Abstract
Human health is intimately connected and tied to the health of our environment and ecosystem, with only a very small fraction of the risk for chronic diseases explained by genetics alone. Companion animals are prone to disease types that are shared with people, including cancers and endocrine disorders, reinforcing the thought that environmental factors contribute to the risks for chronic diseases. These factors include air and water pollution and the built environment. As such, there is increasing interest in pursuing research with companion animals, and specifically dogs, as sentinel species to inform comparative health assessments and identify risk factors for disease. Of the canine diseases for which environmental exposure research has been published, cancers have received the most attention. This review summarizes two main aspects of this comparative approach: (1) cancers that occur in dogs and which are similar to humans and (2) research investigating environmental exposures and health outcomes in dogs. The goal of this review is to highlight the diverse conditions in which pet dogs may provide unique perspectives and advantages to examine relationships between environmental exposures and health outcomes, with an emphasis on chemical pollution and cancer. Furthermore, this review seeks to raise awareness and stimulate discussion around the best practices for the use of companion animals as environmental health sentinels.
Keywords: sentinels, dogs, environmental health, comparative oncology, cancer, alternative models, environmental exposure
Introduction
One Health—One Medicine—One Pathogenesis—One Environmental Health
The concept of One Health has evolved and developed from the philosophies and contributions of people such as Hippocrates, Rudolph Virchow, William Osler, and Calvin Schwabe, who collectively recognized the interconnectedness of health for humans, animals, and the environment. The term “One Health” was defined by the One Health Initiative Task Force in 2008 as the collaborative transdisciplinary effort to attain optimal health for people, animals, and the environment at the local, national, and global level.1,2 Much of the current literature supporting the One Health concept is focused solely on infectious diseases.3 This is likely due to the emergence of zoonotic diseases, wherein the majority of novel infectious diseases in humans have originated in animals, fostered by globalization that connects people across the world.4 Today, after more than a decade of One Health research, gaps remain in our understanding of the multifactorial and noncommunicable chronic diseases that are the leading cause of mortality in humans,1 as well as our understanding of the role of exposure to chemical contaminants on health. Deaths associated with modern pollution (air pollution and toxic chemical exposure) have risen 66% since 2000, and pollution now accounts for over 9 million premature human deaths.5
Historically, much of the research on chronic diseases in humans and the role of genetics in disease etiology has evolved using laboratory models, primarily rodents. Rodent models continue to offer valuable tools and mechanistic insight through the development of transgenic or knockout animals6 or xenograft techniques;7 however, the translational relevance to humans can be complicated. Extrapolation of data from traditional laboratory animal models of disease to human populations can be challenging due to the extent of animal inbreeding and the lack of replicability to complex human gene–environment interactions. While laboratory animal models are important in understanding biochemical and physiological pathogenesis, they may not offer the best representation of naturally occurring disease development in humans and particularly the relationship between real world-environmental exposures and disease.
With a high degree of conservation of biological mechanisms across evolution, it is unsurprising that there would be conserved mechanisms associated with disease processes across species. The domestic dog (Canis familiariz) represents a relatively outbred species with an intact immune system that naturally develops hundreds of diseases (including cancers)8 shared with humans, including those influenced by factors such as sex, age, reproductive history, nutrition, and environmental exposures. In addition, when considering purebred dogs, the breed itself is also considered a predisposing factor for numerous diseases. The human genome is more similar to the canine genome than to the mouse genome,9 and as such, the development of accessible canine specific genomic tools provides support for comparative approaches to be used in assessing pathogenic variables at genomic and molecular levels.
A major challenge associated with conducting contaminant exposure related studies in laboratory animals is the ability to replicate the complexity of real-world exposure to chemical mixtures and the interplay between environmental exposures and natural disease processes. In 2005, a cancer researcher named Christopher Wild recognized this challenge in characterizing environmental influences on chronic disease and urged more attention to this issue. He coined the term “exposome” in his seminal paper, which is defined as the totality of environmental exposures over a lifetimes comprising chemical, physical, biological, and social influences on health.10 Chemicals are an inevitable component of our natural and built environment. In discussing this point, it is important to clarify the difference in the word toxins and toxicants that are often associated with this concept. Toxins are chemicals produced by living organisms (i.e., tetrodotoxin in pufferfish), while toxicants are the chemicals produced intentionally or as a byproduct of anthropogenic activities. Both have the potential to negatively impact human, animal, and environmental health; however, in this review, we focus on chemicals that are toxicants.
All humans and companion animals are exposed to toxicants through inhalation, ingestion, and contact from air, water, food, or other environmental media in their habitat. The acute and chronic effects of these toxicants to each species will vary; some species are likely more vulnerable or sensitive to these effects, developing diseases or conditions sooner than other species and thereby acting as a sentinel system. Assessment of human health effects due to exposure to environmental contaminants, particularly chronic exposures, can be ascertained through sentinel systems in order to determine exposure and hazard.11 The use of companion species as sentinels can provide unique insights into shared environmental exposures that increase risks for certain diseases in humans.12−14 Animals have varying responses to environmental exposures but may function as an effective early indicator of human health effects (i.e., decreased latency periods) or have a health effect at a lower threshold (i.e., increased susceptibility).11,12 It is unlikely that an animal sentinel will exhibit identical response to every exposure as humans do; however, understanding the properties and mechanisms of exposure in nonlaboratory based biological systems can greatly enhance our approaches to treatments, mitigation, and prevention.
For an animal to be considered a sentinel for human health, they could be equal, or more susceptible, or have greater exposure or risk. The important factors to consider an animal as a sentinel species, as defined by the National Research Council, are (1) have a measurable effect, which includes tissue accumulation, (2) have an overlapping range of the area of interest (i.e., shared environmental exposure), and (3) sufficient population and ability to collect appropriate amount of data.11 Pet dogs meet all these criteria and, as such, are considered an excellent sentinel species.
The domestic dog is a relevant model for human health due to the strong synteny between their genomes and their shared environment. As household occupants, our dogs live in the same home, drink the same water, breathe the same indoor and outdoor air, and sometimes eat the same food. In addition, pet dogs are often affected by the socioeconomic status of their owners; for example, food quality, housing conditions, veterinary care, and proximity to point sources of environmental contamination (i.e., landfills, Superfund sites). However, compared to humans, dog have shorter lifespans (typically 1/8th), a more consistent daily routine, and fewer confounding variables such as alcohol consumption, smoking, or job-related exposures (with some exceptions i.e., farming and scent detection dogs). So, in some ways domestic dogs may be better research models than human subjects. Two studies using a cohort of paired owners and dogs demonstrated similar shared exposures in the home environment using noninvasive passive .108,109 In addition, there were greater correlations with the internal dose in dogs compared to humans (likely because they have fewer of these confounding variables). As such, the dog serves as a valuable comparative model for the unique environmental exposure scenarios that need to be further evaluated for disease risk and relevant cancer prevalence. There is a unique opportunity with dogs to combine “omics” studies to establish a more complete understanding of the interplay between genomics, exposomics, etc., and disease etiology.
Review Criteria
This review provides an overview of the progress that has been made in the use of companion dogs as sentinels for environmental exposures and their associated health outcomes. Cancer end points are the major focus of this review because thus far the most progress has been made in this area. Within cancer as a whole, the focus is on cancers where there has been progress demonstrating shared features in diseases with evidence of environmental exposure or potential for shared environmental exposures. A potential limitation of these studies is that many have largely focused solely on survey data, with few reporting objective measurements of exposure. There is a paucity of data regarding objective measurements of exposures and health outcomes using comparative approaches. Studies were excluded that used traditional laboratory colony dogs for experiments or other intentionally dosed animals, except to further amplify the weight of evidence for specific areas of research. Finally, this review concludes with perspectives and recommendations to improve the field.
Comparative Oncology
The study of analogous cancers in companion dogs (i.e., comparative oncology) has led to advancements in clinical and basic science research for several cancers. Clinical trials for novel therapeutics can be completed in a much shorter frame with canine patients than with people. Further, the lack of gold standard treatments in veterinary medicine for many cancers allows for early/investigational and humane studies to be conducted, whereas in human trials, novel therapeutics are often only used following failure of standard of care approaches. For many species and dogs in particular, comparative oncology research has heavily focused on shared genomics, pathobiological features, and therapeutics. A recent review article summarizes these shared genomic features15 and will not be discussed here.
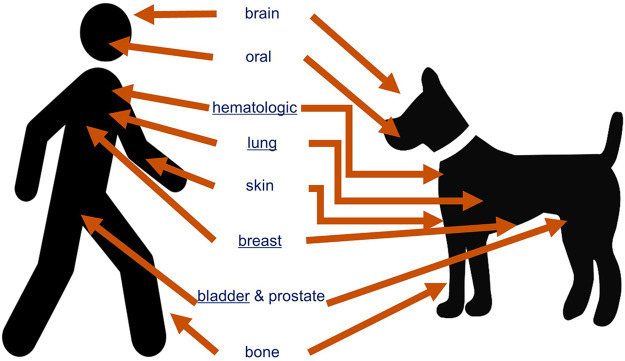
It is estimated that six millions dogs per year are diagnosed with cancer in the United States.16 Many cancers that present as spontaneous disease in pet dogs share similar features with their human equivalents, including morphological appearance, molecular and genetic components, and biological and clinical behaviors.16−21Figure 1 illustrates the corresponding cancers present in humans wherein the dog has been identified as an appropriate comparative model.15,20,22−31
Figure 1.
