Abstract
We investigated the effect of an albumin infusion on the enzyme activity, expression level of cytochrome P450 2E1 (CYP2E1), and oxidative stress in the serum and liver of streptozotocin (STZ)-induced diabetic rats. The STZ treatment enhanced the alanine aminotransferase and aspartate aminotransferase activities in the rat serum compared with those in the untreated rats. Treatment with STZ elevated the expression and catalytic activity of CYP2E1, and the oxidative stress, and decreased the reducing potentials in the liver, suggesting the possibility of diabetes-induced liver injury. Moreover, the antioxidant activity of the serum albumin decreased in the diabetic rats. In contrast, the administration of purified albumin from the intact rats to the diabetic rats restored these deleterious liver indices in an albumin concentration-dependent manner. These results suggest that an exogenous albumin infusion alleviates liver damage induced by type 1 diabetes.
Keywords: cytochrome P450 2E1, diabetes, oxidative stress
Streptozotocin (STZ) is widely used to induce type 1 diabetes mellitus in animal models. It has been suggested that hyperglycaemia, the auto-oxidation of glycated proteins, and the increased production of reactive oxygen species (ROS) lead to increased oxidative stress in STZ-treated rats, which is accompanied by pancreatic β-cell damage (Desco et al. 2002). Moreover, STZ has also been shown to deplete antioxidant pools in cells, making them more susceptible to oxidative damage (Low et al. 1997).
Cytochrome P450 2E1 (CYP2E1) is a classical ethanol-inducible protein that metabolises various endogenous compounds and xenobiotics (Chen et al. 2019) and catalyses the bioactivation of several procarcinogens and protoxins (Guengerich et al. 1991; Surbrook and Olson 1992). CYP2E1 also produces ROS, such as lipid peroxidation (Leung and Nieto 2013), which is known to be involved in the aetiology and pathology of many diseases (Caro and Cederbaum 2004). In relation to diabetes, the increased expression of CYP2E1 in various tissues of STZ-induced diabetic rats, including the liver, has been reported (Raza et al. 2004). CYP2E1 expression has also been linked to the generation of specific pathological conditions, including both alcoholic and non-alcoholic liver disease (Weltman et al. 1998; Niemela et al. 2000), and diabetes is commonly associated with the development of non-alcoholic steatohepatitis. Therefore, the increased expression of CYP2E1 may lead to liver disease in diabetics.
Albumin is the most abundant circulating protein in the plasma, accounting for approximately 60% of the total plasma proteins. Albumin has several important physiological and pharmacological functions, including the transport of metals, fatty acids, cholesterol, bile pigments, and drugs. Albumin also acts as a predominant antioxidant in the plasma, a body compartment that is continuously exposed to oxidative stress (Roche et al. 2008). Previous studies have shown that more than 70% of the free radical-trapping activity of the serum is attributed to the serum albumin (Bourdon and Blache 2001). Thus, hypoalbuminemia patients display a reduced potential for the scavenging of oxygen free radicals (Nicholson et al. 2000).
In this study, we investigated the effect of an external albumin administration on a liver injury in STZ-induced diabetic rats. In addition, the influence of the diabetic occurrence on the serum albumin was re-evaluated.
MATERIAL AND METHODS
Experimental animals
Six-week-old male Sprague Dawley rats weighing 200−250 g were obtained from Samtako Bio Korea (Osan, Republic of Korea). Type 1 diabetes was then induced in the rats with similar body weights after one week of quarantine and acclimation. The rats were housed individually in stainless steel cages in a room with a controlled temperature of 23 ± 1 °C with alternating 12 h periods of light and dark. They were fed commercial rodent chow (Samyang Feed Co., Wonju, Republic of Korea). The experimental design was approved by the Committee for the Care and Use of Laboratory Animals at the Chonnam National University (CNU IACUC-YB-2019-30).
Purification of serum albumin
The albumin in the rat serum was aseptically purified on a clean bench using a modified method reported previously (Raoufinia et al. 2016). Briefly, the blood serum was obtained from the rats, and the serum proteins were precipitated by adding 50% (w/v) ammonium sulfate. After collecting and dialysing the precipitant fractions, the albumin was purified using affinity-column chromatography with anti-albumin antibody-immobilised sepharose. The purity was checked by 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Coomassie blue staining, and densitometry. Fractions with a purity over 98% were then used throughout all the relevant experiments.
Induction of diabetes and albumin administration
Diabetes was induced by a single intraperitoneal injection of STZ (100 mg/kg body weight in a 0.1 M citrate buffer). The rats in the nondiabetic group (control) were injected with an equivalent volume of the 0.1 M citrate buffer only. Five days after injection, the severity of the induced diabetic state was assessed by monitoring the blood glucose levels using Accutrend® reagent strips (Roche Diagnostics GmbH, Mannheim, Germany). Rats with blood glucose levels exceeding 3.0 g/l were classified as diabetic rats. The rat blood was collected from the caudal vena cava. The purified serum albumin from the nondiabetic rats was dissolved in sterile phosphate-buffered saline and co-administered with the STZ to the rats through the tail vein. Albumin (196 mg/ml in the phosphate buffered saline solution) was injected into the rats (approximately 700 mg/kg body weight) for IN-1 (Tables 1 and 2). The same amount of albumin was administered again for IN-2 (Tables 1 and 2), 2 h after the first infusion.
