Abstract
BACKGROUND
Many older adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) have a relapse despite having a measurable residual disease (MRD)–negative complete remission with combination chemotherapy. The addition of blinatumomab, a bispecific T-cell engager molecule that is approved for the treatment of relapsed, refractory, and MRD-positive BCP-ALL, may have efficacy in patients with MRD-negative remission.
METHODS
In a phase 3 trial, we randomly assigned patients 30 to 70 years of age with BCR::ABL1-negative BCP-ALL (with :: indicating fusion) who had MRD-negative remission (defined as <0.01% leukemic cells in bone marrow as assessed on flow cytometry) after induction and intensification chemotherapy to receive four cycles of blinatumomab in addition to four cycles of consolidation chemotherapy or to receive four cycles of consolidation chemotherapy alone. The primary end point was overall survival, and relapse-free survival was a secondary end point.
RESULTS
The data and safety monitoring committee reviewed the results from the third efficacy interim analysis and recommended that they be reported. Complete remission with or without full count recovery was observed in 395 of 488 enrolled patients (81%). Of the 224 patients with MRD-negative status, 112 were assigned to each group. The characteristics of the patients were balanced between the groups. At a median follow-up of 43 months, an advantage was observed in the blinatumomab group as compared with the chemotherapy-only group with regard to overall survival (at 3 years: 85% vs. 68%; hazard ratio for death, 0.41; 95% confidence interval [CI], 0.23 to 0.73; P = 0.002), and the 3-year relapse-free survival was 80% with blinatumomab and 64% with chemotherapy alone (hazard ratio for relapse or death, 0.53; 95% CI, 0.32 to 0.87). A higher incidence of neuropsychiatric events was reported in the blinatumomab group than in the chemotherapy-only group.
CONCLUSIONS
The addition of blinatumomab to consolidation chemotherapy in adult patients in MRD-negative remission from BCP-ALL significantly improved overall survival. (Funded by the National Institutes of Health and others; E1910 ClinicalTrials.gov number, NCT02003222.)
Various developments in the past decade have led to improved outcomes in adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL). These include the use of pediatric-like intensive chemotherapy regimens in adolescents and young adults,1,2 the assessment of measurable residual disease (MRD) for prognostication and management decisions,3 and the development of immunotherapies.4,5 Despite these advances, outcomes in adults with BCP-ALL are inferior to those in children, owing in part to the increased frequency of high-risk genetic abnormalities and to the toxic effects of high-dose chemotherapy.6
Blinatumomab is a bispecific T-cell engager molecule composed of an anti-CD19 variable region linked to an anti-CD3 variable region that brings T cells in proximity to leukemic blasts to form cytolytic synapses, leading to apoptosis and lysis of the blasts.7 A phase 3 randomized trial of blinatumomab as compared with chemotherapy for the treatment of relapsed or refractory BCR::ABL1-negative BCP-ALL (with :: indicating fusion) showed improved outcomes8 and led to the approval of blinatumomab by the Food and Drug Administration (FDA) for this indication in 2014. A subsequent study involving patients with MRD-positive BCP-ALL showed that after one 4-week cycle of blinatumomab, 78% of the patients had MRD-negative status.9 This finding led the FDA to expand the approval of blinatumomab to include MRD-positive BCP-ALL in 2018.
Adults with BCP-ALL who have MRD-negative remission after induction chemotherapy have a better prognosis than patients with MRD-positive status, but they still frequently have a relapse.3 In the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN)–led E1910 trial, which was an international, randomized, phase 3 clinical trial conducted through the National Clinical Trials Network (NCTN) of the National Cancer Institute, we studied the effect of the addition of blinatumomab to standard consolidation chemotherapy in patients 30 to 70 years of age with BCR::ABL1-negative BCP-ALL.
METHODS
PATIENTS
Patients 30 to 70 years of age were enrolled in this trial. We chose this age range to avoid competition for enrollment in an NCTN trial involving adolescents and young adult patients that is being led by the Alliance for Clinical Trials in Oncology group. All the patients provided written informed consent that allowed for preregistration and for the submission of diagnostic bone marrow or peripheral-blood samples to confirm the diagnosis of BCP-ALL and to rule out the presence of the BCR::ABL1 fusion.
Patients were subsequently registered in the trial if they met additional eligibility criteria that were based on hepatic and renal function. Hydroxyurea was allowed for up to 5 days before the initiation of trial therapy. Glucocorticoid treatment was allowed after the submission of baseline samples.
TREATMENTS AND RANDOMIZATION
The chemotherapy backbone was based on that used in our previous trial, MRC UKALL XII/ECOG E2993,10,11 with modification to make it similar to pediatric ALL treatment regimens by the reduction of the daunorubicin dose,1 the replacement of Escherichia coli–derived asparaginase with pegaspargase (with omission of pegaspargase in the two cycles of induction therapy and dose reductions in the intensification and consolidation phases in patients ≥55 years of age), the use of dexamethasone instead of prednisone,12 the extension of cycle 2 of induction therapy from 28 to 42 days, and in a later amendment, the optional addition of rituximab therapy if the patient was CD20-positive (Fig. 1).5
Figure 1. Treatment Schema.
