1380. doi: 10.1007/s12671-018-0886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129-138. [PubMed] [Google Scholar]
- 35.Kean J, Monahan PO, Kroenke K, et al. Comparative responsiveness of the PROMIS Pain Interference Short Forms, Brief Pain Inventory, PEG, and SF-36 Bodily Pain Subscale. Med Care. 2016;54(4):414-421. doi: 10.1097/MLR.0000000000000497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CX, Kroenke K, Stump T, et al. Comparative responsiveness of the PROMIS pain interference short forms with legacy pain measures: results from three randomized clinical trials. J Pain. 2019;20(6):664-675. doi: 10.1016/j.jpain.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238-244. doi: 10.1016/j.pain.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 38.Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89-102. doi: 10.1016/j.jclinepi.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deyo RA, Ramsey K, Buckley DI, et al. Performance of a Patient Reported Outcomes Measurement Information System (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med. 2016;17(2):314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163-173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 41.Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi: 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- 42.Pain Management Collaboratory . Nonpharmacological and self-care approaches measure from PMC (NSCAP). Accessed March 14, 2023. https://painmanagementcollaboratory.org/nonpharmacological-and-self-care-approaches/
- 43.Klein D. MIMRGNS: Stata module to run margins after mi estimate. Ideas. 2022. https://ideas.repec.org/c/boc/bocode/s457795.html
- 44.Burgess D, Calvert C, Bangerter A, et al. Do Mindfulness interventions cause harm: findings from the learning to apply mindfulness to pain study. J Pain. 2023;24(4):63. doi: 10.1016/j.jpain.2023.02.187 [DOI] [Google Scholar]
- 45.Santorelli SF, Meleo-Meyer F, Koerbel L. Mindfulness-Based Stress Reduction (MBSR). Authorized Curriculum Guide; 2017. [Google Scholar]
- 46.Allexandre D, Bernstein AM, Walker E, Hunter J, Roizen MF, Morledge TJ. A web-based mindfulness stress management program in a corporate call center: a randomized clinical trial to evaluate the added benefit of onsite group support. J Occup Environ Med. 2016;58(3):254-264. doi: 10.1097/JOM.0000000000000680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Keeffe M, Cullinane P, Hurley J, et al. What Influences patient-therapist interactions in musculoskeletal physical therapy: qualitative systematic review and meta-synthesis. Phys Ther. 2016;96(5):609-622. doi: 10.2522/ptj.20150240 [DOI] [PubMed] [Google Scholar]
- 48.Krause DJ, Green SA, Koury SP, Hales TW. Solution-focused trauma-informed care (SF-TIC): an integration of models. J Public Child Welf. 2018;12(2):117-135. doi: 10.1080/15548732.2017.1348312 [DOI] [Google Scholar]
- 49.Goldberg SB, Riordan KM, Sun S, Davidson RJ. The Empirical status of mindfulness-based interventions: a systematic review of 44 meta-analyses of randomized controlled trials. Perspect Psychol Sci. 2022;17(1):108-130. doi: 10.1177/1745691620968771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marchand WR, Sandoval K, Lackner R, et al. Mindfulness-based interventions for military veterans: A systematic review and analysis of the literature. Complement Ther Clin Pract. 2021;42:101274. doi: 10.1016/j.ctcp.2020.101274 [DOI] [PubMed] [Google Scholar]
- 51.Sharpe L, Richmond B, Menzies RE, Forrest D, Crombez G, Colagiuri B. A synthesis of meta-analyses of mindfulness-based interventions in pain. Pain. 2024;165(1):18-28. doi: 10.1097/j.pain.0000000000002997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Pain Categories
eTable 2. Operational Definition for Exclusion Based on Chart Review
eTable 3. Intervention Description (based on Template for Intervention Description and Replication (TIDieR)1
eTable 4. Baseline Mental Health Diagnoses, Pain Diagnoses, and Prior Pain Treatment in the Past 3 Months by Randomization Group
eTable 5. Prior Pain Treatment at 10 weeks, 6 months and 12 months by Treatment Arm
eTable 6. Completion of Intervention Activities in a Typical Week, at 10 Weeks, 6 Months, and 12 Months by MBI Group
eTable 7. Side Effect Probabilities (and CIs) by Intervention Arm
Data Sharing Statement