Table 1. Blood biochemical analyses and enzyme activities in the rat serum.
| Assays | Units | Control | STZ | STZ/IN-1 | STZ/IN-2 |
| ALT | μkat/l | 0.81 ± 0.37 | 2.14 ± 0.53* | 1.31 ± 0.44 (1.72 ± 0.51) | 1.07 ± 0.38** (1.20 ± 0.35) |
| AST | μkat/l | 1.93 ± 0.70 | 6.32 ± 1.30* | 3.75 ± 0.94** (4.24 ± 0.98) | 2.96 ± 0.86** (3.47 ± 0.88**) |
| TG | mg/l | 1 338 ± 215 | 2 899 ± 434* | 2 048 ± 389 (2 295 ± 406) | 1 897 ± 402** (1 718 ± 314**) |
| Ketones | mmol/l | 242 ± 58 | 1 224 ± 297* | 661 ± 183** (890 ± 208) | 504 ± 157** (774 ± 181) |
| Albumin | g/l | 43 ± 5 | 39 ± 3 | ND | ND |
| Glucose | g/l | 1 060 ± 320 | 4 780 ± 1 120* | 4 420 ± 950* (ND) | 4 530 ± 1 040* (ND) |
One μkat corresponds to the amount of ALT or AST required to generate 1.0 μmole of pyruvate or glutamate per minute at 37°C, respectively
STZ/IN-1 and STZ/IN-2 represent one (1.0 ml at 196 mg/ml) and two consecutive infusions of albumin, respectively
*P < 0.05 compared with that of control; **P < 0.05 compared with that of STZ
ND = not determined; STZ = streptozotocin; TG = triglycerides
Table 2. Oxidative stress and GST, CAT and SOD enzyme activities in the rat liver.
| Assays | Unit | Control | STZ | STZ/IN-1 | STZ/IN-2 |
| ROS | pmol/mg protein | 3.1 ± 0.9 | 6.8 ± 1.7* | 4.4 ± 1.2 (5.1 ± 1.3) | 3.5 ± 1.0** (3.4 ± 1.2**) |
| GSH | nmol/mg protein | 52.4 ± 18.5 | 33.5 ± 11.4 | 38.2 ± 15.0 (31.7 ± 16.3) | 45.7 ± 16.3 (39.8 ± 13.9) |
| GST | nmol/min/mg protein | 197.6 ± 47.6 | 98.9 ± 28.5* | 138.3 ± 33.7 (152.4 ± 40.2) | 168.5 ± 32.1** (173.5 ± 45.9) |
| CAT | μkat/mg protein | 0.26 ± 0.05 | 0.11 ± 0.02* | 0.16 ± 0.03 (0.13 ± 0.02) | 0.20 ± 0.04** (0.17 ± 0.03**) |
| SOD | μkat/mg protein | 2.52 ± 0.59 | 1.44 ± 0.34* | 1.93 ± 0.43 (1.37 ± 0.37) | 2.16 ± 0.61 (1.80 ± 0.51) |
One μkat of CAT decomposes 1.0 μmole of H2O2 to oxygen and water per minute at a substrate concentration of 50 mM H2O2. One μkat of SOD inhibits the rate of reduction of cytochrome c by 50% in a coupled reaction, using xanthine and xanthine oxidase, at 25 °C
*P < 0.05 compared with that of control; **P < 0.05 compared with that of STZ
CAT = catalase; GSH = glutathione; GST = glutathione transferase; ROS = reactive oxygen species; SOD = superoxide dismutase; STZ = streptozotocin
Serum biochemical analysis
The rats were anaesthetised using a combination of xylazine hydrochloride (Rompun; Bayer Korea, Seoul, Republic of Korea; 10 mg/kg) and ketamine HCl (Yuhan Co., Seoul, Republic of Korea; 40 mg/kg). Blood samples were collected by venepuncture from the posterior vena cava. Each sample was centrifuged at 1 000 × g for 10 min within 30 min after collection, and then the top serum layer was removed. The albumin concentration in the serum was measured using the Albumin (BCG) Assay Kit (Abcam, Cambridge, UK). The concentration of triglycerides (TG) was measured using an ADVIA® 2400 (Siemens, Washington DC, USA). The 3-hydroxybutyrate and acetoacetate concentrations were determined using a method reported previously (McGarry et al. 1970).
Fractionation of liver cells and preparation of pancreas lysates
The cytosolic and microsomal fractions of the liver cells were obtained using a previously described method (Kamath et al. 1971): immediately after decapitation, the liver was excised, rinsed in liquid nitrogen, diced, and homogenised in 2–5 volumes of 100 mM Tris-HCl (pH 7.4, 4 °C) containing 2 mM of phenylmethyl sulfonyl fluoride and pepstatin (12.5 μg/ml), a protease inhibitor, using a homogeniser (IKAm, Staufen, Germany). The homogenate was centrifuged at 10 000 × g for 20 min (4 °C), and the resultant supernatant was centrifuged at 200 000 × g for 60 min (4 °C) to sediment the microsomes. The supernatant was used as the cytosolic fraction and the microsomal pellet was suspended in 100 mM Tris-HCl (pH 7.4, 4 °C). The pancreas lysates were prepared by homogenisation in a modified radioimmunoprecipitation assay (RIPA) buffer (150 mM of NaCl, 1 mM of phenylmethylsulfonyl fluoride, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholic acid, 0.1% of sodium dodecyl sulfate (SDS), 5 μg/ml of aprotinin, 5 μg/ml of leupeptin). The tissue and cell debris were removed by centrifugation (10 000 × g for 20 min, 4 °C).