Cancers highlighted here have been studied using a comparative approach and display similarities among clinical, pathological, histological, genomic, chromosomal, and molecular features. Cancers underlined have explored for shared environmental risk factors. Figure adapted and updated from Schiffman and Breen, 2015.15,20,22−31
Overall, cancers occur at similar life stages in dogs and people, generally being an age associated disease.32 However, one advantage to studying canines as a model for human health is their shorter life span, which translates to a shorter disease latency period compared to humans and a shorter time course for disease progression. In addition, canines share similarities in lifestyles with their owners, such as the indoor environment and even aspects of their diet (drinking water and some foods). Taken together, these similarities provide an opportunity to leverage data derived from our pets as indicators of our health.
True incidence rates for canine cancers are difficult to accurately determine due to the limited screening approaches, variable access to veterinary care, and lack of a comprehensive cancer registry. Another challenge in veterinary medicine is the lack of confirmation of cancer type based on a gold standard histopathological diagnosis. The few canine cancer registries that do exist are restricted to canine populations within a limited geographic range, limited breed inclusion, or limited cancer types.16,33,34
Most of the published studies of environmental risk factors for canine cancers focus on bladder cancer, mammary cancer, and lymphoma (Table 1). Most of those studies used questionnaires or geospatial data to identify potential associations with environmental exposures.
Table 1. Environmental Exposure and Cancer in Companion Animals.
| Cancer Category | Exposure Investigated | Observation Years | Method of Chemical Assessment | Reference |
|---|---|---|---|---|
| Bladder | Trihalomethanes | 1996–1998 | Questionnaire, drinking water utility company data | (47) |
| Obesity and pesticides | 1982–1985 | Interview | (40) | |
| Pesticides - herbicides, insecticides, fungicides, algicides, acaricides, molluscicides | 1995–2003 | Questionnaire | (75) | |
| Industrial activity | 1969–1978 | Observational | (42) | |
| Pesticides, Passive tobacco smoke, household exposures | 2013–2017 | Questionnaire and GST-theta variant genotyping | (41) | |
| Topical flea and tick pesticides | 1995–2003 | Questionnaire | (76) | |
| Vegetable | 1995–2003 | Questionnaire | (77) | |
| Trihalomethanes, arsenic, nitrate, ozone, PM2.5, airborne arsenic, acrylonitrile, benzene, chromium VI, and cadmium | 2013–2017 | GIS mapping residence linked to EWG tap water database, county level EPA air pollution data, EPA NATA outdoor air quality data | (43) | |
| Passive tobacco smoke | Not reported | Questionnaire, urinary cotinine | (39) | |
| Lymphoma | Various environmental factors | 2015–2017 | Questionnaire, GIS mapping | (58) |
| Living in industrial areas, household chemicals - pesticides, paints, solvents | 1996–1998 | Questionnaire | (61) | |
| 2.4-Dichlorophenoxyacetic acid | 1984–1988 | Questionnaire/Interview | (68) | |
| Waste incinerators, polluted sites, and radioactive waste | 2000–2001 | Geospatial analysis with waste incinerators, polluted sites, and radioactive waste | (57) | |
| Passive tobacco smoke, indoor pollution, pesticides, cleaning products | Not reported | Questionnaire and immunocytochemical staining of CD3, PAX5, Ki-67 | (67) | |
| Geospatial analysis with human non-Hodgkin’s lymphoma during the same time period | 2005–2016 | Geospatial distribution at the municipality level of age standardized risk | (56) | |
| Magnetic fields | 1987–1990 | Wire codes and magnetic fields were measured at the homes of dogs, interview | (78) | |
| Radon, pesticides | 2013 | Spatial analysis using Postcode-district level and external sources for environmental exposure data | (55) | |
| Trihalomethanes, arsenic, nitrate, ozone, PM2.5, 1,3-butadiene, benzene, chromium VI, cadmium, carbon tetrachloride, ethylene oxide, formaldehyde, tetrachloroethylene, and polycyclic aromatic hydrocarbons | 2015–2017 | GIS mapping residence linked to EWG tap water database, county level EPA air pollution data, EPA NATA outdoor air quality data | (43) | |
| Flea and tick products, lawn care chemicals, household chemicals | 2006–2007 | Questionnaire | (69) | |
| Various environmental factors | 2010 | Interview | (59) | |
| Living near illegal waste dumping sites | 2003–2008 | Odds ratios between high- and low-danger areas, Questionnaires | (63) | |
| Cumulative exposure burden | 2012–2015 | Geospatial proximity to pollutant sources, county level EPA air pollution data, | (62) | |
| Mammary | Pyrethroids - allethrin, cyhalothrin, cypermethrin, deltamethrin and tetramethrin | Not reported | HPLC measurement of adjacent adipose tissue | (50) |
| Heavy metal and mineral elements | Not reported | ICP-MS - hair samples | (79) | |
| Mycotoxins - aflatoxins, fumonisin, zearalenone | 2010–2012 | HPLC measurement of mycotoxins in animal feed, questionnaire | (80) | |
| Pesticides - γ-HCH, α-HCH, dieldrin, aldrin, heptachlor, butachlor, p,p-DDT, o,p-DDT, p,p-DDD, p,p-DDE, L-cyhalothrin, permethrin, fipronil, and fenitrothion | 2011–2013 | GC-ECD measurement serum, mammary tissue and mammary adipose, questionnaire | (51) | |
| POPs - dioxins, dioxin-like, nondioxin-like polychlorobiphenyls, organochlorine pesticides, brominated flame retardants, perfluorinated alkylated substances | 2012–2013 | GC-HRMS and LC-MS/MS analysis of adipose (n = 115) and/or serum n = 65 (perfluorinated chemicals) | (81) | |
| Respiratory/Nasal/Oral | Black dust matter (anthracosis) | 1980–2005 | Pathology - H&E staining | (82) |
| Asbestos | 1977–1981 | Interview and 3 dogs’ lung tissue analyzed with electron microprobe for fiber content compared to 6 controls | (73) | |
| Asbestos | 1960–1980 | Pathology - Electron microscopy analysis of lung tissues compared to 8 controls for the presence of ferruginous body content | (83) | |
| Urban versus rural | 1953–1968 | Observational from medical records | (74) | |
| Passive smoking | 1985–1987 | Questionnaire | (84) | |
| Lead paint, asbestos, mold, cigarette smoke, aerosols, pesticides, incense/candles, fertilizers, wood shavings | 2002–2012 | Questionnaire | (85) | |
| Indoor air pollution – environmental tobacco smoke, indoor chemical solvents, coal/kerosene heating | 1989–1993 | Questionnaire | (86) | |
| Environmental tobacco smoke | 1986–1990 | Interview | (87) | |
| Liver | Trace elements | 2012–2018 | ICP-MS deparaffinized tissues | (70) |
| Testis | Vietnam military service – Agent Orange/herbicides | 1968–1973 | Medical and service records | (72) |
| Vietnam military service– Agent Orange/herbicides | 1968–1973 | Medical and service records | (71) |
Of the 37 publications that attempted to integrate environmental exposure data and cancer outcomes in dogs, only seven made objective measurements from dogs diagnosed with cancer, and all were either mammary cancer or mesothelioma. Below is a summary of the current literature investigating environmental exposure and specific cancer types in pet dogs.
