Abstract
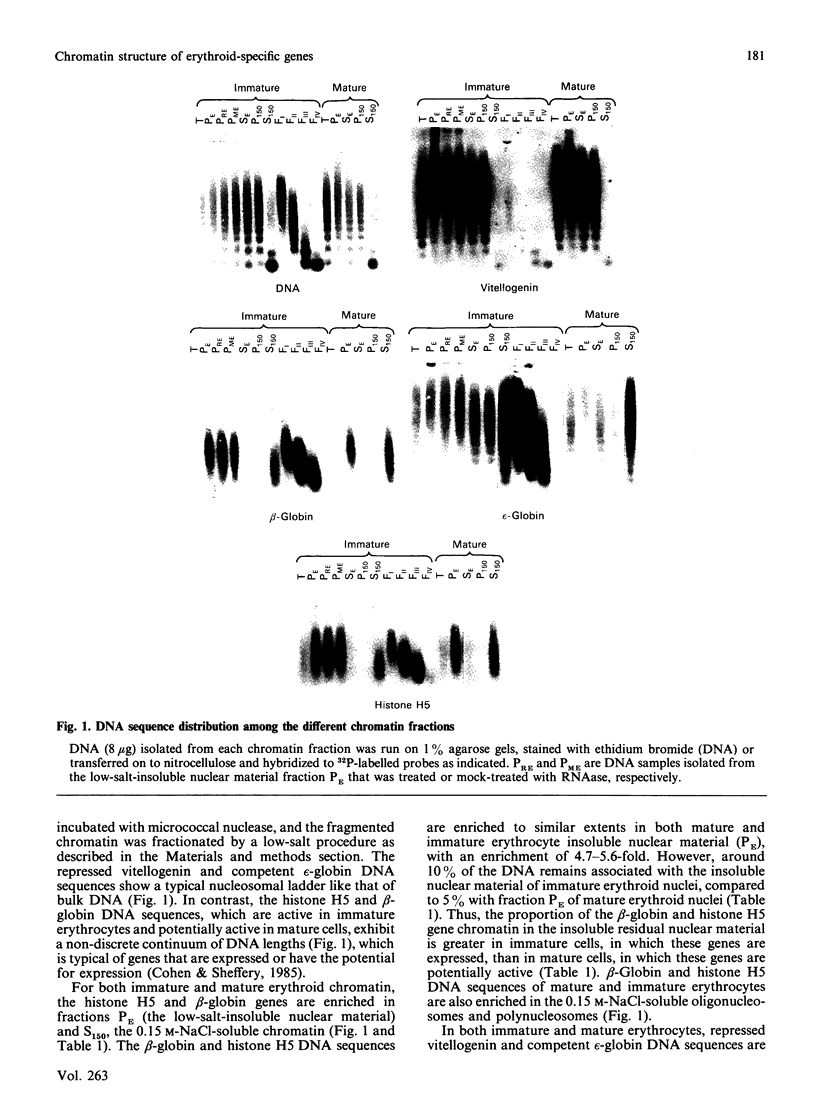
The beta-globin and histone H5 genes are transcriptionally active in immature chicken erythrocytes and potentially active in mature erythrocytes. In both immature and mature erythrocytes, the majority of these erythroid-specific gene sequences are located in two chromatin fractions: the low-salt-insoluble residual nuclear material and the 0.15 M-NaCl-soluble oligo- and poly-nucleosomes. These salt-soluble chromatin fragments are enriched in hyperacetylated species of H4 and H2B, ubiquitinated and polyubiquitinated species of H2A and H2B and are depleted of linker histones H1 and H5. The competent, transcriptionally inactive embryonic epsilon-globin gene, which is part of the DNAase I-sensitive beta-globin domain, is highly enriched in the 0.15 M-NaCl-soluble polynucleosome fraction but not in the insoluble nuclear material. The repressed vitellogenin gene shows no enrichment in either of these fractions. These results suggest that only those genes that are expressed or have the potential for expression are enriched in the low-salt-insoluble nuclear material of immature or mature erythrocytes. The enrichment of active genes in the low-salt-insoluble residual nuclear material of immature erythrocytes is not dependent on on-going transcription, the presence of RNA or changes in the amount of acetylated histone species. Our results are consistent with the hypothesis that active and potentially active genes are insoluble because of the presence of preinitiation transcription complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Côté J., Renaud J., Ruiz-Carrillo A. Regulation of histone and beta A-globin gene expression during differentiation of chicken erythroid cells. Mol Cell Biol. 1987 Oct;7(10):3663–3672. doi: 10.1128/mcb.7.10.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso W. R., Ferris R. C., Zhang D. E., Nelson D. A. Chicken erythrocyte beta-globin chromatin: enhanced solubility is a direct consequence of induced histone hyperacetylation. Nucleic Acids Res. 1987 Nov 25;15(22):9325–9337. doi: 10.1093/nar/15.22.