Assay of enzyme activities
The p-nitrophenol hydroxylase activity of CYP2E1 was measured using the microsomal fractions (Reinke and Moyer 1985): the reaction mixture (2 ml) contained microsomes (approximately 175 μg of proteins), 200 μM of p-nitrophenol dissolved in 50 mM of Tris-HCl (pH 7.4), 5 mM of MgCl2, and 0.8 mM of nicotinamide adenine dinucleotide phosphate (NADPH). The reactions were initiated by the addition of NADPH. After incubation for 20 min at 37 °C, the reactions were terminated by the addition of 0.5 ml of 0.6 N perchloric acid. After the removal of the proteins by centrifugation, the resultant metabolite, 4-nitrocatechol was analysed by high-performance liquid chromatography (HPLC) using the absorbance at 480 nm. The activity values were expressed as nmol hydroxylated nitrophenol/min/mg protein. The thiol-specific peroxidase activity of the serum albumin was measured as previously described (Cha and Kim 1996); the albumin-induced removal of hydrogen peroxide (H2O2) mediated by glutathione (GSH) was analysed in a 100 μl reaction mixture containing 0.5 mM of H2O2, 5 mM of GSH, 50 mM of HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (pH 7.2), and 1.2 mg/ml of albumin. After incubation for 30 min at 37 °C, 0.7 ml of trichloroacetic acid (12.5%, w/v) was added to terminate the reaction. To measure the concentration of the remaining H2O2, 0.2 ml of 10 mM Fe(NH4)2(SO4)2 and 0.1 ml of 2.5 N potassium thiocyanate (KSCN) were added. The absorbance of the purple-coloured sample was monitored at 480 nm. The glutathione 0167S-transferase (GST) activity was measured using an assay kit provided by Sigma Aldrich (St. Louis, MI, USA), based on a method reported previously (Habig et al. 1974). The serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using a Dri-chem® 4000i auto-analyser (Fujifilm Co., Tokyo, Japan). The superoxide dismutase (SOD) activity was determined according to a method previously described (Beauchamp and Fridovich 1971); after homogenisation of the liver sample, the cytosolic fractions (0.4 mg of proteins) were mixed with 50 mM of carbonic buffer (pH 10.2), 0.1 mM of Na2-EDTA, 0.1 mM of xanthine, and 0.025 mM of nitro blue tetrazolium (NBT) and illuminated at 25 °C for 10 minutes. Then, xanthine oxidase (3.3 × 10–6 mM) was added to the reaction mixture, and the reduction of NBT was measured spectrophotometrically at 560 nm. The catalase (CAT) activity was determined using the method described by Aebi (1984) using the cytosolic fractions (0.15 mg proteins). A decrease in the H2O2 absorbance was measured spectrophotometrically at 240 nm in 1 ml of a reaction mixture containing 10 mM of H2O2 and 20 μl of the supernatant in 50 mM of a potassium phosphate buffer (pH 7.0). The CAT activity was expressed as one (l) mole of H2O2 decomposed/mg protein/min at pH 7.0 at 25 °C.
Immunoblot analysis of microsomal CYP2E1 and pancreatic glutamic acid decarboxylase (GAD)
The microsomal liver proteins and pancreas lysate (80 μg/well) were separated using 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the gel was transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated with an anti-CYP2E1 antibody [Abcam, Cambridge, UK; 1/1 000 (v/v)] or anti-GAD 65/67 antibody [Sigma-Aldrich, St. Louis, MO, USA; 1/20 000 (v/v)], and then with an anti-rabbit IgG-HRP secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) for 1 h each. The proteins were visualised using enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK). The protein band intensities were quantified using ImageJ software (National Institute of Health, USA).
Measurement of oxidative stress
The levels of ROS were measured by a spectrofluorimetric method with the cytosolic fractions (1.5 mg proteins) using 2,7-dichlorofluorescein diacetate as a probe according to the method described previously (Bhagwat et al. 1998). The sulfhydryl content in the purified albumin was measured with 5,5-dithio-bis-2-nitrobenzoic acid (ThermoFisher Scientific, Waltham, MA, USA). The reduced GSH levels were also determined with the cytosolic fractions by the protein-free sulfhydryl content using Ellman’s reagent (ThermoFisher Scientific, Waltham, MA, USA), based on a method reported previously (Buttar et al. 1977).
Statistical analysis
The data were analysed using GraphPad InStat v3.0 (GraphPad Software Inc., La Jolla, CA, USA). The results are expressed as the mean ± standard deviation. The differences between groups were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A P-value of < 0.05 was considered to indicate a statistically significant difference.
Protein concentration assay
The protein concentrations were estimated using a bicinchoninic acid procedure (Pierce, Rockford, IL, USA), with a bovine serum albumin solution as the standard.
Tryptophan fluorescence
For the measurement of the intrinsic tryptophan (Trp) fluorescence of the albumin, the emission spectra were obtained in the range of 320−450 nm, with an excitation wavelength of 295 nm, using an RF-5301 PC spectrofluorometer (Shimadzu, Kyoto, Japan) at 30 °C. The commercial albumin purified from the rat serum was obtained from Abcam, and the purity was analysed by SDS-PAGE. All the solutions used in the present study were sterilised by filtration or autoclaving.
RESULTS
STZ is known to exert a detrimental effect on the liver through increased oxidative stress in rats (Kakkar et al. 1998). In the present study, the blood biochemical parameters and activities of the serum ALT and AST, including the production of oxidative stress, were measured to evaluate and confirm liver injury upon the STZ treatment in the rats. The concentrations of ketone bodies (3-hydroxybutyrate and acetoacetate) and TG as well as the glucose level were enhanced in the serum of STZ-treated rats compared with those of the normal rats, indicating the development of type 1 diabetes (Table 1). In addition, the activities of serum ALT and AST increased by approximately 2.6- and 3.3-fold, respectively, in the STZ-treated rats compared to those in the normal rats. Table 1 also shows that the albumin level in the serum of the STZ-treated rats decreased by approximately 9% compared with that of the normal rats, although the difference was not statistically significant.