Bladder
Canine bladder cancer is considered a good model for high-risk muscle invasive bladder cancer in humans.23 Urothelial carcinoma (previously termed transitional cell carcinoma or TCC) accounts for most bladder cancer in both species. Human bladder cancer is one of the top ten most common cancer sites in humans with an estimated 82 thousand cases of diagnosed in people in the United States each year,35 while urothelial carcinoma in the dog is the most common malignancy in the urogenital tract with greater than 60,000 diagnoses in the United States each year.23 In addition to shared cytogenetic and genomic aberrations, canine urothelial carcinoma exhibits the same luminal and basal transcriptional subtypes as those seen in human urothelial carcinoma. Among other molecular targets, overexpression of EGFR is seen in greater than 70% of urothelial carcinomas in both species.15 A homologous mutation in exon 15 of the BRAF gene that is highly prevalent in canine urothelial carcinoma, although uncommon in most human bladder cancers, has recently been reported to have a higher incidence in humans with higher-risk tumors (i.e., larger masses and/or metastasis).19 A cohort of humans with high-risk urothelial carcinoma, defined as a rate of 55% with a 2-year metastasis, had BRAF mutations on 25% of cases.36 A recent study found that dogs with urothelial carcinoma that are BRAF V595E undetected have other alterations within the MAPK pathway, including short in-frame deletions within BRAF exon 12 and MAPK1 exons 2 and 3.37
Although smoking is one of the principal risk factors for bladder cancer in humans,38 pet dogs do not inhale tobacco smoke directly but can be exposed to passive tobacco smoke. A recent study reported Scottish Terriers with bladder cancer had greater odds of living in a home with a cigarette smoker (OR 6.34, p < 0.05).39 For all homes with cigarette smokers present, dogs with bladder cancer were exposed to significantly greater amounts of cigarettes (determined by quantity per day and years of exposure) compared to dogs with no bladder cancer.39 Dogs with quantifiable urinary cotinine concentrations had significantly higher incidence of bladder cancer compared to dogs without quantifiable urinary cotinine concentrations (p < 0.05), however they found no correlations between reported passive tobacco smoke exposure and urinary cotinine levels most likely due to reported smoking cessation.39 Two other previous studies found no evidence that passive tobacco smoke exposure is associated with canine bladder cancer based on questionnaire data.40,41 Other possible risk factors include air pollution. One study from the 1980s showed that human and canine cancers share similar geographic distribution, and reported a significant positive correlation between proportional morbidity ratios for bladder cancer in dogs and the overall level of industrial activity in the host county of the corresponding veterinary hospital.42 No specific pollutants were described in that study. A more recent study found that dogs with bladder cancer lived in areas with higher ambient ozone levels compared to controls.43 Although ozone is not a direct anthropogenic pollutant, it is influenced by solar radiation and the presence of airborne anthropogenic pollutants, such as volatile organic compounds, and is considered a marker for poor air quality. These data are consistent with human studies that have shown urban living environments and air pollution are associated with increased risk of bladder cancer incidence and mortality.44
Pesticides have also been implicated in the development of canine bladder cancer. In the 1980s, based on interviews with pet owners, and collection of data via surveys, significant associations between pesticide use and canine bladder cancer were observed.40 Several studies have shown relationships between owner reported use or known pesticide exposures and proximity to potential point sources (i.e., farms and marshes). A link between occupational exposure to various pesticides and bladder cancer has also been reported in several studies, such as exposure to aromatic amine pesticides and chlorinated pesticides.45,46
Exposure to disinfection byproducts (DBPs), chemicals produced during drinking water treatment, are also being investigated as a potential risk factor for human bladder cancer. Only two contradictory studies are available that assess associations between DBPs in drinking water, based on estimated exposure to total trihalomethanes (a major class of DBPs), and canine bladder cancer. Using reports of DBP levels in water from municipal records, Backer et al.47 found no statistically significant differences in potential DBP exposure in bladder cancer cases vs controls. In contrast, Smith et al.43 reported 3-fold higher levels of DBPs in the drinking water of canine bladder cancer cases compared to controls. However, these data were based on county level total trihalomethane levels reported by the drinking water utility during an annual water test and so do not necessarily reflect levels of exposure experienced by the dogs themselves. Levels of DBPs can change throughout the year, and point of use water filtration within homes (e.g., kitchen sink water filters) may eliminate or reduce DBPs and therefore exposure. Differences could also be attributable to the different study designs. Backer et al.47 matched cases and controls by age and sex, and their assessment of DBP exposure was based on historical water reports for each dog that was based on their residential history over approximately eight years or a five-year period ending two years prior to diagnosis. Smith et al. matched age and sex but also accounted for breed and spay/neuter status. In their study, however, they only estimated a snapshot of DBP exposure based on the most recent county level water utility data.43 More research is needed to understand the role of DBP exposure in the incidence and severity.
Mammary/Breast
Similar to breast cancer in women, mammary neoplasia in dogs is the most prevalent cancer in females and has been shown to present similarly, follow similar disease course, and exhibit molecular similarities.48 The prevalence, genomic, and clinical similarities of breast and mammary cancer have been summarized in a recent review article.22 This review highlights both germline and somatic mutations in genes including TP53, BRCA1/2, and PTEN, all of which have been implicated in human mammary carcinoma.22 Additionally, estrogen exposure is an important factor in both species; dogs that undergo ovariohysterectomy procedures earlier in life have lower risk of mammary cancer.22 Some geographic differences in mammary neoplasia in dogs can be attributed to regional differences in the practice of ovariohysterectomy, which is chosen for the majority of dogs living in the US but is much less common in dogs living in Europe.49
Contrary to most other cancers explored here, environmental exposures studied in pet dogs with mammary tumors have all objectively measured contaminant levels in biological tissues. A small study of nine dogs detected pyrethroids in adipose tissue adjacent to malignant mammary tumors in the three dogs with the most aggressive tumor types.50 Another study used a case-control study design and measured 14 pesticides in biological tissues.51 This study, based in India, included 36 cases of malignant mammary cancer and six tumor-free female dogs with critical conditions undergoing euthanasia. The authors collected blood samples prior to any sedation or euthanasia and collected mammary and adipose tissues during surgery (cases) or immediately following euthanasia (controls). Although the results of this study were not statistically significant, the authors reported higher odds of total pesticide concentration (n = 14 pesticides quantified) in mammary tissues from dogs with malignant mammary cancer (β = 4.99). A major limitation of this study was the small control sample size due to the use of critical care terminal dogs and the logistics of owners being willing and able to provide consent for participation.
Lymphoma
Lymphomas are a group of cancers that arise from lymphocytes, and although the cancer can affect any organ, most cases arise in immune system organs, most commonly in the lymph nodes. Canine lymphomas are similar to human non-Hodgkin lymphoma (NHL). The most frequent subtype in both species is diffuse large-cell lymphoma (DLBCL). Subtype in dogs is often breed associated, for example, boxers are particularly predisposed to T-cell lymphoma and B-cell is more common in Rottweilers and Dobermanns.52,53 Studies have shown similar genomic aberrations in these cancers between species, specifically in alterations in the NF-κB pathway and the MYC gene. Standard therapeutic protocols, using CHOP-based chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone), are considered standard of care in both species and there have been major success in clinical trials for treatment in dogs using immunotherapeutic approaches.54 Geographical distributions of lymphoma and specific subtypes of lymphoma in humans and dogs, suggest there may be shared environmental risk factors.53,55−58
Several of the published canine lymphoma studies that use different approaches and specifically target unique chemicals share one common denominator: chemicals that contribute to air pollution. A study in Brazil found the greatest association with canine lymphomas was with dogs that were permanently kept outdoors in close proximity to high traffic areas (Odds Ratio, OR 3.1, p < 0.01).59 Although vehicle emissions are complex mixtures, benzene is a major component of vehicle pollution, and recently reported as a key pollutant associated with the risk of human NHL.60 A study in Italy found that dogs with lymphoma were more likely to reside in industrial areas (OR 8.5, 95% confidence interval (CI) 2.3–30.9) or have owners that use chemicals in the home, specifically paints, solvents fuels, and oils (OR 5.5, CI 2.0–15.0).61
Three studies focused exclusively on a single breed when examining exposures associated with lymphoma, two using boxers and one using golden retrievers.43,58,62 Two used geospatial data to assess proximity to potential point sources of environmental exposures, including manufacturer, chemical plants or suppliers, incinerators, crematoriums, bus depots, landfills, farms, golf courses, nuclear power plants, coal plants, or mines. Boxers with lymphoma were found to be at increased odds if they lived within two miles of a chemical industry manufacturer or supplier (OR 2.28, p < 0.05) or a crematorium (OR 2.17, p < 0.05), or with ten miles of a nuclear power plant (OR 5.76, p < 0.01).58 In another study, using this same boxer population, dogs with lymphoma were found to have greater average ground level ozone concentrations (OR 2.66, p < 0.05) based on EPA county level data. The National Air Toxics Assessment (NATA) database additionally suggests that those counties have higher exposure risk for 1,3-butadiene and formaldehyde, which can be found in emissions from vehicle exhaust and pollution from chemical industries and incineration.43 However, these same results could not be replicated in a case-control study using golden retrievers, which had an even distribution of dogs with B- and T-cell lymphoma, from the Golden Retriever Lifetime Study (GRLS).62 Within a population of golden retrievers, it was found that although no individual point source was significant, dogs with lymphoma had greater odds of living in close proximity to three or more pollution sources (OR 2.6, p = 0.053). Authors suggested there could be breed differences or potentially differences in dogs with B- versus T-cell subtypes, as they noted nonsignificant differences in exposure patterns between dogs with the different subtypes.62
Similar to what has been reported in the human literature, increased rates of cancer have been reported in canine residents living in close proximity to sites where illegal dumping and incineration of waste occurs.63 In this study, Marconato et al.63 attributed an increased risk in developing canine cancer to high-risk areas in Italy (areas with poor waste management practices) for all tumor types combined (OR 1.55, p < 0.05) and specifically to increased rates of lymphoma (OR 2.39, p < 0.01). However, no increased risk of mast cell tumors or mammary cancer was observed in dogs residing in these areas. The authors suggested that dioxins generated by the incineration of waste might be a contributing chemical exposure, on the basis that another study in the region linked waste incineration to dioxin in livestock milk. Dioxins are highly toxic, persistent organic pollutants and known carcinogens, specifically associated with NHL in humans exposed occupationally or accidentally.60 A study of humans found increased odds of NHL in people with higher serum concentrations of dioxins, furans and PCBs, which was also associated with proximity to waste incineration.64,65 Although dioxin is certainly a potential contributing factor, waste incineration releases many chemicals into the environment, which may have an additive or synergistic effect on lymphoma risk in people and dogs.
Tobacco smoke is another factor that may contribute to cancer incidence. Dogs may be exposed to passive tobacco smoke through inhalation or oral ingestion of chemicals deposited on their fur during grooming. Although there are mixed findings regarding the potential link between active tobacco smoking and the follicular subtype of NHL in humans, there is also evidence suggesting individuals with no history of smoking but exposure to passive tobacco smoke have an elevated risk of follicular NHL.66 The latter would likely be a better comparison to the exposure scenario in pet dogs. Marconato et al. also reported an increased odds for canine lymphoma with exposure to passive tobacco smoke (OR 3.37, p < 0.01).63 A small study of 19 dogs with lymphoma observed a significant association between number of smokers in the home and cellular proliferation of fine needle aspiration biopsies from lymph nodes, determined via immunohistochemistry for Ki-67 expression.67 Combined, these studies suggest that passive exposure to tobacco smoke in nonsmokers may be a risk factor for lymphoma.