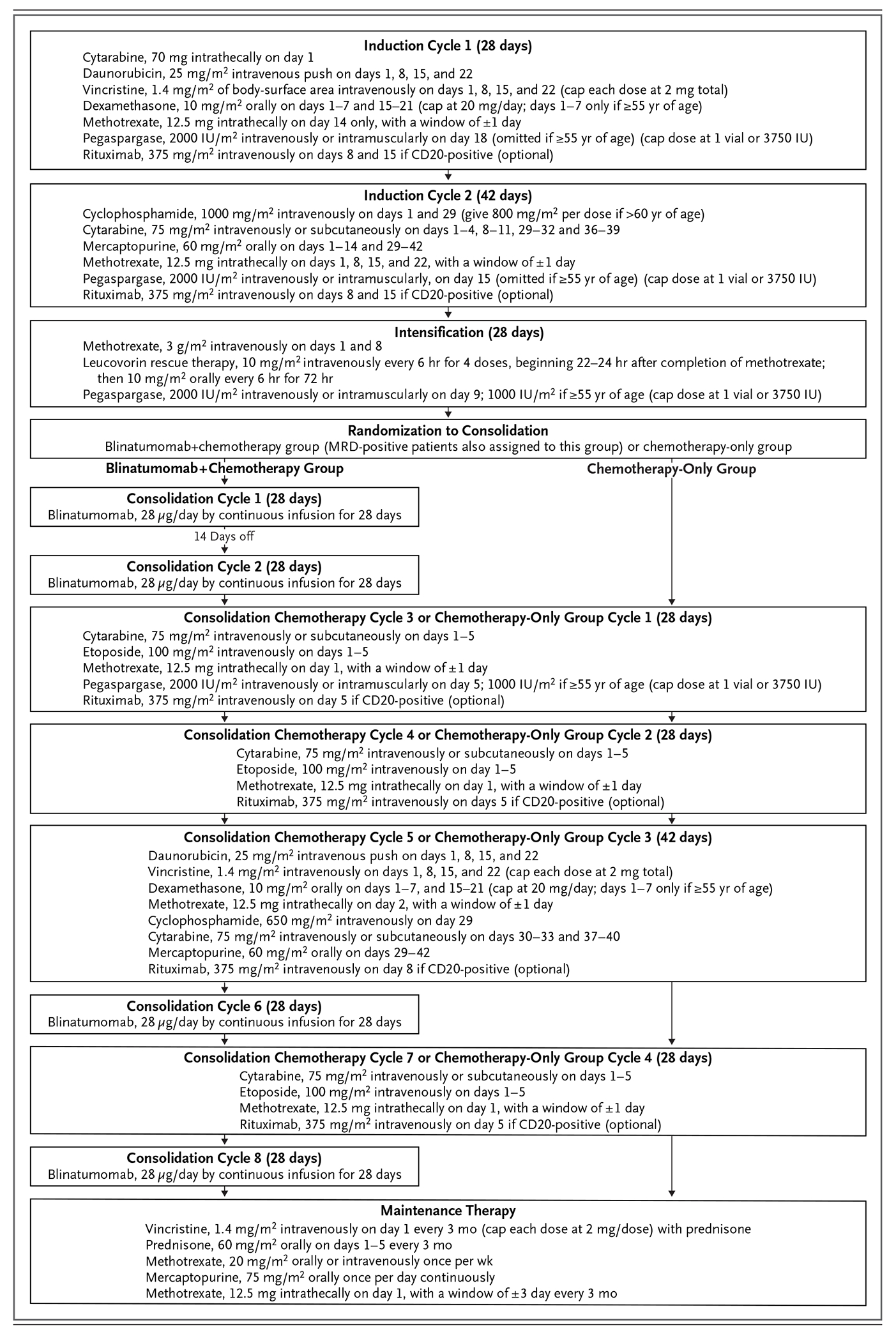
If leukemia was present in the central nervous system at diagnosis, methotrexate (at a dose of 12.5 mg, administered intrathecally or by means of an Ommaya reservoir) was to be given twice weekly until blasts were not present in cerebrospinal fluid. If the patient subsequently had a complete remission, a total dose of 1800 cGy of cranial irradiation, administered in 10 daily fractions of 180 cGy per fraction, was to be given during the first cycle of maintenance therapy. Maintenance therapy was planned to continue for 2.5 years from the start of the intensification phase. IU denotes international units.
Induction treatment consisted of two cycles of chemotherapy. Patients who had a morphologic complete remission with or without complete count recovery at the end of induction proceeded to the intensification phase, which involved a 28-day cycle of high-dose intravenous methotrexate and pegaspargase for central nervous system prophylaxis.
Bone marrow evaluation that was performed after count recovery from the intensification treatment was assessed centrally for MRD by means of six-color flow cytometry (Tables S1 through S4 in Supplementary Appendix 1, available with the full text of this article at NEJM.org). Patients in morphologic remission with MRD of less than 0.01% leukemic cells in the bone marrow were considered to have MRD-negative status and were randomly assigned either to the control group, in which they received four cycles of consolidation chemotherapy (chemotherapy-only group), or to the blinatumomab group, in which they received the same four cycles of chemotherapy plus four cycles of blinatumomab. Randomization was stratified according to patient age (30 to 54 years or ≥55 years), CD20 status (positive or negative), rituximab use (yes or no), and whether transplantation was intended (yes or no).
Patients who were assigned to the blinatumomab group received two cycles of blinatumomab at a dose of 28 μg per day for 4 weeks with a 2-week interval between cycles, followed by four cycles of chemotherapy and two additional cycles of blinatumomab. Patients who were assigned to the chemotherapy-only group received the same four cycles of consolidation chemotherapy.
In the initial trial design, patients with MRD-positive status also underwent randomization. After the approval of blinatumomab therapy for patients with MRD-positive status, a protocol amendment assigned all subsequent patients with MRD-positive status to the blinatumomab group.
Patients were allowed to receive an allogeneic transplant from any donor and to receive any conditioning regimen after at least two cycles of blinatumomab in the blinatumomab group or at any time after the commencement of consolidation chemotherapy in the chemotherapy-only group. After the receipt of consolidation therapy, patients proceeded to maintenance therapy, which continued for 2.5 years from the start of the intensification phase. The planned duration of treatment was the same in the two trial groups.
TRIAL DESIGN AND OVERSIGHT
The trial was conducted in Canada, Israel, and the United States. The protocol, which is available at NEJM.org, was approved by the central institutional review board of the National Cancer Institute. The trial was conducted in accordance with the principles of the Declaration of Helsinki.
The trial was funded by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute, designed by ECOG-ACRIN, and approved by CTEP and Amgen. Data collection and monitoring procedures are described in Supplementary Appendix 1. Amgen had no role in data collection and analysis or in the writing or submission of the manuscript. The principal investigator (first author) reviewed case-report forms for all the patients enrolled in the trial, wrote the manuscript with the approval of all the authors and without the assistance of nonauthors, and made the decision to submit the manuscript for publication.
ASSESSMENT OF MRD AND CYTOGENETIC AND MOLECULAR ANALYSES
Patient eligibility was confirmed centrally with the use of bone marrow or peripheral-blood samples assessed by means of flow cytometry and polymerase-chain-reaction assay to confirm the diagnosis of BCP-ALL and negativity of the BCR::ABL1 fusion, respectively. MRD in bone marrow was assessed by means of the different-from-normal approach and the standardized six-color Children’s Oncology Group BCP-ALL MRD flow cytometry assay.13 Before randomization, the required sensitivity level of 0.01% was achieved in all patient samples.
Local results of cytogenetic and fluorescent in situ hybridization analyses were reviewed by the ECOG-ACRIN cytogenetics committee. Total RNA stranded transcriptome sequencing was performed on 419 of 481 eligible diagnostic patient samples with the use of the TruSeq library preparation and HiSeq 2000 or 2500 or NovaSeq 6000 sequencers (Illumina). BCP-ALL subtypes were assigned as described in our analyses of the E2993 cohort (Fig. S1 in Supplementary Appendix 1).14,15
END POINTS
The primary objective was to compare, in the MRD-negative subpopulation, overall survival (primary end point) among patients who received blinatumomab plus chemotherapy with that among patients who received chemotherapy alone. Overall survival was defined as the time between randomization and death from any cause. A secondary end point was relapse-free survival, which was defined as the time between randomization and relapse or death (whichever occurred first). Safety was assessed according to the Common Terminology Criteria for Adverse Events, version 4, of the National Cancer Institute.