The STZ treatment resulted in an approximately 2.2-fold elevation in the production of ROS in the cytosolic fractions of the rat liver (Table 2). Moreover, the GSH concentration and GST, CAT, and SOD enzyme activities in the STZ-treated rats were 64, 50, 42, and 57%, respectively, of the control values in the normal rats. These results collectively imply that STZ mediated the elevation of the oxidative stress and a decrease in the reducing potentials of the liver in the STZ-diabetic rats. The parameters shown in Tables 1 and 2 were measured 15 days after the diabetes occurrence (as judged by the blood glucose level and at a time point of 20 days in Figures 1, 2, and 3), and therefore the values may have changed with the progression in diabetes severity.
Figure 1. Time-dependent changes in the peroxidase activities (A), tryptophan fluorescence at λmax (B), and the numbers of free thiol group in the albumin (C).
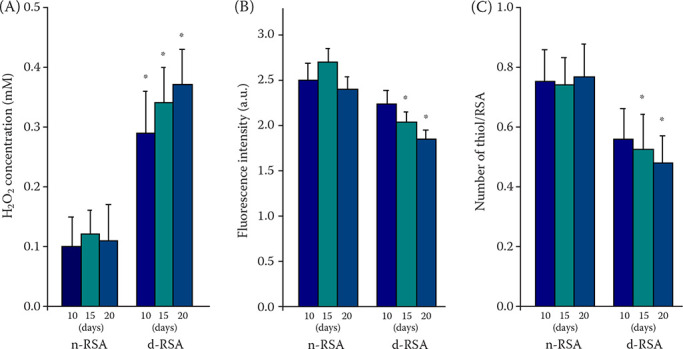
All the experiments were performed with purified serum albumin at each indicated day after treatment of the rats with STZ (d-RSA) or with a citrate buffer (n-RSA). The diabetic occurrence was confirmed based on the blood glucose levels (over 3.0 g/l) five days after the STZ treatment (therefore, the days in the figures represent each indicated time after the STZ injection). The data are represented as the mean ± SD, n = 7
*P < 0.05 indicates a comparison with values of n-RSA
In (A), 0.5 mM of H2O2 was added to the reaction mixtures, and, after a 30 min incubation at 37 °C, the concentration of remaining H2O2 was measured to determine peroxidase activity. In (B), the a.u. in the y-axis represents arbitrary unit. In (C), the number of thiol/RSA in the y-axis represents the number of thiol groups per albumin molecule
STZ = streptozotocin
Figure 2. Immunoblots (A), their quantification (B), and catalytic activity (C) for CYP2E1.
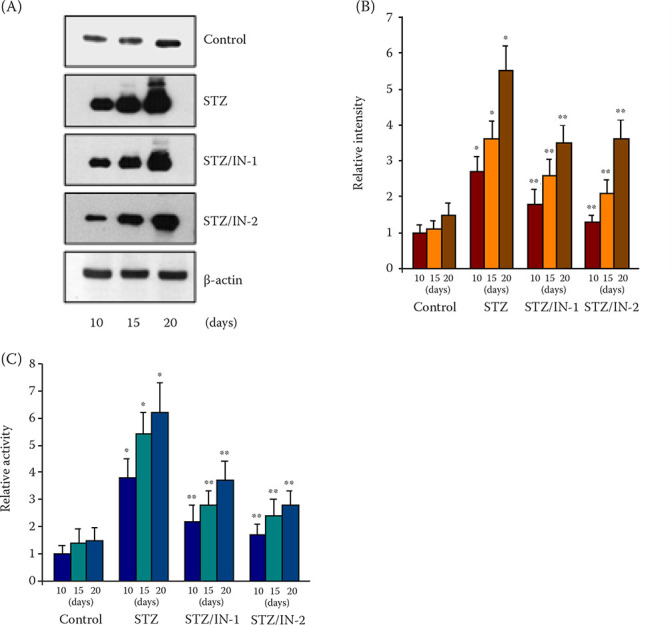
In (B), the relative band intensities of CYP2E1 were determined using ImageJ software; the control sample at 10 days was set to one (1). In (C), the y-axis represents the relative activity of CYP2E1; the level of rat microsomes 10 days after injection of the citrate buffer was set to one (1). In (A), (B), and (C), five individual samples in each group were collected and used for the experiments
*P < 0.05, **P < 0.05 indicates a comparison with values of the normal and STZ-diabetic rats, respectively
Control, STZ, STZ/IN-1, and STZ/IN-2 represent the results for the immunoblot and catalytic activity with liver microsomes of the normal, STZ-diabetic, and STZ/albumin (IN-1 and IN-2)-treated rats, respectively
STZ = streptozotocin
Figure 3. Immunoblots (A) and their quantification (B) for glutamic acid decarboxylase (GAD).