Another factor that may contribute to human and canine lymphoma is pesticide exposures. In a UK based study, canine lymphoma showed highly variable geographic distributions with some mild clustering near London and the southwest of England which they reported as similar to the distribution of NHL in men.55 Using pesticide data at the census ward level herbicides and fungicides levels were shown to have a weak association with canine lymphoma cases, and after adjusting for breed and age, associations with moderate herbicide exposure (135–754 kg usage per census ward) and lymphoma diagnosis (OR 1.55, p < 0.05) were evident. A questionnaire-based study found that yearly number of owner applied lawn applications of 2,4-D with either short- or long-term use was associated with canine lymphoma (OR 1.9, p < 0.05).68 Another study reported that some lawn care chemicals may increase dogs’ risk for lymphoma for professionally applied pesticides (OR 1.7, p < 0.05) or personal application of insect growth regulators (OR 2.7, p < 0.05).69 However, no association with the personal application of other pesticides or herbicides was reported. In contrast, Gavazza et al.61 found no association between canine lymphoma and pesticides exposure, but they attributed their lack of findings to absence of participant knowledge regarding the specific types of products used and thus they could only reasonably assess the broad effect of any and all pesticide applications.61
Other Cancers
There are limited studies that investigated the role of environmental exposure and various other neoplasia in pet dogs. As documented in humans, imbalances in hepatic trace mineral concentrations have been reported in a study of canine hepatocellular carcinoma (HCC).70 Specifically, archived liver tissues in dogs with hepatocellular carcinoma had significantly higher levels of copper and significantly lower levels of zinc compared to a control population (n = 170; p < 0.03). While copper and zinc are considered essential metals, excess or deficient concentrations of either can be a cause or a consequence of disease. Although there are many explanations that could account for these imbalances, some possibilities may be abnormal mineral transport proteins, abnormal cellular metabolism in neoplastic liver tissues, impaired cholestasis, or differential patterns of exposure to metals from diet or the environment. Further studies are required to assess potential relationships between HCC and trace metals. Another study investigated testicular tumors and environmental exposures in working dogs and soldiers. Although no definitive associations have been made, it has been postulated that environmental exposures associated with the Vietnam War, such as herbicides (e.g., Agent Orange which contained chlorinated dioxins), may have contributed to increased incidence of testicular tumors.71,72
In contrast to humans, primary cancers in the respiratory tract are uncommon in pet dogs, and little is known about risk factors for dogs. It is possible that the low incidence of lung cancers in the canine population is a reflection of a broad study design that included all types of primary cancers of the lung. Focusing on specific types of cancer may improve the findings. For example, mesothelioma has been linked to asbestos exposure in humans and dogs.73 Some of the earliest reports of environmental risk factors for canine respiratory tract cancers found that dogs living in urban environments compared to rural had higher prevalence of tonsillar carcinoma compared to gastrointestinal cancers (χ2 = 10.2, p < 0.01) and compared to the total hospital population (χ2 = 3.8, p < 0.05).74
One of the biggest challenges that may affect the mixed findings in these questionnaire-based studies is exposure misclassification. For example, owner reported use of pesticides is subject to both recall bias and the owner’s potentially limited knowledge of pesticide classifications or use of specific products. There is tremendous variability in the amount of product, duration, and frequency of use, and the extent of access the dog has to treated areas. Furthermore, many of the case control studies used controls that had either other forms of cancer or chronic diseases and may or may not have matched controls based on varying factors such as age, sex, and breed.
Other Chronic Diseases—Emerging Areas
Kidney Disease
While cancers are the most well studied, the value of companion animals as a model of environmental health extends to many other areas. For example, higher concentrations of chromium and cadmium have been reported in liver tissue of dogs with chronic kidney disease compared to dogs with normal kidney function.88 Synergistic effects of heavy metals, including chromium with cadmium and lead, have been associated with decreased renal function in humans.89
Endocrine Disorders
Companion animals may also provide unique insights into disease incidence based on exposure to endocrine disrupting chemicals (EDCs) because there are opportunities to investigate effects in the presence and absence of normal endogenous hormones due to the practice of gonadectomy. A recent review article looked closely at chemical exposures associated with endocrine disruption and the “shared risk” scenario, which implicates pet dogs and cats as sentinels for studying the effect of endocrine disrupting chemicals.90 This review highlights reproductive and thyroid disorders in companion animals as a model for human health.
Reproductive/Fertility
Dogs and humans are subject to similar conditions that are often attributed to endocrine disruption including fertility issues, hypospadias, and cryptorchidism. Given that several studies are now suggesting a link between environmental chemical exposures to EDCs and loss of fertility, it seems prudent and crucial to expand these studies in dogs to support successful breeding programs and their long-term health. Furthermore, there may be a unique opportunity to investigate the transgenerational effects or developmental origins of adult diseases.
Aging
All mammals, including dogs and humans, experience similar developmental stages of life beginning with embryogenesis, then birth, and infancy, followed by juvenile, adolescence, adulthood, and finally geriatric stages. Aging is the single greatest risk factor for mortality and many chronic diseases, including cancer, arthritis, and neurogenerative disease. The factors that affect aging are complex, and while some strides have been made in terms of the molecular and environmental influences on aging, there is still a lot to be learned. Dogs are an emerging model of aging. Patterns of age-related mortality and morbidity are similar between dogs and humans.32 There are immense opportunities to investigate how environmental exposures not only contribute to disease but also impact aging. Environmental chemicals that have the capacity to accelerate physiological aging are known as gerontogens.91 In longitudinal studies accessing data from the pet dog population, it should be possible to investigate how gerontogens may accelerate aging in dogs. Cross species analyses of DNA methylomes across physiologic life stages have shown that there is a nonlinear epigenetic signature that is conserved across dogs and humans.92 DNA methylation, which is a hallmark of aging, can be used in cross-species studies to assess how various environmental factors influence aging and age-related diseases.
Neurodegeneration
In addition to physiologic aging and age-related risk of various morbidities, dogs offer a unique opportunity to study age-related cognitive declines. Senior dogs tend to exhibit age-related cognitive abnormalities similar to human neurodegenerative diseases with symptoms including: apathy, anxiety, disorientation and confusion, altered sleep cycles, impaired social interactions, and incontinence.93 Typically, this is clinically referred to as Canine Cognitive Dysfunction Syndrome (CCD) and has been linked to both tau tangles and β-amyloid accumulations like those seen in humans with neurodegenerative diseases.94,95 Tau and β-amyloid are natural occurring proteins, but when they abnormally accumulate, they can interfere with cognition and memory and are considered hallmarks of Alzheimer’s disease. Although no specific studies were identified linking a specific environmental exposure to a neurodegenerative disease in pet dogs, studies conducted in Mexico City with mongrel dogs linked exposure to urban air pollution, with brain abnormalities consistent with neurodegenerative disorders like Alzheimer’s disease.96,97
Other Outcomes
Further, studies have reported that concurrent chronic immune related diseases occur in cohabitating dogs and people, including allergies,98,99 obesity,100−102 diabetes,103 and elevated chronic stress.104 While environmental exposures could be involved in these, it is possible that other shared lifestyle factors, such as exercise and feeding habits, may also contribute to shared disease risk. Another facet of research that is increasingly being considered for its role in overall health and particularly immune related diseases is the microbiome. Studies have shown cohabitating people and dogs have shared microbiomes.105,106 These areas all deserve further research to understand the role of the environment on both human and pet health.
Perspectives on Wearable Sensors for Advancing Research
This review highlights areas of research where companion dogs have been used as a model for environmental health research, specifically focusing on health outcomes in dogs that have been associated with environmental exposures. While there is increasing recognition and value attributed to the use of pet dogs as sentinel species for environmental health, one of the most consistent challenges is lack of statistical power, which may be attributed to the historically limited availability of funds to support these types of studies. Furthermore, there has been a major reliance on observational data, and future studies need objective environmental exposure data for their participants.
Wearable technologies (i.e., personal samplers) are an excellent tool for companion animal research (Figure 2). For example, silicone dog tags have been used as a complementary method to the silicone wristband used in human exposure assessment studies.107 These have successfully been used in domestic cats and dogs.108−111 There are several reviews that have been published in the past few years that describe the use of passive silicone samplers for environmental exposure assessments, detailing their applications in environmental health research, best practices for their use, advantages and challenges, comparisons with other methods and matrices used in exposure assessment and analytical capabilities.112−115 Some of the key advantages of their utility with pets include the following:
Correlation with internal biomarkers of exposure
Noninvasive and simplicity of use by owners
Shown to correlate with owner exposures
Transportability and stability; easily transported/mailed between study participants and researchers
Wide range of exposure measures– data sets can be curated to look at thousands of chemicals in a single sample
Integrated measures over the sampling time period
Can support prospective measures over time/seasons
Figure 2.