Subgroup analyses of overall survival were performed in subgroups defined according to age (<55 vs. ≥55 years), combined molecular risk (favorable vs. intermediate vs. unfavorable; see below), BCR::ABL1-like genotype (yes vs. no), intention to undergo transplantation (yes vs. no), CD20 status (positive vs. negative), and rituximab use (yes vs. no). The subgroup analysis of combined molecular risk was conducted post hoc. The combined molecular risk categories were defined as follows: favorable risk as DUX4-rearranged, high-hyperdiploid, TCF3::PBX1, or PAX5 P80R; intermediate risk as PAX5-altered, PAX5::ETV6, MEF2D-rearranged, or ZNF384-rearranged; and unfavorable risk as KMT2A-rearranged, low-hypodiploid or near-haploid, BCR::ABL1-like, BCL2-rearranged or MYC-rearranged (or both), ETV6::RUNX1-like with IGH::CRLF2 fusion, and high-hyperdiploid with BCR::ABL1-like, CRLF2-rearranged. Further details are provided in the Definition of Combined Risk Assignment section in Supplementary Appendix 1.
STATISTICAL ANALYSIS
We intended to enroll 488 patients with BCR::ABL1-negative ALL in the trial. On the basis of available data, we assumed that at least 190 of 488 patients (39%) would have an MRD-negative remission and undergo randomization. We calculated that, with adjustment for sequential monitoring, the trial would have 80% power to detect a 45% lower risk in the blinatumomab group relative to the chemotherapy-only group, using a one-sided log-rank test at the significance level of 0.025, with the number of deaths needed for the analysis set at 94.
Estimates of overall survival and relapse-free survival, including medians and confidence intervals, were calculated by means of the Kaplan–Meier method. Comparison of overall survival between the treatment groups was conducted with the use of the two-sided stratified log-rank test with the stratification factors of age, CD20 status, rituximab use, and intention to receive a transplant. Using the same stratification factors as above, we used stratified Cox proportional-hazards models to assess the treatment effect on overall survival and relapse-free survival with adjustment for possible clinical and biologic risk factors. The interaction between the treatment groups and the logarithm of survival time was included in the Cox model as a time-varying covariate to test the proportional-hazards assumptions, and the assumptions were confirmed to be valid. To further account for the potential effect of transplant receipt on the comparison of overall survival, a sensitivity analysis was conducted with the use of a Cox model, with stratification according to MRD status, patient age, CD20 status, and rituximab use; the model included receipt of a transplant as a time-varying covariate. Full details are provided in Supplementary Appendix 1. The widths of the confidence intervals that are reported here were not adjusted for multiplicity and may not be used in place of hypothesis testing.
RESULTS
ENROLLMENT AND PATIENT CHARACTERISTICS
The trial began in December 2013 and met the enrollment goal in October 2019. A total of 766 patients registered for the initial screening, and 488 were enrolled. Most of the patients who were found to be ineligible at the initial screening had BCR::ABL1-positive ALL. BCR::ABL1-positive disease was subsequently identified in 6 enrolled patients, and T-cell ALL in 1 enrolled patient. All 488 patients were included in the subsequent analyses (Fig. 2). Details of the 481 eligible patients are presented in Table S5 in Supplementary Appendix 2, available at NEJM.org (see also Supplementary Appendix 1). A total of 44 patients with MRD-positive status underwent randomization under the initial trial protocol; after the protocol amendment, 18 subsequent patients with MRD-positive status were assigned to the blinatumomab group.
Figure 2. Randomization and Treatment Phases.
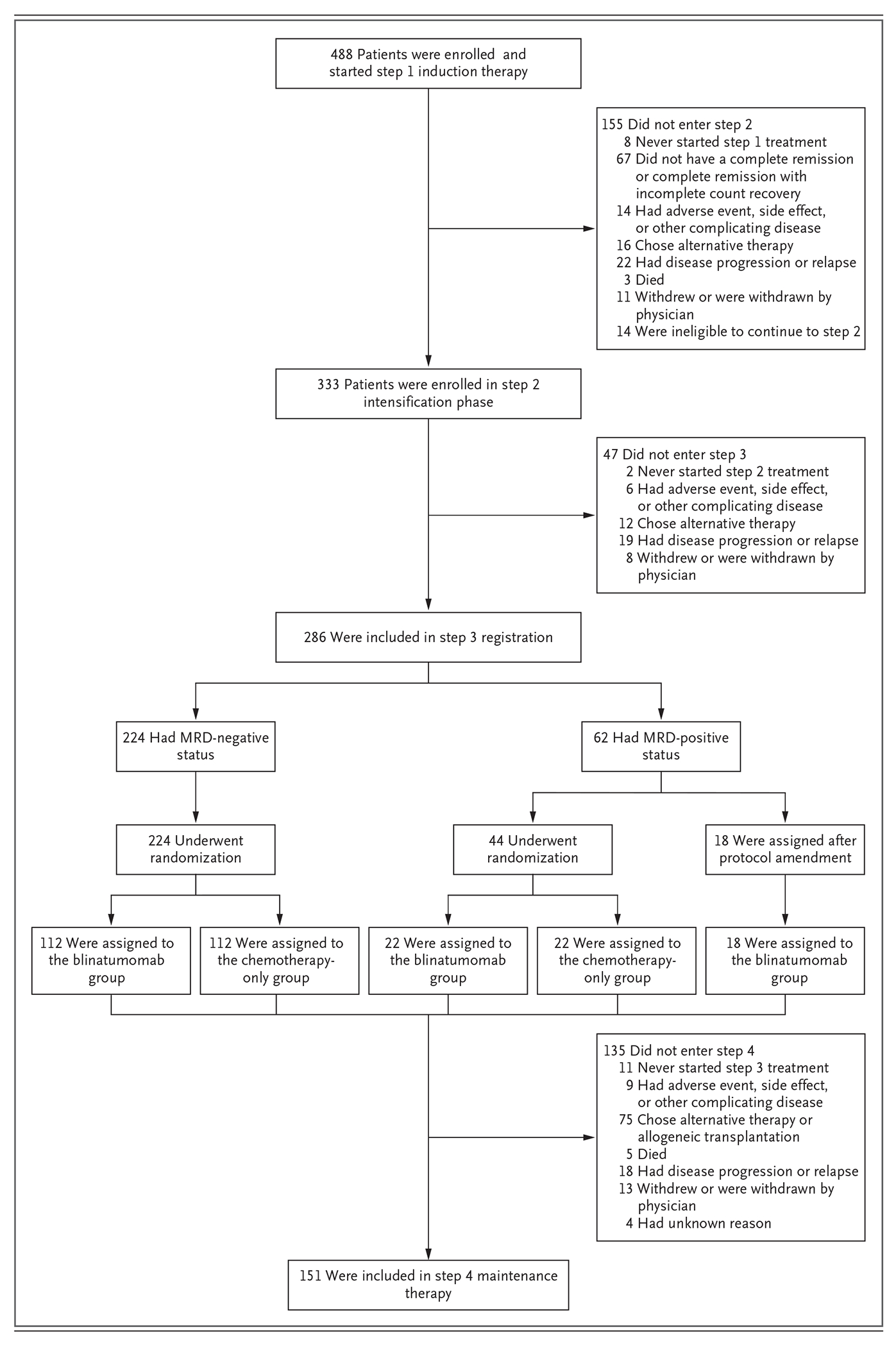
Shown is the status of patients throughout the course of the trial from initial registration (488 patients), followed by 2.5 months of induction therapy (step 1), along with the numbers and reasons why patients did not proceed to intensification (step 2, which included 333 patients) and the following intensification phase and the numbers and reasons why patients did not proceed to the step 3 registration (which included 286 patients). In step 3, patients were randomly or nonrandomly (after protocol amendment 14) assigned to a trial group. Patients who were positive for measurable residual disease (MRD) who had undergone the initial randomization were all later assigned to the blinatumomab group in the trial. The reasons that patients did not proceed to step 4 maintenance therapy after consolidation therapy are listed.