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K., Mähr R., Björkroth B., Daneholt B. Rapid reformation of the thick chromosome fiber upon completion of RNA synthesis at the Balbiani ring genes in Chironomus tentans. Chromosoma. 1982;87(1):33–48. doi: 10.1007/BF00333508. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Chan S., Attisano L., Lewis P. N. Histone H3 thiol reactivity and acetyltransferases in chicken erythrocyte nuclei. J Biol Chem. 1988 Oct 25;263(30):15643–15651. [PubMed] [Google Scholar]
- Cohen R. B., Sheffery M. Nucleosome disruption precedes transcription and is largely limited to the transcribed domain of globin genes in murine erythroleukemia cells. J Mol Biol. 1985 Mar 5;182(1):109–129. doi: 10.1016/0022-2836(85)90031-2. [DOI] [PubMed] [Google Scholar]
- Conklin K. F., Groudine M. Varied interactions between proviruses and adjacent host chromatin. Mol Cell Biol. 1986 Nov;6(11):3999–4007. doi: 10.1128/mcb.6.11.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Wides R. J., Sollner-Webb B. Eucaryotic transcription complexes are specifically associated in large sedimentable structures: rapid isolation of polymerase I, II, and III transcription factors. Mol Cell Biol. 1985 Jul;5(7):1582–1590. doi: 10.1128/mcb.5.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R. Two-dimensional gel systems for rapid histone analysis for use in minislab polyacrylamide gel electrophoresis. Anal Biochem. 1982 Mar 1;120(2):276–281. doi: 10.1016/0003-2697(82)90348-7. [DOI] [PubMed] [Google Scholar]
- Ericsson C., Goldknopf I. L., Daneholt B. Inhibition of transcription does not affect the total amount of ubiquitinated histone 2A in chromatin. Exp Cell Res. 1986 Nov;167(1):127–134. doi: 10.1016/0014-4827(86)90210-7. [DOI] [PubMed] [Google Scholar]
- Fath K. R., Lasek R. J. Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. J Cell Biol. 1988 Aug;107(2):613–621. doi: 10.1083/jcb.107.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenz C. R., Nelson D. A. N-Butyrate incubation of immature chicken erythrocytes preferentially enhances the solubility of beta A chromatin. Nucleic Acids Res. 1985 Mar 25;13(6):1977–1995. doi: 10.1093/nar/13.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