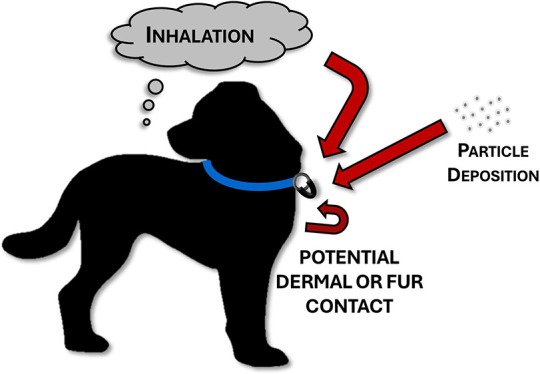
Personal passive samplers are typically deployed on the collar of companion animals and sample both inhalation and dermal routes of exposure.
However, there are some limitations to wearable sensors. For example, it is important to note that these wearables do not capture dietary exposures (i.e., chemicals in drinking water or food). Furthermore, they are not capable of capturing exposure to metals, which can have major health impacts. Lastly, it is very important for studies to include experts with experience in analytical chemistry and exposure assessments to ensure the proper interpretation of data. The data are unlikely to identify a single source of exposure for most chemicals. Despite these limitations, silicone samplers offer a unique and valuable tool for assessing personal exposures, particularly for the use of comparative studies because they measure external exposures which are not influenced by xenobiotic metabolism.
Diet and drinking water represent significant pathways for environmental exposure in dogs and people. While silicone samplers offer a noninvasive method for assessing external exposures, they are unable to capture chemicals ingested through diet or drinking water. Instead, exposure assessments through blood and urine collection or the analysis of hair and nails for select chemicals provide valuable insights into internal exposures. Urine samples offer a snapshot of recent exposures to certain compounds with shorter half-lives. However, it is essential to note the limitations of single spot urine samples, as they may not fully reflect long-term average exposure patterns. Blood samples can provide an assessment of both recent and cumulative exposures and offer insights into longer-term exposure patterns; however, chemicals that are rapidly metabolized can be difficult to detect in the blood and urine. Furthermore, some metabolites are not specific to one parent compound and can confound interpretation of the exposures. Prospective studies or biobanks aiming to investigate these exposure pathways should plan ahead for sample collection, ensuring that the methods and materials are conducive to future analytical techniques. Nevertheless, it is important to recognize that collecting blood samples can be invasive and cumbersome for pet owners, often requiring the assistance of veterinary professionals, which may incur additional costs.
Recommendations for Enhancing Future Studies
As household dogs share much of their own daily environment, there is enormous potential to gather an abundance of data from our pets as we address possible links between exposure and health outcomes. There is value in information obtained that links exposures and conditions in both species as well as the absence of associations in one or the other. There is increasing pressure to reduce the primary reliance on laboratory animal testing to alternative approaches under the rubric of New Approach Methodologies (NAM), and the use of naturally occurring diseases in companions complements that movement. Companion animal exposure science is on the precipice of tremendous opportunity that needs support and greater recognition within the scientific community.
Companion animal veterinary studies should not exist in a bubble; the full potential of the companion animal model will be realized when experts from both human and veterinary sciences incorporate these types of studies on a larger scale in a One Health effort. Interdisciplinary studies using companion animals would benefit from experts in veterinary, human, and basic science focused fields. Long-term studies should be designed with input from experts in exposure science, toxicology, and statistics from the outset to better optimize experimental design and prioritize types of sample collection for analyses. This may help to reduce the consistent reports of insufficient statistical power in studies and maximize the potential data obtained from each study.
Despite the advances that have been made using companion animals for comparative environmental health studies, there are major design issues that need to improve. First, proper selection and definition of cases versus controls are critical to evaluating associations. Second, recruitment and inclusion of animals needs to be properly considered, and we should focus more on pets rather than animals housed in an animal shelter, which may not be reflective of normal human conditions. Third, characterizing and comprehensively reporting demographic data for pets used in the studies should consider the NIH expectations for human studies including sex and age but should also include factors such as spay/neuter status and breed. While there are quite a few studies that examine urban versus rural environments, none of these studies factor in the socioeconomics of the home in which these pets reside in. The following recommendations for best practices should be considered when using companion animals as sentinels:
Selecting appropriate controls for studies of chronic diseases based on age, breed, and sex (including spay/neuter status)
Enrolling a greater number of participants to improve statistical power
Obtaining objective measurements of environmental exposures (e.g., collection and analysis of blood, urine, nail clippings or silicone tags)
Improved characterization of the home (e.g., age of home, type of flooring) and socioeconomic status of the homeowners
Only animals living with a shared environment with people should be included (i.e., shelter animals are in a much different environment that does not reflect the general population)
Conclusions
As the age of “big data” progresses, our ability to interrogate complex multispecies data sets will vastly improve, allowing better understanding of putative pathogenic genes and toxicants that dictate the development and progression of diseases and aging. The role of cross-species analysis to fill in gaps for undiscovered or poorly characterized mechanisms of pathogenesis should be recognized. Further, companion animals offer a unique opportunity to integrate various ‘omics to address the complexity of real-world relevant studies on mixtures or the total burden of exposures and changes in health-related variables, such as genomic, transcriptomic, metabolomic, etc. perturbations. Capitalizing on the use of companion animal models may provide advances in disease prevention in both medical and veterinary science. Further, there is a unique opportunity to investigate the role of early life exposures on later in life health outcomes in a shortened time frame.
Environmental exposure remains a critical global issue, and linking exposures to health outcomes in humans can be challenging. There is now a growing body of literature documenting links between exposures and various adverse health outcomes in companion animals. The use of naturally occurring health end point with companion animals as a model to study shared diseases and shared environmental exposures is an invaluable opportunity that needs further support from the scientific community and funding bodies. The pet dog is the modern-day canary in the coal mine with the ability to alert us to not only potential acute dangers but also subtler long-term consequences of environmental exposures and greater public health outcomes, otherwise known as the canine on the couch.
Acknowledgments
The authors acknowledge financial support from the V Foundation and the Consortium for Canine Comparative Oncology (M.B., NCSU; H.M.S., Duke).
The authors declare no competing financial interest.
References
- Destoumieux-Garzon D.; Mavingui P.; Boetsch G.; Boissier J.; Darriet F.; Duboz P.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front Vet Sci. 2018, 5, 14. 10.3389/fvets.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. J.; Anderson L. R.; Blackmore C. G.; Blackwell M. J.; Lautner E. A.; Marcus L. C.; et al. Executive summary of the AVMA One Health Initiative Task Force report. J. Am. Vet Med. Assoc 2008, 233 (2), 259–261. 10.2460/javma.233.2.259. [DOI] [PubMed] [Google Scholar]
- Day M. J. One health: the importance of companion animal vector-borne diseases. Parasit Vectors 2011, 4, 49. 10.1186/1756-3305-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. E.; Patel N. G.; Levy M. A.; Storeygard A.; Balk D.; Gittleman J. L.; et al. Global trends in emerging infectious diseases. Nature 2008, 451 (7181), 990–993. 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R.; Landrigan P. J.; Balakrishnan K.; Bathan G.; Bose-O’Reilly S.; Brauer M.; et al. Pollution and health: a progress update. Lancet Planet Health 2022, 6 (6), e535–e547. 10.1016/S2542-5196(22)00090-0. [DOI] [PubMed] [Google Scholar]
- Politi K.; Pao W. How genetically engineered mouse tumor models provide insights into human cancers. J. Clin Oncol 2011, 29 (16), 2273–2281. 10.1200/JCO.2010.30.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N. E.; Depinho R. A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discov 2006, 5 (9), 741–754. 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- Shearin A. L.; Ostrander E. A. Leading the way: canine models of genomics and disease. Dis Model Mech 2010, 3 (1–2), 27–34. 10.1242/dmm.004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad-Toh K.; Wade C. M.; Mikkelsen T. S.; Karlsson E. K.; Jaffe D. B.; Kamal M.; et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005, 438 (7069), 803–819. 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- Wild C. P. Complementing the genome with an ″exposome″: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 2005, 14 (8), 1847–1850. 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee on Animals as Monitors of Environmental Hazards . Animals as sentinels of environmental health hazards/Committee on Animals as Monitors of Environmental Hazards, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council; National Academy Press, 1991.