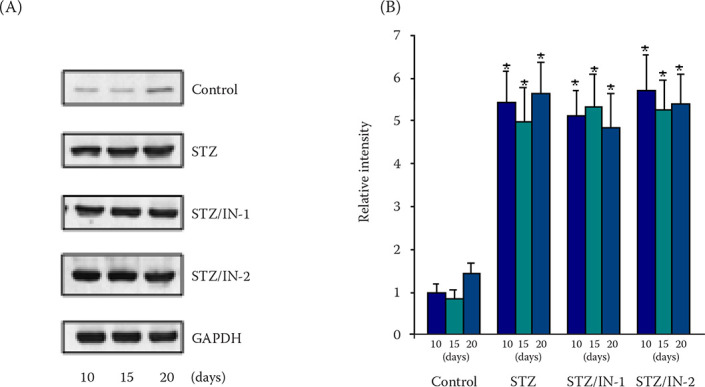
The amounts of GAD in the pancreas lysates were determined by immunoblotting using the anti-GAD 65/67 antibody. The protein bands on the immunoblots were analysed by ImageJ software and the results were expressed as the relative intensity in the y-axis; the STZ-treated sample was set to one (1)
*P < 0.05 indicates a comparison with values of normal rats
Control, STZ, STZ/IN-1, and STZ/IN-2 represent the results for the immunoblot with pancreas lysates of the normal, STZ-diabetic, and STZ/albumin (IN-1 and IN-2)-treated rats, respectively
GAPDH = glyceraldehyde 3-phosphase dehydrogenase; STZ = streptozotocin
In contrast to the changes in the observed variables in the STZ-treated rats, the co-administration of the exogenous albumin, purified from the normal rats, together with the STZ significantly reduced the elevated TG and ketones levels and the ALT and AST activities, although the molecular mechanisms for these effects are currently unclear (IN-1 in Table 1). The treatment with IN-2 further decreased these values. In addition, the albumin infusion reduced the STZ-induced production of ROS (IN-1 and IN-2 in Table 2). The albumin co-administration also restored the decreased GST, CAT, and SOD activities, including the reduced GSH level. However, the albumin-induced changes in the values shown in Tables 1 and 2 did not approach the normal ranges, and the changes in the GSH and SOD values were not significant. Furthermore, the albumin concentration-dependency was not assessed in more detail, and the albumin administration had a negligible effect on blood glucose levels in the STZ-diabetic rats, regardless of the amount of administered albumin. Despite the distinct effect that the albumin infusion had on the indices tested, there remains a possibility that certain components in the albumin fraction could have influenced the results, because the albumin was purified from the blood of normal rats. To test this possibility and confirm the effect of the external albumin administration, we repeated the experiments using commercially obtained rat albumin at the same concentrations as those for IN-1 and IN-2 and observed similar results to those of the purified samples (the values in parentheses in Tables 1 and 2).
The antioxidant activity, such as the free radical scavenging, is a key biological function of serum albumin (Roche et al. 2008) and we, therefore, measured the peroxidase activity of the albumin purified from the rat serum. Figure 1A shows that the enzyme activity of the serum albumin from the STZ-diabetic rats was significantly lower than that of the untreated normal rats. Moreover, the activity was reduced in a time-dependent manner, whereas the normal albumin retained a similar activity regardless of the sampling time. These results suggest that the oxidation and/or glycation of the albumin impaired the antioxidant activity, as reported previously (Bourdon et al. 1999). To confirm the result shown in Figure 1A, the tryptophan fluorescence and number of free thiol groups in the albumin were also analysed. Figure 1B shows that the emission intensity of the albumin purified from the diabetic rats at λmax (the emission wavelength showing the maximal fluorescence intensity) was lower than that of normal albumin at the same concentration and decreased as a function of time for the blood sampling, indicating a conformational change in the protein with time, as suggested previously (Tayeh et al. 2009).
The measurement of the free thiol groups in the protein further supported this structural modification in the albumin (Figure 1C), in which the reduced albumin has one free sulfhydryl group at Cys-34, whereas the oxidised albumin has a ligand bound to the sulfhydryl group (Hayashi et al. 2002). The serum albumin purified from the normal rats was found to have 0.83 free thiol groups per albumin molecule on average, and this value was independent of the time from blood sample collection. In contrast, the value of the STZ-diabetic albumin was 0.62 and gradually decreased with an increasing time after the diabetes occurrence. As a control, the value for free thiol groups in the commercial albumins was determined to be 0.78 while the emission intensity at λmax was very similar to the albumin purified from intact rats used in the present study (results not shown).
Enhanced oxidative stress in diabetes accompanies an increased expression of CYP2E1 (Mari and Cederbaum 2001), and CYP2E1 itself produces ROS (Lieber 1997). Figure 2A and 2B shows that the expression of CYP2E1 was markedly enhanced in the liver microsomes of the STZ-treated rats with the increased time of sampling, whereas this was independent of the time in the untreated samples and lower than that of the diabetic rats. In contrast, the administration of normal albumin (IN-1) to the diabetic rats reduced the protein expression, and the treatment with IN-2 induced a more increased suppression of CYP2E1 expression. However, the albumin infusions did not completely suppress the elevated expression. The results for the CYP2E1 expression were supported by the p-nitrophenol hydroxylase activities of CYP2E1 in the microsomes of the STZ-diabetic rats, which were higher than those of intact rats and increased as a function of the sampling time (Figure 2C). Conversely, the albumin infusion reduced the enhanced activities and IN-2 was more efficient in this attenuation than IN-1. However, the albumin treatment still showed higher CYP2E1 activities than that in the intact rats. As a control experiment, the expression and activity of CYP3A4, another member of the cytochrome P450 superfamily, were also assessed and did not display any effect after the STZ administration or the co-administration of STZ with albumin (results not shown). These results collectively suggest that the external albumin specifically plays an important role in the suppression of the CYP2E1 expression and catalytic activity.