The median age of the enrolled patients was 51 years (range, 30 to 70), with 59% of the patients being younger than 55 years of age (median in this subgroup, 42 years) and with 41% being 55 years of age or older (median in this subgroup, 62 years) (Table 1). On the basis of a combined cytogenetic and molecular risk profile, outcome risk according to genomic leukemia subtype was successfully assigned in 409 of the 481 eligible patients (85%); no risk could be assigned in 72 patients (15%).15 The majority of the patients (57%) were deemed to be at unfavorable risk (see the definition of combined risk assessment in Supplementary Appendix 1).
Table 1.
Characteristics of the Patients with MRD-Negative Status at Enrollment.*
| Characteristic | Blinatumomab + Chemotherapy (N = 112) | Chemotherapy Only (N = 112) | Total (N = 224) |
|---|---|---|---|
| Age | |||
| Median age (range) — yr | 51.5 (30–69) | 50 (30–70) | 51 (30–70) |
| Distribution — no. (%) | |||
| <55 yr | 66 (59) | 65 (58) | 131 (58) |
| ≥55 yr | 46 (41) | 47 (42) | 93 (42) |
| Sex — no. (%) | |||
| Female | 57 (51) | 56 (50) | 113 (50) |
| Male | 55 (49) | 56 (50) | 111 (50) |
| ECOG performance-status score — no. (%)† | |||
| 0 or 1 | 106 (95) | 101 (90) | 207 (92) |
| >2 | 6 (5) | 11 (10) | 17 (8) |
| Leukocyte count | |||
| Median (range) — ×10−9/liter | 3.6 (1–295) | 4.0 (1–112) | 3.7 (1–295) |
| Distribution — no. (%) | |||
| <10×109/liter | 88 (79) | 84 (75) | 172 (77) |
| ≥10×109/liter | 24 (21) | 28 (25) | 52 (23) |
| Platelet count | |||
| Median (range) — ×10−9/liter | 49.5 (13–347) | 48 (24–319) | 48.5 (13–347) |
| Distribution — no. (%) | |||
| <10×109/liter | 5 (4) | 11 (10) | 16 (7) |
| ≥10×109/liter | 107 (96) | 101 (90) | 208 (93) |
| Hemoglobin | |||
| Median (range) — g/dl | 8.6 (6–15) | 8.4 (6–14) | 8.5 (6–15) |
| Distribution — no. (%) | |||
| <10 g/dl | 86 (77) | 89 (79) | 175 (78) |
| ≥10 g/dl | 26 (23) | 23 (21) | 49 (22) |
| Median peripheral blood blasts (range) — % | 12 (0–93) | 19 (0–98) | 16 (0–98) |
| Median bone marrow blasts (range) — % | 84 (1–100) | 84 (5–100) | 84 (1–100) |
| Intention to undergo transplantation at randomization — no. (%) | |||
| Yes | 36 (32) | 35 (31) | 71 (32) |
| No | 76 (68) | 77 (69) | 153 (68) |
| CD20 status at randomization — no. (%)‡ | |||
| Positive | 45 (40) | 46 (41) | 91 (41) |
| Negative | 26 (23) | 26 (23) | 52 (23) |
| Rituximab use at randomization — no. (%)‡ | |||
| Yes | 33 (29) | 36 (32) | 69 (31) |
| No | 38 (34) | 36 (32) | 74 (33) |
| Immunophenotype — no. (%) | |||
| CD10-positive, early pre-B | 95 (85) | 93 (83) | 188 (84) |
| CD10-negative, pro-B | 16 (14) | 18 (16) | 34 (15) |
| Missing data | 1 (1) | 1 (1) | 2 (1) |
| Combined risk — no. (%)§ | |||
| Favorable | 19 (17) | 29 (26) | 48 (21) |
| Intermediate | 22 (20) | 19 (17) | 41 (18) |
| Unfavorable | 50 (45) | 44 (39) | 94 (42) |
| No risk assigned | 21 (19) | 20 (18) | 41 (18) |
Percentages may not total 100 because of rounding.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
The stratification factors of CD20 status and rituximab use were added in protocol addendum 9. Data were available for 143 patients (71 in the blinatumomab group and 72 in the chemotherapy-only group).
Details of the genetic and genomic subtypes for each risk group are provided in the Results section.
A total of 19 of 488 patients (4%) died from toxic effects during induction treatment. The causes of death included sepsis, intracranial hemorrhage, pulmonary mucormycosis, hepatic failure, myocardial infarction, stroke, and respiratory failure. At the completion of two cycles of induction therapy, 395 patients (81%) had either a complete remission (364 patients [75%]) or a complete remission with incomplete count recovery (31 patients [6%]) and were continuing in the trial. Of the 395 patients who had a remission, 109 did not reach the next trial registration after the intensification phase because of death, recurrent disease, adverse events, or withdrawal from the trial (Table S6 in Supplementary Appendix 1).