- Halliday J. E.; Meredith A. L.; Knobel D. L.; Shaw D. J.; Bronsvoort B. M.; Cleaveland S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R Soc. Interface 2007, 4 (16), 973–984. 10.1098/rsif.2007.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Ma X.; Tian L.; Kong A.; Wang N.; Huang C.; et al. Chronic co-exposure to low levels of brominated flame retardants and heavy metals induces reproductive toxicity in zebrafish. Toxicol Ind. Health 2018, 34 (9), 631–639. 10.1177/0748233718779478. [DOI] [PubMed] [Google Scholar]
- Schmidt P. L. Companion animals as sentinels for public health. Vet Clin North Am. Small Anim Pract 2009, 39 (2), 241–250. 10.1016/j.cvsm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Oh J. H.; Cho J. Y. Comparative oncology: overcoming human cancer through companion animal studies. Exp Mol. Med. 2023, 55 (4), 725–734. 10.1038/s12276-023-00977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H. L.; Fenger J. M.; London C. A. Dogs as a Model for Cancer. Annu. Rev. Anim Biosci 2016, 4, 199–222. 10.1146/annurev-animal-022114-110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M. I.; Raposo T. P.; Silva-Carvalho R.; Pires I.; Prada J.; Gregorio H.; et al. The Dog as a Model to Study the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1329, 123–152. 10.1007/978-3-030-73119-9_7. [DOI] [PubMed] [Google Scholar]
- Garden O. A.; Volk S. W.; Mason N. J.; Perry J. A. Companion animals in comparative oncology: One Medicine in action. Vet J. 2018, 240, 6–13. 10.1016/j.tvjl.2018.08.008. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. K.; Mazcko C. N. Improving human cancer therapy through the evaluation of pet dogs. Nat. Rev. Cancer 2020, 20 (12), 727–742. 10.1038/s41568-020-0297-3. [DOI] [PubMed] [Google Scholar]
- Schiffman J. D.; Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos. Trans R Soc. Lond B Biol. Sci. 2015, 370 (1673), 20140231. 10.1098/rstb.2014.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone L.; Barutello G.; Iussich S.; Giacobino D.; Quaglino E.; Buracco P.; et al. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer Immunol Immunother 2019, 68 (11), 1839–1853. 10.1007/s00262-019-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J. Y.; Moskwa N.; Kang W.; Fan T. M.; Lee C. Canine as a Comparative and Translational Model for Human Mammary Tumor. J. Breast Cancer 2023, 26 (1), 1–13. 10.4048/jbc.2023.26.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp D. W.; Dhawan D.; Ramos-Vara J. A.; Ratliff T. L.; Cresswell G. M.; Utturkar S.; et al. Naturally-Occurring Invasive Urothelial Carcinoma in Dogs, a Unique Model to Drive Advances in Managing Muscle Invasive Bladder Cancer in Humans. Front Oncol 2020, 9, 1493. 10.3389/fonc.2019.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery A. C. The Genetic and Molecular Basis for Canine Models of Human Leukemia and Lymphoma. Front Oncol 2020, 10, 23. 10.3389/fonc.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedan B.; Rault M.; Abadie J.; Ulve R.; Botherel N.; Devauchelle P.; et al. PTPN11 mutations in canine and human disseminated histiocytic sarcoma. Int. J. Cancer 2020, 147 (6), 1657–1665. 10.1002/ijc.32991. [DOI] [PubMed] [Google Scholar]
- Ito D.; Frantz A. M.; Modiano J. F. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications. Vet. Immunol. Immunopathol. 2014, 159 (3–4), 192–201. 10.1016/j.vetimm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi L.; Scotlandi K.; Pettinari I.; Benassi M. S.; Porcellato I.; Pazzaglia L. MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells 2021, 10 (2), 428. 10.3390/cells10020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch G.; Sivaprakasam K.; Zismann V.; Perdigones N.; Contente-Cuomo T.; Nazareno A.; et al. Identification of Recurrent Activating HER2Mutations in Primary Canine Pulmonary Adenocarcinoma. Clin. Cancer Res. 2019, 25 (19), 5866–5877. 10.1158/1078-0432.CCR-19-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megquier K.; Turner-Maier J.; Swofford R.; Kim J. H.; Sarver A. L.; Wang C.; et al. Comparative Genomics Reveals Shared Mutational Landscape in Canine Hemangiosarcoma and Human Angiosarcoma. Mol. Cancer Res. 2019, 17 (12), 2410–2421. 10.1158/1541-7786.MCR-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouteau A.; Andre C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes (Basel) 2019, 10 (7), 501. 10.3390/genes10070501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilatis D. M.; Lucchesi C. A.; Ghosh P. M. Molecular Similarities and Differences between Canine Prostate Cancer and Human Prostate Cancer Variants. Biomedicines 2023, 11 (4), 1100. 10.3390/biomedicines11041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. M.; Creevy K. E.; Franks A.; O’Neill D. G.; Promislow D. E. L. The companion dog as a model for human aging and mortality. Aging Cell 2018, 17 (3), e12737 10.1111/acel.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalko J. M.; Kruglyak K. M.; McCleary-Wheeler A. L.; Goyal V.; Phelps-Dunn A.; Wong L. K.; et al. Age at cancer diagnosis by breed, weight, sex, and cancer type in a cohort of more than 3,000 dogs: Determining the optimal age to initiate cancer screening in canine patients. PLoS One 2023, 18 (2), e0280795 10.1371/journal.pone.0280795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baioni E.; Scanziani E.; Vincenti M. C.; Leschiera M.; Bozzetta E.; Pezzolato M.; et al. Estimating canine cancer incidence: findings from a population-based tumour registry in northwestern Italy. BMC Vet Res. 2017, 13 (1), 203. 10.1186/s12917-017-1126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Wagle N. S.; Jemal A. Cancer statistics, 2023. CA Cancer J. Clin 2023, 73 (1), 17–48. 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- Longo T.; McGinley K. F.; Freedman J. A.; Etienne W.; Wu Y.; Sibley A.; et al. Targeted Exome Sequencing of the Cancer Genome in Patients with Very High-risk Bladder Cancer. Eur. Urol 2016, 70 (5), 714–717. 10.1016/j.eururo.2016.07.049. [DOI] [PubMed] [Google Scholar]
- Thomas R.; Wiley C. A.; Droste E. L.; Robertson J.; Inman B. A.; Breen M. Whole exome sequencing analysis of canine urothelial carcinomas without BRAF V595E mutation: Short in-frame deletions in BRAF and MAP2K1 suggest alternative mechanisms for MAPK pathway disruption. PLoS Genet 2023, 19 (4), e1010575 10.1371/journal.pgen.1010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letasiova S.; Medve’ova A.; Sovcikova A.; Dusinska M.; Volkovova K.; Mosoiu C.; et al. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11 (Suppl 1), S11. 10.1186/1476-069X-11-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp D. W.; Dhawan D.; Ruple A.; Cooper B. R.; Zhang M.; Liu D.; et al. Association between cigarette smoke exposure and urinary bladder cancer in Scottish terriers in a cohort study. Vet J. 2024, 303, 106044 10.1016/j.tvjl.2023.106044. [DOI] [PubMed] [Google Scholar]
- Glickman L. T.; Schofer F. S.; McKee L. J.; Reif J. S.; Goldschmidt M. H. Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J. Toxicol Environ. Health 1989, 28 (4), 407–414. 10.1080/15287398909531360. [DOI] [PubMed] [Google Scholar]
- Luethcke K. R.; Ekena J.; Chun R.; Trepanier L. A. Glutathione S-transferase theta genotypes and environmental exposures in the risk of canine transitional cell carcinoma. J. Vet Intern Med. 2019, 33 (3), 1414–1422. 10.1111/jvim.15504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes H. M. Jr.; Hoover R.; Tarone R. E. Bladder cancer in pet dogs: a sentinel for environmental cancer?. Am. J. Epidemiol 1981, 114 (2), 229–233. 10.1093/oxfordjournals.aje.a113186. [DOI] [PubMed] [Google Scholar]
- Smith N.; Luethcke K. R.; Craun K.; Trepanier L. Risk of bladder cancer and lymphoma in dogs is associated with pollution indices by county of residence. Vet Comp Oncol 2022, 20 (1), 246–255. 10.1111/vco.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. D.; Prasad S. M.; Patel A. R.; Weiner A. B.; Pariser J. J.; Razmaria A.; et al. Bladder Cancer Mortality in the United States: A Geographic and Temporal Analysis of Socioeconomic and Environmental Factors. J. Urol 2016, 195 (2), 290–296. 10.1016/j.juro.2015.07.091. [DOI] [PubMed] [Google Scholar]
- Boulanger M.; Tual S.; Lemarchand C.; Guizard A. V.; Velten M.; Marcotullio E.; et al. Agricultural exposure and risk of bladder cancer in the AGRIculture and CANcer cohort. Int. Arch Occup Environ. Health 2017, 90 (2), 169–178. 10.1007/s00420-016-1182-y. [DOI] [PubMed] [Google Scholar]
- Koutros S.; Silverman D. T.; Alavanja M. C.; Andreotti G.; Lerro C. C.; Heltshe S.; et al. Occupational exposure to pesticides and bladder cancer risk. Int. J. Epidemiol 2016, 45 (3), 792–805. 10.1093/ije/dyv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer L. C.; Coss A. M.; Wolkin A. F.; Flanders W. D.; Reif J. S. Evaluation of associations between lifetime exposure to drinking water disinfection by-products and bladder cancer in dogs. J. Am. Vet Med. Assoc 2008, 232 (11), 1663–1668. 10.2460/javma.232.11.1663. [DOI] [PubMed] [Google Scholar]