To test the possibility that the albumin co-administration may deactivate the efficacy of STZ in inducing diabetes in rats as the albumin binds to various endogenous and exogenous ligands, an immunoblot analysis for GAD, a candidate biomarker of type 1 diabetes (Jun et al. 2002), was performed. The GAD protein band intensity did not significantly differ between the STZ treated and STZ/albumin co-administered rats, although the expression was markedly enhanced compared with that of control sample (Figure 3). Moreover, the expression levels of GAD were similar in both groups regardless of the time intervals after treatment with STZ or STZ/albumin. Based on this result and similar blood glucose levels in both samples (Table 1), the possibility of an albumin-caused decrease in the STZ effectiveness in developing diabetes could be excluded.
DISCUSSION
The present study shows that an albumin infusion could attenuate STZ-mediated liver damage, which may be attributed to the decreased levels and modification of albumin, and the resultant increase in ROS. When considered together with previous reports, the current results indicate that the functional activation of CYP2E1 in STZ-diabetic rats contributes to tissue injury. Inversely, our results also suggest that administration of normal albumin can potentially restore the detrimental effects of diabetes in the liver in rats.
The STZ treatment caused a decrease in the serum albumin level compared with that of intact rats (Table 1). Consistent with this result, a previous study reported that the albumin level decreased in the peripheral blood mononuclear cells of STZ-induced diabetic rats, which resulted in an increased production of oxidative stress in the cells (Park et al. 2014). As serum albumin is synthesised in the liver, an STZ treatment may affect the liver functions involved in the synthesis and secretion of albumin, as well as inducing liver damage; this would explain the 2.6- to 3.3-fold increase in the serum ALT and AST activities in STZ-diabetic rats. This is consistent with previous reports (Ahn et al. 2006), although the enhanced degrees of the parameters were different from the former results. As the ALT and AST activities in the serum are used as an index for liver damage, these results suggest liver injury in the STZ-diabetic rats. Moreover, the decrease in the serum albumin level supports STZ-induced liver injury. However, it has also been reported that there was no significant difference in the serum albumin level between the intact and STZ-diabetic rats (Omoruyi et al. 2013). In addition to the possibility that STZ downregulates the synthesis and secretion of albumin in the liver, an insulin deficiency can also cause decreases in these functions in diabetes (Bae et al. 2013). Moreover, low albumin levels in the serum increase the protein glycation in the plasma (Bhonsle et al. 2012), which may be responsible for the loss of the albumin function, as it has been shown to induce structural and functional changes in the albumin (Anguizola et al. 2013).
Because albumin is the most abundant protein in the serum and has antioxidant properties, an albumin malfunction may result in the ineffective attenuation of oxidative stress in cells. The elevated levels of oxidants can also lead to an increase in the oxidation of albumin. Figure 1A and 1B support the inter-relationship: the albumin in diabetic rats was more oxidised than that of the normal albumin and that oxidation may be responsible for the structural change and decreased antioxidant activity of the albumin in diabetic rats. These results also imply the that the STZ-induced changes in serum albumin were closely correlated with the severity of the diabetes. However, the total number of albumins damaged structurally and functionally by the STZ could not be deduced from the current results. Nonetheless, the STZ-mediated decrease in the albumin concentration and protein deformation can explain the elevated oxidative stress in the diabetic rats. Taken together, STZ may be considered to induce serial events in rats: a reduced albumin level in the serum, a detrimental modification of the serum albumin, and a concomitant increase in ROS.
As oxidative stress has been implicated in liver diseases and albumin infusions are widely used in liver cirrhosis (Carvalho and Verdelho Machado 2018), the current findings regarding the attenuation of an STZ-induced liver injury through albumin supplementation in diabetic rats may be closely related to these previous reports. However, the molecular mechanism(s) for the protective effect of albumin remains unknown and the therapeutic effect of albumin on liver cirrhosis is believed to be a result of its oncotic properties. In addition, the degree of STZ-induced liver injury shown in Table 1 might not be significant because 2- to 3-fold higher than normal ALT and AST activities in the serum are generally considered as reflecting a mild liver injury.
Regarding the dose-dependent effectiveness of albumin, it should be noted that the rats were probably overdosed with albumin (196 mg for IN-1 in Table 1), considering that physiological albumin concentrations in male rats are between 30 g/l to 51 g/l. Moreover, a 2-fold higher amount of albumin (IN-2) than that for IN-1 resulted in the more efficient remediation of the liver injury and elimination of the ROS. Whether the efficacy of this dose is specific to the STZ-mediated liver damage in rats is not currently known, and therefore, administering similar doses of albumin used in this study to other animal models may be difficult. A dose with half the amount of albumin in IN-1 (Table 1) had a minimal effect in alleviating the STZ-induced liver damage (results not shown). Although there is no direct relation with the present study, albumin has been administered to 70 kg-adults at doses of 105 g on day 1 and 70 g on day 3 in a study on the effect of an albumin infusion on the renal function and survival of patients with cirrhosis and spontaneous bacterial peritonitis (Sort et al. 1999).
At present, there is no direct evidence to show that oxidative stress induces the expression of CYP2E1, while a lot of reports have proved that CYP2E1 generates free radicals. Although CYP2E1-dependent oxidative stress following the induction of CYP2E1 by ethanol is one of the main causes of hepatotoxicity (Jaeschke et al. 2002), and CYP2E1 catalyses the ethanol oxidation in the liver (Osna and Donohue 2013), alcohol is also metabolised by other enzymes, such as alcohol dehydrogenase, catalase, and a combination of NADPH-oxidase/catalase, resulting in oxidative stress (Comporti et al. 2010). These reports suggest that oxidative stress itself can trigger the expression of CYP2E1 and that albumin supplementation may thus suppress the expression of CYP2E1 by reducing the STZ-mediated ROS generation.