After the intensification phase, 224 patients had MRD-negative status and were randomly assigned in a 1:1 ratio to the blinatumomab group or the chemotherapy-only group (112 patients in each group). The characteristics of the patients at diagnosis, including the combined risk profile, were balanced between the groups (Table 1 and Table S7A, S7B, and S7C in Supplementary Appendix 1). The BCR::ABL1-like genotype (a genotype subset that lacks the translocation but carries a transcriptional profile that resembles BCR::ABL1-positive disease and has a poor prognosis) was present in 14% of the patients who had been assigned to the blinatumomab group and in 13% of those who had been assigned to the chemotherapy-only group.
EFFICACY OUTCOMES
In September 2022, the ECOG-ACRIN data and safety monitoring committee reviewed the results of the third efficacy interim analysis at a median follow-up of 43 months and recommended that the results be released. The results of this third efficacy interim analysis are presented in this article. Among the 224 patients with MRD-negative status who underwent randomization, 22 in each group underwent allogeneic transplantation during the trial. After stopping trial treatment and going on to receive alternate treatment, an additional 6 patients in the blinatumomab group underwent transplantation (for a total of 28 patients in the blinatumomab group who underwent transplantation), as compared with an additional 10 patients in the chemotherapy-only group (for a total of 32 patients in the chemotherapy-only group). No substantial between-group difference was observed in the percentage of patients who underwent transplantation (Tables S8 and S9 in Supplementary Appendix 1).
The primary end point of the trial was overall survival from the time of randomization, assessed among patients with MRD-negative status. There were 17 deaths in the blinatumomab group (8 deaths from relapse and 9 from non–relapse-related causes, largely from infection) and 40 deaths in the chemotherapy-only group (31 deaths from relapse and 7 from non–relapse-related causes, also largely from infection, as well as 2 from unknown causes). The 3-year overall survival in the blinatumomab group was 85%, as compared with 68% in the chemotherapy-only group. Treatment with blinatumomab significantly improved overall survival as compared with chemotherapy alone (hazard ratio for death, 0.41; 95% confidence interval [CI], 0.23 to 0.73; P = 0.002) (Fig. 3A), a result that crossed the stopping boundary for efficacy (a two-sided P value of 0.007) at the data-cutoff date for this interim analysis. Results of the analysis of relapse-free survival were similar to those of the overall survival analysis. The 3-year relapse-free survival was 80% in the blinatumomab group and 64% in the chemotherapy-only group (hazard ratio for relapse or death, 0.53; 95% CI, 0.32 to 0.87) (Fig. S2 in Supplementary Appendix 1).
Figure 3. Overall Survival According to Trial Group and Clinical and Molecular Features.
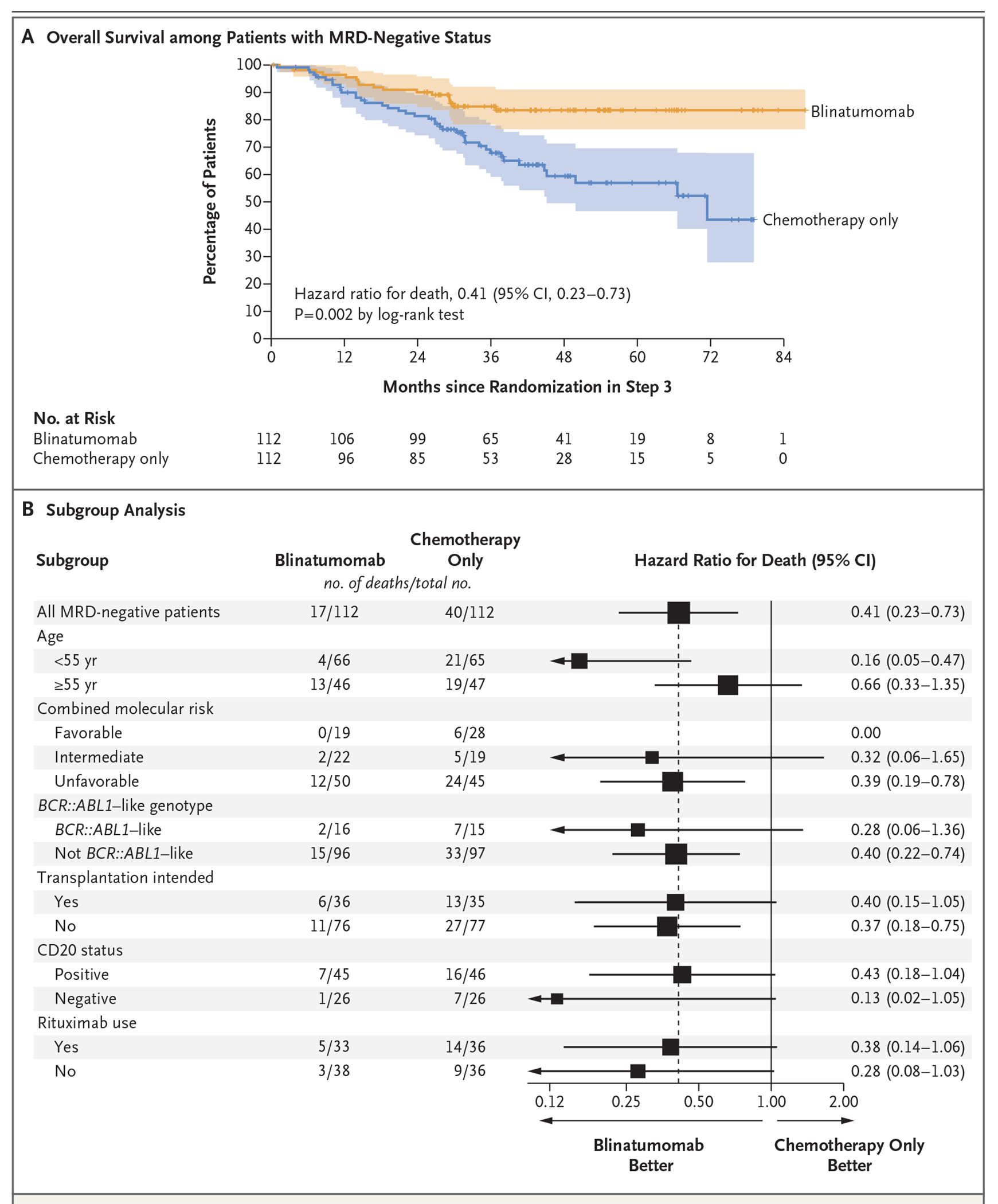
Panel A shows the comparison of median overall survival among the MRD-negative patients. The widths of the confidence intervals (shaded areas) were not adjusted for multiplicity and may not be used in place of hypothesis testing. Panel B shows the subgroup analysis of overall survival between the blinatumomab group and the chemotherapy-only group among the MRD-negative patients. The stratification factors of CD20 status and rituximab use were added in protocol addendum 9. The subgroup analysis that was defined according to combined molecular risk was post hoc. The size of the squares is inversely proportional to the standard error of the hazard ratio estimates, and arrows indicate confidence intervals that exceed the graphed space.