- Abdelmegeed S. M.; Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018, 15 (6), 8195–8205. 10.3892/ol.2018.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer S. R.; Kaeberlein M. Desexing Dogs: A Review of the Current Literature. Animals (Basel) 2019, 9 (12), 1086. 10.3390/ani9121086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade F. H.; Figueiroa F. C.; Bersano P. R.; Bissacot D. Z.; Rocha N. S. Malignant mammary tumor in female dogs: environmental contaminants. Diagn Pathol 2010, 5, 45. 10.1186/1746-1596-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S.; Sood N. K.; Gupta K.; Joshi C.; Gill K. K.; Kaur R.; et al. Bioaccumulation of pesticide contaminants in tissue matrices of dogs suffering from malignant canine mammary tumors in Punjab, India. Heliyon 2020, 6 (10), e05274 10.1016/j.heliyon.2020.e05274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska U.; Jagielski D.; Czopowicz M.; Sapierzynski R. The animal-dependent risk factors in canine T-cell lymphomas. Vet Comp Oncol 2017, 15 (2), 307–314. 10.1111/vco.12164. [DOI] [PubMed] [Google Scholar]
- Comazzi S.; Marelli S.; Cozzi M.; Rizzi R.; Finotello R.; Henriques J.; et al. Breed-associated risks for developing canine lymphoma differ among countries: an European canine lymphoma network study. BMC Vet Res. 2018, 14 (1), 232. 10.1186/s12917-018-1557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J. N. R.; Andre A. S.; Aguiar S. I.; Gil S.; Tavares L.; Aires-da-Silva F. Immunotherapeutic Strategies for Canine Lymphoma: Changing the Odds Against Non-Hodgkin Lymphoma. Front Vet Sci. 2021, 8, 621758 10.3389/fvets.2021.621758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield I.; Stevens K. B.; Pittaway C.; O’Neill D. G.; Fecht D.; Dobson J. M.; et al. Geographic distribution and environmental risk factors of lymphoma in dogs under primary-care in the UK. J. Small Anim Pract 2019, 60 (12), 746–754. 10.1111/jsap.13075. [DOI] [PubMed] [Google Scholar]
- Pinello K. C.; Niza-Ribeiro J.; Fonseca L.; de Matos A. J. Incidence, characteristics and geographical distributions of canine and human non-Hodgkin’s lymphoma in the Porto region (North West Portugal). Vet J. 2019, 245, 70–76. 10.1016/j.tvjl.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Pastor M.; Chalvet-Monfray K.; Marchal T.; Keck G.; Magnol J. P.; Fournel-Fleury C.; et al. Genetic and environmental risk indicators in canine non-Hodgkin’s lymphomas: breed associations and geographic distribution of 608 cases diagnosed throughout France over 1 year. J. Vet Intern Med. 2009, 23 (2), 301–310. 10.1111/j.1939-1676.2008.0255.x. [DOI] [PubMed] [Google Scholar]
- Craun K.; Ekena J.; Sacco J.; Jiang T.; Motsinger-Reif A.; Trepanier L. A. Genetic and environmental risk for lymphoma in boxer dogs. J. Vet Intern Med. 2020, 34 (5), 2068–2077. 10.1111/jvim.15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini D. A.; Kimura K. C.; Nishiya A. T.; Ubukata R.; Leandro R. M.; Brito C. P. d.; et al. Environmental risk factors related to the development of canine non-Hodgkin’s lymphoma. Ciência Rural 2013, 43 (7), 1302–1308. 10.1590/S0103-84782013005000089. [DOI] [Google Scholar]
- Boyle J.; Ward M. H.; Cerhan J. R.; Rothman N.; Wheeler D. C. Modeling historic environmental pollutant exposures and non-Hodgkin lymphoma risk. Environ. Res. 2023, 224, 115506 10.1016/j.envres.2023.115506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazza A.; Presciuttini S.; Barale R.; Lubas G.; Gugliucci B. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J. Vet Intern Med. 2001, 15 (3), 190–195. 10.1111/j.1939-1676.2001.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Luethcke K. R.; Trepanier L. A.; Tindle A. N.; Labadie J. D. Environmental exposures and lymphoma risk: a nested case-control study using the Golden Retriever Lifetime Study cohort. Canine Med. Genet 2022, 9 (1), 10. 10.1186/s40575-022-00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconato L.; Leo C.; Girelli R.; Salvi S.; Abramo F.; Bettini G.; et al. Association between waste management and cancer in companion animals. J. Vet Intern Med. 2009, 23 (3), 564–569. 10.1111/j.1939-1676.2009.0278.x. [DOI] [PubMed] [Google Scholar]
- Viel J. F.; Floret N.; Deconinck E.; Focant J. F.; De Pauw E.; Cahn J. Y. Increased risk of non-Hodgkin lymphoma and serum organochlorine concentrations among neighbors of a municipal solid waste incinerator. Environ. Int. 2011, 37 (2), 449–453. 10.1016/j.envint.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Viel J. F.; Daniau C.; Goria S.; Fabre P.; de Crouy-Chanel P.; Sauleau E. A.; et al. Risk for non Hodgkin’s lymphoma in the vicinity of French municipal solid waste incinerators. Environ. Health 2008, 7, 51. 10.1186/1476-069X-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odutola M. K.; van Leeuwen M. T.; Turner J.; Bruinsma F.; Seymour J. F.; Prince H. M. Associations between Smoking and Alcohol and Follicular Lymphoma Incidence and Survival: A Family-Based Case-Control Study in Australia. Cancers (Basel) 2022, 14 (11), 2710. 10.3390/cancers14112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinello K. C.; Santos M.; Leite-Martins L.; Niza-Ribeiro J.; de Matos A. J. Immunocytochemical study of canine lymphomas and its correlation with exposure to tobacco smoke. Vet World 2017, 10 (11), 1307–1313. 10.14202/vetworld.2017.1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes H. M.; Tarone R. E.; Cantor K. P.; Jessen C. R.; McCurnin D. M.; Richardson R. C. Case-control study of canine malignant lymphoma: positive association with dog owner’s use of 2,4-dichlorophenoxyacetic acid herbicides. J. Natl. Cancer Inst 1991, 83 (17), 1226–1231. 10.1093/jnci/83.17.1226. [DOI] [PubMed] [Google Scholar]
- Takashima-Uebelhoer B. B.; Barber L. G.; Zagarins S. E.; Procter-Gray E.; Gollenberg A. L.; Moore A. S.; et al. Household chemical exposures and the risk of canine malignant lymphoma, a model for human non-Hodgkin’s lymphoma. Environ. Res. 2012, 112, 171–176. 10.1016/j.envres.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro C. C.; Smedley R. C.; Buchweitz J. P.; Langlois D. K. Hepatic copper and other trace mineral concentrations in dogs with hepatocellular carcinoma. J. Vet Intern Med. 2019, 33 (5), 2193–2199. 10.1111/jvim.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes H. M.; Tarone R. E.; Casey H. W. A cohort study of the effects of Vietnam service on testicular pathology of U.S. military working dogs. Mil Med. 1995, 160 (5), 248–255. 10.1093/milmed/160.5.248. [DOI] [PubMed] [Google Scholar]
- Hayes H. M.; Tarone R. E.; Casey H. W.; Huxsoll D. L. Excess of seminomas observed in Vietnam service U.S. military working dogs. J. Natl. Cancer Inst 1990, 82 (12), 1042–1046. 10.1093/jnci/82.12.1042. [DOI] [PubMed] [Google Scholar]
- Glickman L. T.; Domanski L. M.; Maguire T. G.; Dubielzig R. R.; Churg A. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res. 1983, 32 (2), 305–313. 10.1016/0013-9351(83)90114-7. [DOI] [PubMed] [Google Scholar]
- Reif J. S.; Cohen D. The environmental distribution of canine respiratory tract neoplasms. Arch. Environ. Health 1971, 22 (1), 136–140. 10.1080/00039896.1971.10665823. [DOI] [PubMed] [Google Scholar]
- Glickman L. T.; Raghavan M.; Knapp D. W.; Bonney P. L.; Dawson M. H. Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J. Am. Vet Med. Assoc 2004, 224 (8), 1290–1297. 10.2460/javma.2004.224.1290. [DOI] [PubMed] [Google Scholar]
- Raghavan M.; Knapp D. W.; Dawson M. H.; Bonney P. L.; Glickman L. T. Topical flea and tick pesticides and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J. Am. Vet Med. Assoc 2004, 225 (3), 389–394. 10.2460/javma.2004.225.389. [DOI] [PubMed] [Google Scholar]
- Raghavan M.; Knapp D. W.; Bonney P. L.; Dawson M. H.; Glickman L. T. Evaluation of the effect of dietary vegetable consumption on reducing risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J. Am. Vet Med. Assoc 2005, 227 (1), 94–100. 10.2460/javma.2005.227.94. [DOI] [PubMed] [Google Scholar]
- Reif J. S.; Lower K. S.; Ogilvie G. K. Residential exposure to magnetic fields and risk of canine lymphoma. Am. J. Epidemiol 1995, 141 (4), 352–359. 10.1093/aje/141.4.352. [DOI] [PubMed] [Google Scholar]
- Badea E.; Goran G. V.; ŢOCA C.; Crivineanu V. Assessment of Heavy Metal and Mineral Levels in Hair Samples from Dogs with Mammary Neoplasms. Bulletin UASVM Food Science and Technology 2018, 75 (1), 1. 10.15835/buasvmcn-fst:0007. [DOI] [Google Scholar]
- Frehse M. S.; Martins M. I.; Ono E. Y.; Bracarense A. P.; Bissoqui L. Y.; Teixeira E. M.; et al. Aflatoxins ingestion and canine mammary tumors: There is an association?. Food Chem. Toxicol. 2015, 84, 74–78. 10.1016/j.fct.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Severe S.; Marchand P.; Guiffard I.; Morio F.; Venisseau A.; Veyrand B.; et al. Pollutants in pet dogs: a model for environmental links to breast cancer. Springerplus 2015, 4, 27. 10.1186/s40064-015-0790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini G.; Morini M.; Marconato L.; Marcato P. S.; Zini E. Association between environmental dust exposure and lung cancer in dogs. Vet J. 2010, 186 (3), 364–369. 10.1016/j.tvjl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Harbison M. L.; Godleski J. J. Malignant mesothelioma in urban dogs. Vet Pathol 1983, 20 (5), 531–540. 10.1177/030098588302000504. [DOI] [PubMed] [Google Scholar]