Ketones and other small organic molecules are both substrates and inducers of CYP2E1 (Barnett et al. 1992). The production of ketones in diabetes has been suggested to result in the increased expression and catalytic activity of CYP2E1 (Chalasani et al. 2003; Abdelmegeed et al. 2005). Consistent with these reports, the present results showed that an albumin infusion led to a decrease in the levels of ketones in the blood and expression of CYP2E1. Hence, although ketones are not the sole inducers of CYP2E1 in diabetes and the molecular relationship between the albumin treatment and the ketone levels in blood is unclear, the suppression of the ketone production by the albumin infusion could be a mechanism for a reduction in the CYP2E1 expression in diabetic rats. However, conflicting results have also been reported, showing that fatty acid treatment downregulated the CYP2E1 expression at the mRNA level and enzymatic activity in human hepatocytes, but did not induce any significant change in the enzyme expression (Donato et al. 2007; Aljomah et al. 2015). Therefore, the role of ketone bodies inducing the expression of CYP2E1 remains unknown because these molecules are produced by the liver from fatty acids in type 1 diabetes.
A variety of xenobiotics and natural extracts have been suggested to show protective effects on STZ-induced liver injury, and several of these have been used as drugs for remediation of other diseases (Yanardag et al. 2005; Afrin et al. 2015). Regardless of their pharmacological effectiveness on the liver damage, these molecules may also elicit toxic and other side effects in cells. For example, although metformin is a well-known medication for the treatment of type 2 diabetes and was also suggested to have protective effects on liver injury in STZ-diabetic rats (Yanardag et al. 2005), this can also induce lactic acidosis and other deleterious symptoms (DeFronzo et al. 2016). In contrast, an albumin administration is believed to have excellent safety, and serious adverse events in albumin recipients are therefore very rare (von Hoegen and Waller 2001; Vincent et al. 2003).
Considering the overall results obtained, it can be concluded that treatment with exogenous normal albumin may reduce the oxidative stress and restore the liver injury induced by diabetes. This is also supported by the albumin-induced decrease in the ketone levels, as a high level of ketones in the blood is a typical indicator of type 1 diabetes. However, further experiments, such as histological studies, should be performed to estimate the exact liver state. In addition, there remains a possibility that the production of ROS and liver damage were induced by the STZ treatment per se. Nonetheless, the present study demonstrates that an STZ-mediated liver injury could be recovered by administration of external normal albumin, which can thus serve as an alternative remedy for liver damage induced by type 1 diabetes. Moreover, the current results may be applied to other tissue and cellular injuries, as increased oxidative stress plays an important role in the aetiology and pathogenesis of many diseases (Liguori et al. 2018). However, several issues, such as the determination of the optimal dose and application to other animals, still remain to be addressed before an external albumin administration can be used to clinically treat liver damage.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Abdelmegeed MA, Carruthers NJ, Woodcroft KJ, Kim SK, Novak RF. Acetoacetate induces CYP2E1 protein and suppresses CYP2E1 mRNA in primary cultured rat hepatocytes. J Pharmacol Exp Ther. 2005 Oct;315(1):203-13. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6. [DOI] [PubMed] [Google Scholar]
- Afrin R, Arumugam S, Soetikno V, Thandavarayan RA, Pitchaimani V, Karuppagounder V, Sreedhar R, Harima M, Suzuki H, Miyashita S, Nomoto M, Suzuki K, Watanabe K. Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats. Free Radic Res. 2015 Mar;49(3):279-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn T, Yun CH, Oh DB. Tissue-specific effect of ascorbic acid supplementation on the expression of cytochrome P450 2E1 and oxidative stress in streptozotocin-induced diabetic rats. Toxicol Lett. 2006 Sep 30;166(1):27-36. [DOI] [PubMed] [Google Scholar]
- Aljomah G, Baker SS, Liu W, Kozielski R, Oluwole J, Lupu B, Baker RD, Zhu L. Induction of CYP2E1 in non-alcoholic fatty liver diseases. Exp Mol Pathol. 2015 Dec;99(3):677-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M, Hage DS. Review: Glycation of human serum albumin. Clin Chim Acta. 2013 Oct 21;425:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JC, Seo SH, Hur KY, Kim JH, Lee MS, Lee MK, Lee WY, Rhee EJ, Oh KW. Association between serum albumin, insulin resistance, and incident diabetes in nondiabetic subjects. Endocrinol Metab (Seoul). 2013 Mar;28(1):26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CR, Petrides L, Wilson J, Flatt PR, Ioannides C. Induction of rat hepatic mixed-function oxidases by acetone and other physiological ketones: Their role in diabetes-induced changes in cytochrome P450 proteins. Xenobiotica. 1992 Dec;22(12):1441-50. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276-87. [DOI] [PubMed] [Google Scholar]
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998 Oct 1;56(7):831-9. [DOI] [PubMed] [Google Scholar]
- Bhonsle HS, Korwar AM, Kote SS, Golegaonkar SB, Chougale AD, Shaik ML, Dhande NL, Giri AP, Shelgikar KM, Boppana R, Kulkarni MJ. Low plasma albumin levels are associated with increased plasma protein glycation and HbA1c in diabetes. J Proteome Res. 2012 Feb 3;11(2):1391-6. [DOI] [PubMed] [Google Scholar]
- Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. 2001 Apr;3(2):293-311. [DOI] [PubMed] [Google Scholar]
- Bourdon E, Loreau N, Blache D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999 Feb;13(2):233-44. [DOI] [PubMed] [Google Scholar]