A multivariable analysis was performed to examine the treatment effect, with adjustment for sex, white-cell count, platelet count, hemoglobin level, peripheral blood blasts, bone marrow blasts, ECOG performance-status score, and combined molecular risk category. Age, CD20 status, intended rituximab use, and intention to receive an allogeneic transplant were used as stratification factors. In this analysis, the hazard ratio for death with blinatumomab as compared with chemotherapy alone was 0.44 (95% CI, 0.23 to 0.81). A multivariable analysis of relapse-free survival showed a hazard ratio for relapse or death in the blinatumomab group as compared with the chemotherapy-only group of 0.57 (95% CI, 0.33 to 0.98). In sensitivity analyses in which receipt of a transplant was included as a time-varying covariate, the hazard ratio for death with blinatumomab as compared with chemotherapy alone was 0.43 (95% CI, 0.24 to 0.79), and the hazard ratio for relapse or death was 0.53 (95% CI, 0.31 to 0.91).
Among the 132 patients younger than 55 years of age, overall survival and relapse-free survival appeared to be improved among those who received blinatumomab as compared with those who received chemotherapy alone. In this subgroup, the 3-year overall survival was 95% with blinatumomab and 70% with chemotherapy alone (hazard ratio for death, 0.16; 95% CI, 0.05 to 0.47), and the 3-year relapse-free survival was 87% and 70%, respectively (hazard ratio for relapse or death, 0.31; 95% CI, 0.14 to 0.69). Among the 93 patients 55 years of age or older, treatment with blinatumomab appeared to improve both overall survival (at 3 years: 70%, vs. 65% with chemotherapy alone; hazard ratio for death, 0.66; 95% CI, 0.33 to 1.35) and relapse-free survival (at 3 years: 69% vs. 57%; hazard ratio for relapse or death, 0.74; 95% CI, 0.39 to 1.43). Details are provided in Figure 3B and Figures S3, S4, and S5 in Supplementary Appendix 1. Although the magnitude of benefit of blinatumomab was larger in patients younger than 55 years of age, benefit was also observed in patients 55 years of age or older.
Integrated genetic and genomic analysis14,15 enabled the subgrouping of patients into favorable risk (defined as DUX4-rearranged [17 patients], high-hyperdiploid [24 patients], TCF3::PBX1 [13 patients], or PAX5 P80R [12 patients]), intermediate risk (defined as PAX5-altered [34 patients], PAX5::ETV6 [3 patients], MEF2D-rearranged [8 patients], or ZNF384-rearranged [14 patients]), and unfavorable risk (defined as KMT2A-rearranged [66 patients], low-hypodiploid or near-haploid [90 patients], BCR::ABL1-like [100 patients], BCL2-rearranged or MYC-rearranged [10 patients], ETV6::RUNX1-like with IGH::CRLF2 fusion [2 patients], and high-hyperdiploid with BCR::ABL1-like, CRLF2-rearranged [1 patient]). In a post hoc subgroup analysis, blinatumomab therapy appeared to improve both overall survival and relapse-free survival in all three risk groups (Fig. 3B and Figs. S6, S7, and S8 in Supplementary Appendix 1).
ADVERSE EVENTS
Data on treatment-related adverse events were available for 479 enrolled patients. Treatment-related adverse events that occurred during consolidation therapy in patients with MRD-negative status are reported in Table 2. Among the patients who had MRD-negative status and received blinatumomab, a treatment-related non-hematologic toxic effect of grade 3 occurred in 43%, of grade 4 in 14%, and of grade 5 in 2%, as compared with 36%, 15%, and 1%, respectively, among patients with MRD-negative status in the chemotherapy-only group (P = 0.87). A treatment-related neurologic or psychiatric adverse event of grade 3 or higher occurred in 23% of the 111 patients who started treatment with blinatumomab, as compared with 5% of the 112 patients in the chemotherapy-only group (P<0.001). Causes of death are listed in Table S10 in Supplementary Appendix 1. Details of all the treatment-related toxic effects of grade 3, 4, or 5 according to each step of the trial and according to treatment group are provided in Table S11 in Supplementary Appendix 2.
Table 2.
Treatment-Related Toxic Effects during Consolidation Therapy in Patients with MRD-Negative Status.*
| Event | Blinatumomab + Chemotherapy (N = 112) | Chemotherapy Only (N = 112) | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| percentage of patients | ||||||
| Anemia | 20 | 1 | 0 | 35 | 2 | 0 |
|
| ||||||
| Leukopenia | 4 | 27 | 0 | 2 | 52 | 0 |
|
| ||||||
| Neutropenia | 3 | 55 | 0 | 1 | 86 | 0 |
|
| ||||||
| Lymphopenia | 3 | 8 | 0 | 6 | 17 | 0 |
|
| ||||||
| Thrombocytopenia | 9 | 40 | 0 | 10 | 59 | 0 |
|
| ||||||
| Febrile neutropenia | 16 | 1 | 0 | 21 | 2 | 0 |
|
| ||||||
| Sepsis | 0 | 4 | 1 | 0 | 6 | 1 |
|
| ||||||
| Hyperglycemia | 3 | 1 | 0 | 6 | 2 | 0 |
|
| ||||||
| Fatigue | 3 | 0 | 0 | 4 | 0 | 0 |
|
| ||||||
| ALT increased | 3 | 0 | 0 | 5 | 1 | 0 |
|
| ||||||
| AST increased | 1 | 0 | 0 | 1 | 2 | 0 |
|
| ||||||
| Hypertriglyceridemia | 0 | 3 | 0 | 1 | 3 | 0 |
|
| ||||||
| Nausea | 3 | 0 | 0 | 1 | 0 | 0 |
|
| ||||||
| Vomiting | 2 | 0 | 0 | 3 | 0 | 0 |
|
| ||||||
| Headache | 3 | 0 | 0 | 5 | 0 | 0 |
|
| ||||||
| Syncope | 3 | 0 | 0 | 3 | 0 | 0 |
|
| ||||||
| Other infection | 2 | 1 | 0 | 2 | 1 | 0 |
|
| ||||||
| Catheter-related infection | 1 | 0 | 0 | 3 | 1 | 0 |
|
| ||||||
| Upper respiratory tract infection | 1 | 0 | 0 | 3 | 0 | 0 |
Grade 3 to 5 adverse events that were reported in at least 3% of the patients in either group are listed. The worst grade of event was summarized by consolidating the reports of a given type of adverse event for a patient over all cycles during consolidation therapy.
ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
DISCUSSION
This randomized phase 3 trial showed an overall survival benefit with blinatumomab added to consolidation chemotherapy in patients with BCR::ABL1-negative disease who were 30 and 70 years of age and had BCP-ALL in MRD-negative remission. Because these patients have a better prognosis than those with MRD-positive remission, an intentional prolonged follow-up was necessary to have enough events to show a benefit with regard to overall survival. Although the trial was not powered for subgroup analyses, patients younger than 55 years of age appeared to have the greatest benefit.
Other trials of blinatumomab have shown a benefit in patients with MRD-positive disease, including the BLAST trial, which showed that after one 4-week cycle of blinatumomab, 78% of 113 evaluable patients had a complete MRD response; this result led to an accelerated FDA approval of blinatumomab in MRD-positive BCP-ALL.9 At a median of 22 months, improved event-free survival with blinatumomab was seen among children with a first relapse of BCP-ALL who had completed induction therapy and two cycles of consolidation therapy and had been randomly assigned to receive additional consolidation with blinatumomab, as compared with those who had been assigned to receive additional chemotherapy, before allogeneic transplantation.16 With longer follow-up, an overall survival advantage with blinatumomab was also seen in that trial, and the benefit of blinatumomab as compared with chemotherapy was seen in both MRD-negative and MRD-positive patients with regard to both event-free survival and overall survival.17 The Children’s Oncology Group randomly assigned patients up to 30 years of age with a first relapse to receive either blinatumomab or chemotherapy in consolidation after they had a remission with chemotherapy; although the trial did not meet its prespecified end point, it showed a significant improvement in the likelihood of an MRD-negative status with blinatumomab.18 These two trials led to the full FDA approval of blinatumomab for MRD-positive disease.
Single-group phase 2 trials are exploring the use of blinatumomab as first-line treatment. A study involving 38 patients (median age, 37 years) who received four cycles of hyper-CVAD chemotherapy (which includes cyclophosphamide, vincristine sulfate, doxorubicin, dexamethasone, methotrexate, and cytarabine) followed by four cycles of blinatumomab showed an estimated 3-year relapse-free survival of 73%. A total of 32 of 33 patients (97%) had an MRD-negative status over the course of therapy.19 The GIMEMA (Gruppo Italiano Malattie Ematologiche Dell’Adulto) group designed a phase 2 trial of a pediatric-intensive regimen involving 149 patients up to 65 years of age (median age, 41 years), with dose adjustments for patients older than 55 years of age, that added two cycles of blinatumomab to consolidation chemotherapy. After the first cycle of blinatumomab, the percentage of patients with MRD-negative status increased, from 70% (85 patients) before the receipt of blinatumomab to 93% (102 patients) after the receipt of blinatumomab (P<0.001). At a median follow-up of 37.5 months, overall survival was 71% and disease-free survival was 66% — findings that are higher than those seen in historical controls.20 The GMALL (German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia) group treated patients 56 to 76 years of age (median age, 66 years), in whom three cycles of standard consolidation chemotherapy was replaced with three cycles of blinatumomab.21 After the first cycle of blinatumomab, the incidence of complete remission increased, from 76% before the receipt of blinatumomab to 85% after the receipt of blinatumomab, with the incidence of MRD-negative status increasing from 18% to 82%. Overall survival was 80% at 1 year and 67% at 3 years. The percentage of patients with MRD-negative status was 82% with blinatumomab and 55% in a historical control group (P = 0.006); 3-year overall survival was 67% and 49%, respectively (P = 0.08), and among patients with a complete remission, 83% and 55%, respectively, remained in complete remission at 3 years (P = 0.06).21
In the present trial, morphologic complete remission with or without complete count recovery after induction was observed in 395 patients (81%). Only 286 of these patients reached the randomization or assignment step after the intensification phase. The remainder of the patients did not do so for reasons that included relapse, toxic effects, death, withdrawal of consent, and transplantation. Of the 488 patients who had been initially registered, 280 (57%) were categorized on the basis of genomic analysis as having high-risk disease, which probably accounts for the relatively low percentage of patients with a complete remission, as well as the fact that many of these patients did not reach randomization. Trials currently in development are exploring the use of blinatumomab earlier in the course of disease, including a trial of blinatumomab alternating with low-intensity chemotherapy as compared with standard-care chemotherapy among older adults with newly diagnosed BCR::ABL1-negative disease (ClinicalTrials.gov number, NCT04994717).
In this randomized trial, we found a significant improvement in overall survival among patients 30 to 70 years of age who had an MRD-negative remission of BCP-ALL and received blinatumomab plus chemotherapy in consolidation, as compared with chemotherapy alone. The incidence of reported neuropsychiatric adverse events was higher with blinatumomab than with chemotherapy alone.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute of the National Institutes of Health (U10CA180820, U10CA180794, U10CA180821, U10CA180888, U10CA180868, UG1CA189856, UG1CA189859, UG1CA189869, UG1CA232760, UG1CA233180, UG1CA233198, UG1CA233234, UG1CA233253, UG1CA233277, UG1CA233290, UG1CA233320, UG1CA233330, UG1CA233196, UG1CA233331, UG1CA233337, UG1CA233339, UG1CA239767, U10CA180863, P30CA021765, and R35CA197695), from the National Institute of General Medical Sciences of the National Institutes of Health (P50GM115279), and from the Canadian Cancer Society (704970) and in part by Amgen.