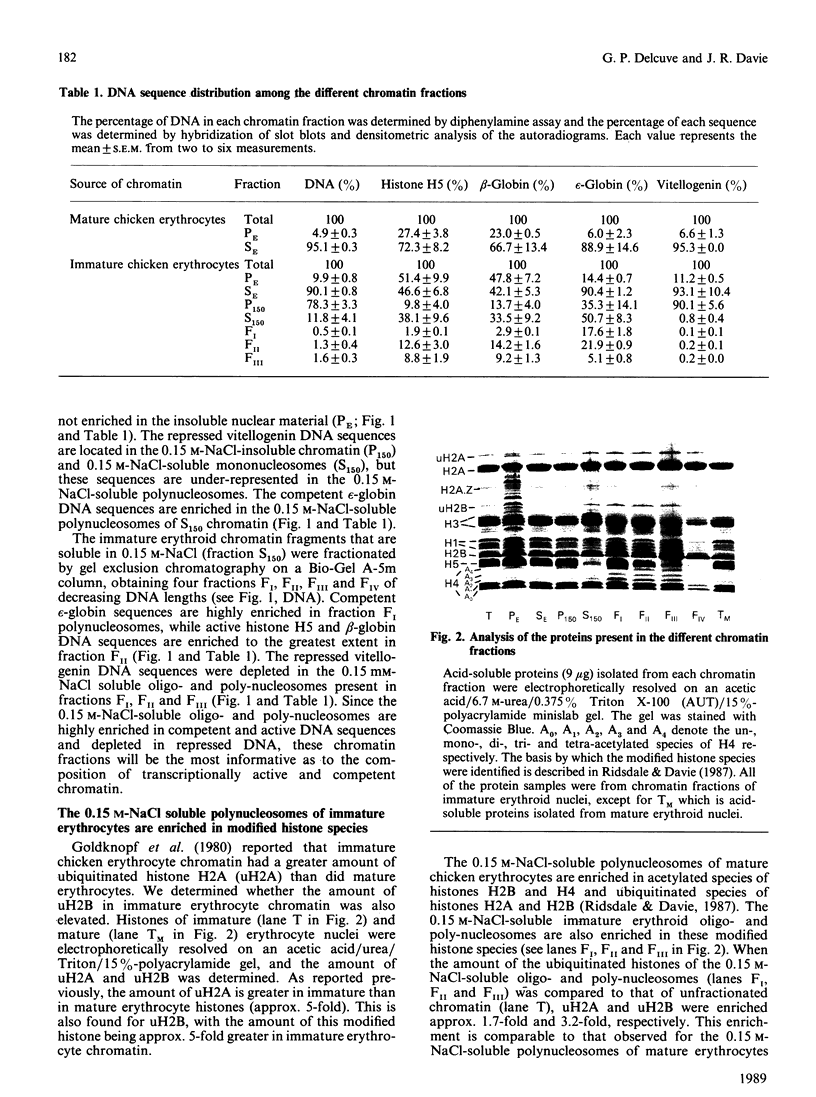
- Goldknopf I. L., Wilson G., Ballal N. R., Busch H. Chromatin conjugate protein A24 is cleaved and ubiquitin is lost during chicken erythropoiesis. J Biol Chem. 1980 Nov 25;255(22):10555–10558. [PubMed] [Google Scholar]
- Harvey R. P., Whiting J. A., Coles L. S., Krieg P. A., Wells J. R. H2A.F: an extremely variant histone H2A sequence expressed in the chicken embryo. Proc Natl Acad Sci U S A. 1983 May;80(10):2819–2823. doi: 10.1073/pnas.80.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Okret S., Wikström A. C., Gustafsson J. A., Shaper J. H. Binding of the glucocorticoid receptor to the rat liver nuclear matrix. The role of disulfide bond formation. J Biol Chem. 1986 Sep 15;261(26):11962–11967. [PubMed] [Google Scholar]
- LaFond R. E., Woodcock H., Woodcock C. L., Kundahl E. R., Lucas J. J. Generation of an internal matrix in mature avian erythrocyte nuclei during reactivation in cytoplasts. J Cell Biol. 1983 Jun;96(6):1815–1819. doi: 10.1083/jcb.96.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafond R. E., Woodcock C. L. Status of the nuclear matrix in mature and embryonic chick erythrocyte nuclei. Exp Cell Res. 1983 Aug;147(1):31–39. doi: 10.1016/0014-4827(83)90268-9. [DOI] [PubMed] [Google Scholar]
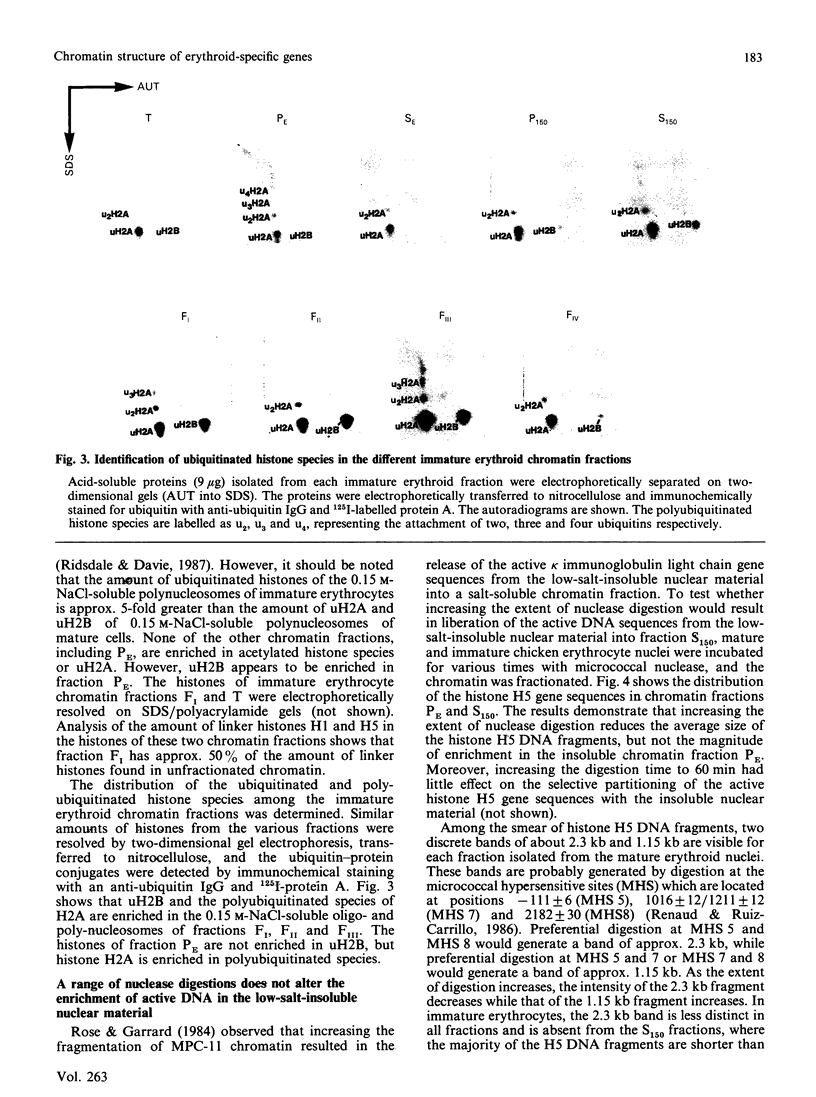
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Ferris R. C., Zhang D. E., Ferenz C. R. The beta-globin domain in immature chicken erythrocytes: enhanced solubility is coincident with histone hyperacetylation. Nucleic Acids Res. 1986 Feb 25;14(4):1667–1682. doi: 10.1093/nar/14.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Davie J. R. Structure of polyubiquitinated histone H2A. Biochemistry. 1989 Feb 7;28(3):964–968. doi: 10.1021/bi00429a007. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Davie J. R. The protamine gene chromatin in developing trout testis exists in an altered state. Biochim Biophys Acta. 1989 Jan 23;1007(1):23–29. doi: 10.1016/0167-4781(89)90125-5. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Roth S. Y., Cook R. G., Allis C. D., Davie J. R. Changes in the histone H2A variant H2A.Z and polyubiquitinated histone species in developing trout testis. Biochemistry. 1987 Jul 14;26(14):4417–4421. doi: 10.1021/bi00388a034. [DOI] [PubMed] [Google Scholar]
- Nickerson J. A., Krochmalnic G., Wan K. M., Penman S. Chromatin architecture and nuclear RNA. Proc Natl Acad Sci U S A. 1989 Jan;86(1):177–181. doi: 10.1073/pnas.86.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Renaud J., Ruiz-Carrillo A. Fine analysis of the active H5 gene chromatin of chicken erythroid cells at different stages of differentiation. J Mol Biol. 1986 May 5;189(1):217–226. doi: 10.1016/0022-2836(86)90392-x. [DOI] [PubMed] [Google Scholar]
- Ridsdale J. A., Davie J. R. Chicken erythrocyte polynucleosomes which are soluble at physiological ionic strength and contain linker histones are highly enriched in beta-globin gene sequences. Nucleic Acids Res. 1987 Feb 11;15(3):1081–1096. doi: 10.1093/nar/15.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale J. A., Rattner J. B., Davie J. R. Erythroid-specific gene chromatin has an altered association with linker histones. Nucleic Acids Res. 1988 Jul 11;16(13):5915–5926. doi: 10.1093/nar/16.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M., Dahmus M. E., Bradbury E. M. Chromosomal loop/nuclear matrix organization of transcriptionally active and inactive RNA polymerases in HeLa nuclei. J Mol Biol. 1988 Jun 5;201(3):545–555. doi: 10.1016/0022-2836(88)90636-5. [DOI] [PubMed] [Google Scholar]
- Rose S. M., Garrard W. T. Differentiation-dependent chromatin alterations precede and accompany transcription of immunoglobulin light chain genes. J Biol Chem. 1984 Jul 10;259(13):8534–8544. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Affolter M., Renaud J. Genomic organization of the genes coding for the six main histones of the chicken: complete sequence of the H5 gene. J Mol Biol. 1983 Nov 15;170(4):843–859. doi: 10.1016/s0022-2836(83)80191-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Strätling W. H., Dölle A., Sippel A. E. Chromatin structure of the chicken lysozyme gene domain as determined by chromatin fractionation and micrococcal nuclease digestion. Biochemistry. 1986 Jan 28;25(2):495–502. doi: 10.1021/bi00350a033. [DOI] [PubMed] [Google Scholar]
- Strätling W. H. Gene-specific differences in the supranucleosomal organization of rat liver chromatin. Biochemistry. 1987 Dec 1;26(24):7893–7899. doi: 10.1021/bi00398a053. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen R., van Venrooij W., Ramaekers F. The nuclear matrix: structure and composition. J Cell Sci. 1988 May;90(Pt 1):11–36. doi: 10.1242/jcs.90.1.11. [DOI] [PubMed] [Google Scholar]
- Villeponteau B., Landes G. M., Pankratz M. J., Martinson H. G. The chicken beta globin gene region. Delineation of transcription units and developmental regulation of interspersed DNA repeats. J Biol Chem. 1982 Sep 25;257(18):11015–11023. [PubMed] [Google Scholar]
- Williams A. F. DNA synthesis in purified populations of avian erythroid cells. J Cell Sci. 1972 Jan;10(1):27–46. doi: 10.1242/jcs.10.1.27. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Felsenfeld G. Chromatin structure of the chicken beta-globin gene region. Sensitivity to DNase I, micrococcal nuclease, and DNase II. J Biol Chem. 1982 Jul 10;257(13):7730–7736. [PubMed] [Google Scholar]
- Zandomeni R., Bunick D., Ackerman S., Mittleman B., Weinmann R. Mechanism of action of DRB. III. Effect on specific in vitro initiation of transcription. J Mol Biol. 1983 Jul 5;167(3):561–574. doi: 10.1016/s0022-2836(83)80098-9. [DOI] [PubMed] [Google Scholar]
- Zandomeni R., Zandomeni M. C., Shugar D., Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986 Mar 5;261(7):3414–3419. [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified. Biochem J. 1988 Feb 15;250(1):233–240. doi: 10.1042/bj2500233. [DOI] [PMC free article] [PubMed] [Google Scholar]