- Reif J. S.; Dunn K.; Ogilvie G. K.; Harris C. K. Passive smoking and canine lung cancer risk. Am. J. Epidemiol 1992, 135 (3), 234–239. 10.1093/oxfordjournals.aje.a116276. [DOI] [PubMed] [Google Scholar]
- Zierenberg-Ripoll A.; Pollard R. E.; Stewart S. L.; Allstadt S. D.; Barrett L. E.; Gillem J. M.; et al. Association between environmental factors including second-hand smoke and primary lung cancer in dogs. J. Small Anim Pract 2018, 59 (6), 343–349. 10.1111/jsap.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. A.; Wartenberg D.; Goldschmidt M. Environmental causes for sinonasal cancers in pet dogs, and their usefulness as sentinels of indoor cancer risk. J. Toxicol Environ. Health A 1998, 54 (7), 579–591. 10.1080/009841098158719. [DOI] [PubMed] [Google Scholar]
- Reif J. S.; Bruns C.; Lower K. S. Cancer of the nasal cavity and paranasal sinuses and exposure to environmental tobacco smoke in pet dogs. Am. J. Epidemiol 1998, 147 (5), 488–492. 10.1093/oxfordjournals.aje.a009475. [DOI] [PubMed] [Google Scholar]
- Passlack N.; Mainzer B.; Lahrssen-Wiederholt M.; Schafft H.; Palavinskas R.; Breithaupt A.; et al. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in the liver and kidneys of dogs according to age, gender, and the occurrence of chronic kidney disease. J. Vet Sci. 2015, 16 (1), 57–66. 10.4142/jvs.2015.16.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T. L.; Kuo C. C.; Pan W. H.; Chung Y. T.; Chen C. Y.; Wu T. N.; et al. The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 2017, 92 (3), 710–720. 10.1016/j.kint.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Pocar P.; Grieco V.; Aidos L.; Borromeo V. Endocrine-Disrupting Chemicals and Their Effects in Pet Dogs and Cats: An Overview. Animals (Basel) 2023, 13 (3), 378. 10.3390/ani13030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino J. A.; Sanoff H. K.; Sharpless N. E. Defining the toxicology of aging. Trends Mol. Med. 2014, 20 (7), 375–384. 10.1016/j.molmed.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Ma J.; Hogan A. N.; Fong S.; Licon K.; Tsui B.; et al. Quantitative Translation of Dog-to-Human Aging by Conserved Remodeling of the DNA Methylome. Cell Syst 2020, 11 (2), 176–185.e6. 10.1016/j.cels.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor S.; Kubinyi E. Genetic Pathways of Aging and Their Relevance in the Dog as a Natural Model of Human Aging. Front Genet 2019, 10, 948. 10.3389/fgene.2019.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insua D.; Suarez M. L.; Santamarina G.; Sarasa M.; Pesini P. Dogs with canine counterpart of Alzheimer’s disease lose noradrenergic neurons. Neurobiol Aging 2010, 31 (4), 625–635. 10.1016/j.neurobiolaging.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Smolek T.; Madari A.; Farbakova J.; Kandrac O.; Jadhav S.; Cente M.; et al. Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment. J. Comp Neurol 2016, 524 (4), 874–895. 10.1002/cne.23877. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L.; Maronpot R. R.; Torres-Jardon R.; Henriquez-Roldan C.; Schoonhoven R.; Acuna-Ayala H.; et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol 2003, 31 (5), 524–538. 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L.; Reynoso-Robles R.; Vargas-Martinez J.; Gomez-Maqueo-Chew A.; Perez-Guille B.; Mukherjee P. S.; et al. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ. Res. 2016, 146, 404–417. 10.1016/j.envres.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Hakanen E.; Lehtimaki J.; Salmela E.; Tiira K.; Anturaniemi J.; Hielm-Bjorkman A.; et al. Urban environment predisposes dogs and their owners to allergic symptoms. Sci. Rep 2018, 8 (1), 1585. 10.1038/s41598-018-19953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimaki J.; Sinkko H.; Hielm-Bjorkman A.; Laatikainen T.; Ruokolainen L.; Lohi H. Simultaneous allergic traits in dogs and their owners are associated with living environment, lifestyle and microbial exposures. Sci. Rep 2020, 10 (1), 21954 10.1038/s41598-020-79055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornvad C. R.; Gloor S.; Johansen S. S.; Sandoe P.; Lund T. B. Neutering increases the risk of obesity in male dogs but not in bitches - A cross-sectional study of dog- and owner-related risk factors for obesity in Danish companion dogs. Prev Vet Med. 2019, 170, 104730 10.1016/j.prevetmed.2019.104730. [DOI] [PubMed] [Google Scholar]
- Nijland M. L.; Stam F.; Seidell J. C. Overweight in dogs, but not in cats, is related to overweight in their owners. Public Health Nutr 2010, 13 (1), 102–106. 10.1017/S136898000999022X. [DOI] [PubMed] [Google Scholar]
- Suarez L.; Bautista-Castano I.; Pena Romera C.; Montoya-Alonso J. A.; Corbera J. A. Is Dog Owner Obesity a Risk Factor for Canine Obesity? A ″One-Health″ Study on Human-Animal Interaction in a Region with a High Prevalence of Obesity. Vet Sci. 2022, 9 (5), 243. 10.3390/vetsci9050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delicano R. A.; Hammar U.; Egenvall A.; Westgarth C.; Mubanga M.; Byberg L.; et al. The shared risk of diabetes between dog and cat owners and their pets: register based cohort study. BMJ. 2020, 371, m4337. 10.1136/bmj.m4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundman A. S.; Van Poucke E.; Svensson Holm A. C.; Faresjo A.; Theodorsson E.; Jensen P.; et al. Long-term stress levels are synchronized in dogs and their owners. Sci. Rep 2019, 9 (1), 7391. 10.1038/s41598-019-43851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. J.; Lauber C.; Costello E. K.; Lozupone C. A.; Humphrey G.; Berg-Lyons D.; et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013, 2, e00458 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels S. U.; Strachan C. R.; Conrady B.; Wagner M.; Burgener I. A.; Viranyi Z.; et al. Wolves, dogs and humans in regular contact can mutually impact each other’s skin microbiota. Sci. Rep 2021, 11 (1), 17106 10.1038/s41598-021-96160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell S. G.; McCartney M. A.; Paulik L. B.; Allan S. E.; Tidwell L. G.; Wilson G.; et al. Improvements in pollutant monitoring: optimizing silicone for co-deployment with polyethylene passive sampling devices. Environ. Pollut. 2014, 193, 71–78. 10.1016/j.envpol.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise C. F.; Hammel S. C.; Herkert N.; Ma J.; Motsinger-Reif A.; Stapleton H. M.; et al. Comparative Exposure Assessment Using Silicone Passive Samplers Indicates That Domestic Dogs Are Sentinels To Support Human Health Research. Environ. Sci. Technol. 2020, 54 (12), 7409–7419. 10.1021/acs.est.9b06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise C. F.; Hammel S. C.; Herkert N. J.; Ospina M.; Calafat A. M.; Breen M.; et al. Comparative Assessment of Pesticide Exposures in Domestic Dogs and Their Owners Using Silicone Passive Samplers and Biomonitoring. Environ. Sci. Technol. 2022, 56 (2), 1149–1161. 10.1021/acs.est.1c06819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse C. M.; Herbstman J. B.; Peterson M. E.; Gordon J.; Soboroff P. H.; Holmes D.; et al. Silicone Pet Tags Associate Tris(1,3-dichloro-2-isopropyl) Phosphate Exposures with Feline Hyperthyroidism. Environ. Sci. Technol. 2019, 53 (15), 9203–9213. 10.1021/acs.est.9b02226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo D. M.; Lo S.; Krop E.; Meijer J.; Beeltje H.; Lamoree M. H.; et al. Do cats mirror their owner? Paired exposure assessment using silicone bands to measure residential PAH exposure. Environ. Res. 2023, 222, 115412 10.1016/j.envres.2023.115412. [DOI] [PubMed] [Google Scholar]
- Waclawik M.; Rodzaj W.; Wielgomas B. Silicone Wristbands in Exposure Assessment: Analytical Considerations and Comparison with Other Approaches. Int. J. Environ. Res. Public Health 2022, 19 (4), 1935. 10.3390/ijerph19041935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samon S. M.; Hammel S. C.; Stapleton H. M.; Anderson K. A. Silicone wristbands as personal passive sampling devices: Current knowledge, recommendations for use, and future directions. Environ. Int. 2022, 169, 107339 10.1016/j.envint.2022.107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes Z. C.; Schwartz Y. L.; Robuck A. R.; Walker D. I. Operationalizing the Exposome Using Passive Silicone Samplers. Curr. Pollut Rep 2022, 8 (1), 1–29. 10.1007/s40726-021-00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeme J. O.; Koelmel J. P.; Johnson E.; Lin E. Z.; Gao D.; Pollitt K. J. G. Wearable Passive Samplers for Assessing Environmental Exposure to Organic Chemicals: Current Approaches and Future Directions. Curr. Environ. Health Rep 2023, 10 (2), 84–98. 10.1007/s40572-023-00392-w. [DOI] [PubMed] [Google Scholar]