We thank Peter J. O’Dwyer, M.D., and Mitchell D. Schnall, M.D., Ph.D. (co-chairs of the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network [ECOG-ACRIN] Cancer Research Group), as well as Gerhard Zugmaier, M.D., Executive Medical Director Global Development at Amgen, for contributions to the development and conduct of the trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Mark R. Litzow, M.D., Zhuoxin Sun, Ph.D., Ryan J. Mattison, M.D., Elisabeth M. Paietta, Ph.D., Kathryn G. Roberts, Ph.D., Yanming Zhang, M.D., Janis Racevskis, Ph.D., Hillard M. Lazarus, M.D., Jacob M. Rowe, M.D., Daniel A. Arber, M.D., Matthew J. Wieduwilt, M.D., Ph.D., Michaela Liedtke, M.D., Julie Bergeron, M.D., Brent L. Wood, M.D., Ph.D., Yaqi Zhao, M.Sc., Gang Wu, Ph.D., Ti-Cheng Chang, Ph.D., Wenchao Zhang, Ph.D., Keith W. Pratz, M.D., Shira N. Dinner, M.D., Noelle Frey, M.D., Steven D. Gore, M.D., Bhavana Bhatnagar, D.O., Ehab L. Atallah, M.D., Geoffrey L. Uy, M.D., Deepa Jeyakumar, M.D., Tara L. Lin, M.D., Cheryl L. Willman, M.D., Daniel J. DeAngelo, M.D., Ph.D., Shejal B. Patel, D.O., Michelle A. Elliott, M.D., Anjali S. Advani, M.D., Dimitrios Tzachanis, M.D., Ph.D., Pankit Vachhani, M.D., Rupali R. Bhave, M.D., Elad Sharon, M.D., M.P.H., Richard F. Little, M.D., Harry P. Erba, M.D., Ph.D., Richard M. Stone, M.D., Selina M. Luger, M.D., Charles G. Mullighan, M.B., B.S., M.D., and Martin S. Tallman, M.D.
The authors’ affiliations are as follows: the Mayo Clinic, Rochester, MN (M.R.L., C.L.W., M.A.E.); Dana–Farber Cancer Institute, Boston (Z.S., D.J.D., R.M.S.); the University of Wisconsin Carbone Cancer Center, Madison (R.J.M.), and the Medical College of Wisconsin, Milwaukee (E.L.A.); Montefiore Medical Center Moses Campus (E.M.P., J.R.) and Memorial Sloan Kettering Cancer Center (Y. Zhang, M.S.T.) — both in New York; the Department of Pathology and the Center for Excellence for Leukemia Studies (K.G.R., Y. Zhao, C.G.M.) and the Center for Applied Bioinformatics (G.W., T.-C.C., W.Z.), St. Jude’s Children’s Research Hospital, Memphis, TN; Case Western Reserve University (H.M.L.) and Cleveland Clinic Foundation (A.S.A.), Cleveland, and the Ohio State University Comprehensive Cancer Center, Columbus (B.B.) — all in Ohio; Shaare Zedek Medical Center, Jerusalem, Israel (J.M.R.); Stanford Cancer Institute, Palo Alto (D.A.A., M.L.), the University of California, San Diego, Moores Cancer Center, La Jolla (M.J.W., D.T.), and the University of California, Irvine, Health Cancer Center-Newport, Orange (D.J.) — all in California; the University of Chicago (D.A.A.) and Northwestern University (S.N.D.) — both in Chicago; Hopital Maisonneuve-Rosemont, Montreal (J.B.); the University of Washington, Seattle (B.L.W.); Johns Hopkins University Sidney Kimmel Cancer Center, Baltimore (K.W.P.), and the National Cancer Institute, National Institutes of Health, Bethesda (E.S., R.F.L.) — both in Maryland; the University of Pennsylvania Abramson Cancer Center, Philadelphia (N.F., S.M.L.); Yale School of Medicine, New Haven, CT (S.D.G.); the Washington University in St. Louis School of Medicine, St. Louis (G.L.U.); the University of Kansas Cancer Center, Westwood (T.L.L.); Virginia Commonwealth University Massey Cancer Center, Richmond (S.B.P.); the University of Alabama at Birmingham, Birmingham (P.V.); and Wake Forest University Health Sciences, Winston-Salem (R.R.B.), and Duke University Medical Center, Durham (H.P.E.) — both in North Carolina.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
A Quick Take is available at NEJM.org
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 2019;133:1548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 2015;29:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol 2017;3(7):e170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh SA, Litzow MR. Philadelphia chromosome-negative acute lymphoblastic leukemia: therapies under development. Future Oncol 2014;10:2201–12. [DOI] [PubMed] [Google Scholar]
- 5.Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med 2016;375:1044–53. [DOI] [PubMed] [Google Scholar]
- 6.Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet 2020;395:1146–62. [DOI] [PubMed] [Google Scholar]
- 7.Newman MJ, Benani DJ. A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract 2016;22:639–45. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018;131:1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008;111:1827–33. [DOI] [PubMed] [Google Scholar]
- 11.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005;106:3760–7. [DOI] [PubMed] [Google Scholar]
- 12.Teuffel O, Kuster SP, Hunger SP, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia 2011;25:1232–8. [DOI] [PubMed] [Google Scholar]
- 13.Borowitz MJ, Wood BL, Keeney M, Hedley BD. Measurable residual disease detection in B-acute lymphoblastic leukemia: the Children’s Oncology Group (COG) method. Curr Protoc 2022;2(3):e383. [DOI] [PubMed] [Google Scholar]
- 14.Jeha S, Choi J, Roberts KG, et al. Clinical significance of novel subtypes of acute lymphoblastic leukemia in the context of minimal residual disease-directed therapy. Blood Cancer Discov 2021;2:326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paietta E, Roberts KG, Wang V, et al. Molecular classification improves risk assessment in adult BCR-ABL1-negative B-ALL. Blood 2021;138:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locatelli F, Zugmaier G, Rizzari C, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA 2021;325:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locatelli F, Eckert C, Hrusak O, et al. Blinatumomab overcomes poor prognostic impact of measurable residual disease in pediatric high-risk first relapse B-cell precursor acute lymphoblastic leukemia. Pediatr Blood Cancer 2022;69(8):e29715. [DOI] [PubMed] [Google Scholar]
- 18.Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA 2021;325:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabbour E, Short NJ, Jain N, et al. Hyper-CVAD and sequential blinatumomab for newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: a single-arm, single-centre, phase 2 trial. Lancet Haematol 2022;9(12):e878–e885. [DOI] [PubMed] [Google Scholar]
- 20.Chiaretti S, Della Starza I, Santoro A, et al. Sequential chemotherapy and blinatumomab to improve minimal residual disease in adult Ph- B-lineage acute lymphoblastic leukemia. Final results of the phase II Gimema LAL2317 trial. Blood 2023;142:Suppl 1:826. abstract (https://ashpublications.org/blood/article/142/Supplement%201/826/503873/Sequential-Chemotherapy-and-Blinatumomab-to). [Google Scholar]
- 21.Goekbuget N, Schwartz S, Faul C, et al. Dose reduced chemotherapy in sequence with blinatumomab for newly diagnosed older patients with Ph/BCR::ABL negative B-precursor adult lymphoblastic leukemia (ALL): preliminary results of the GMALL bold trial. Blood 2023;142:Suppl 1:964. abstract (https://ashpublications.org/blood/article/142/Supplement%201/964/499580/Dose-Reduced-Chemotherapy-in-Sequence-with). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