- Buttar HS, Chow AY, Downie RH. Glutathione alterations in rat liver after acute and subacute oral administration of paracetamol. Clin Exp Pharmacol Physiol. 1977 Jan-Feb;4(1):1-6. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004 Feb;44:27-42. [DOI] [PubMed] [Google Scholar]
- Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol. 2018 Jul-Aug;17(4):547-60. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003 Mar;37(3):544-50. [DOI] [PubMed] [Google Scholar]
- Cha MK, Kim IH. Glutathione-linked thiol peroxidase activity of human serum albumin: A possible antioxidant role of serum albumin in blood plasma. Biochem Biophys Res Commun. 1996 May 15;222(2):619-25. Erratum in: Biochem Biophys Res Commun 1996 Aug 14;225(2): 695. [DOI] [PubMed] [Google Scholar]
- Chen J, Jiang S, Wang J, Renukuntla J, Sirimulla S, Chen J. A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab Rev. 2019 May;51(2):178-95. [DOI] [PubMed] [Google Scholar]
- Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B. Ethanol-induced oxidative stress: Basic knowledge. Genes Nutr. 2010 Jun;5(2):101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016 Feb;65(2):20-9. [DOI] [PubMed] [Google Scholar]
- Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: Protection by allopurinol. Diabetes. 2002 Apr;51(4):1118-24. [DOI] [PubMed] [Google Scholar]
- Donato MT, Jimenez N, Serralta A, Mir J, Castell JV, Gomez-Lechon MJ. Effects of steatosis on drug-metabolizing capability of primary human hepatocytes. Toxicol In Vitro. 2007 Mar;21(2):271-6. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168-79. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130-9. [PubMed] [Google Scholar]
- Hayashi T, Suda K, Imai H, Era S. Simple and sensitive high-performance liquid chromatographic method for the investigation of dynamic changes in the redox state of rat serum albumin. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 May 25;772(1):139-46. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002 Feb;65(2):166-76. [DOI] [PubMed] [Google Scholar]
- Jun HS, Khil LY, Yoon JW. Role of glutamic acid decarboxylase in the pathogenesis of type 1 diabetes. Cell Mol Life Sci. 2002 Nov;59(11):1892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci (Lond). 1998 Jun;94(6):623-32. [DOI] [PubMed] [Google Scholar]
- Kamath SA, Kummerow FA, Narayan KA. A simple procedure for the isolation of rat liver microsomes. FEBS Lett. 1971 Sep 15;17(1):90-2. [DOI] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013 Feb;58(2):395-8. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: Its physiological and pathological role. Physiol Rev. 1997 Apr 1;77(2):517-44. [DOI] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018 Apr 26;13:757-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997 Sep;46(Suppl_2):S38-42. [DOI] [PubMed] [Google Scholar]
- Mari M, Cederbaum AI. Induction of catalase, alpha, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology. 2001 Mar;33(3):652-61. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Guest MJ, Foster DW. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382-90. [PubMed] [Google Scholar]
- Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000 Oct;85(4):599-610. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000 Dec;33(6):893-901. [DOI] [PubMed] [Google Scholar]
- Omoruyi FO, Budiaman A, Eng Y, Olumese FE, Hoesel JL, Ejilemele A, Okorodudu AO. The potential benefits and adverse effects of phytic acid supplement in streptozotocin-induced diabetic rats. Adv Pharmacol Sci. 2013;2013:172494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Donohue TM Jr. CYP2E1-catalyzed alcohol metabolism: Role of oxidant generation in interferon signaling, antigen presentation and autophagy. Subcell Biochem. 2013;67:177-97. [DOI] [PubMed] [Google Scholar]
- Park KT, Yun CH, Bae CS, Ahn T. Decreased level of albumin in peripheral blood mononuclear cells of streptozotocin-induced diabetic rats. J Vet Med Sci. 2014 Aug;76(8):1087-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoufinia R, Mota A, Keyhanvar N, Safari F, Shamekhi S, Abdolalizadeh J. Overview of albumin and its purification methods. Adv Pharm Bull. 2016 Dec;6(4):495-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: Tissue-specific variations and roles in oxidative stress. Diabetes. 2004 Jan;53(1):185-94. [DOI] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985 Sep-Oct;13(5):548-52. [PubMed] [Google Scholar]
- Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008 Jun 11;582(13):1783-7. [DOI] [PubMed] [Google Scholar]
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Gines P, Rodes J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999 Aug 5;341(6):403-9. [DOI] [PubMed] [Google Scholar]
- Surbrook SE Jr, Olson MJ. Dominant role of cytochrome P-450 2E1 in human hepatic microsomal oxidation of the CFC-substitute 1,1,1,2-tetrafluoroethane. Drug Metab Dispos. 1992 Jul-Aug;20(4):518-24. [PubMed] [Google Scholar]
- Tayeh N, Rungassamy T, Albani JR. Fluorescence spectral resolution of tryptophan residues in bovine and human serum albumins. J Pharm Biomed Anal. 2009 Sep 8;50(2):107-16. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Wilkes MM, Navickis RJ. Safety of human albumin – Serious adverse events reported worldwide in 1998–2000. Br J Anaesth. 2003 Nov;91(5):625-30. [DOI] [PubMed] [Google Scholar]
- von Hoegen I, Waller C. Safety of human albumin based on spontaneously reported serious adverse events. Crit Care Med. 2001 May;29(5):994-6. [DOI] [PubMed] [Google Scholar]
- Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998 Jan;27(1):128-33. [DOI] [PubMed] [Google Scholar]
- Yanardag R, Ozsoy-Sacan O, Bolkent S, Orak H, Karabulut-Bulan O. Protective effects of metformin treatment on the liver injury of streptozotocin-diabetic rats. Hum Exp Toxicol. 2005 Mar;24(3):129-35. [DOI] [PubMed] [Google Scholar]


