Abstract
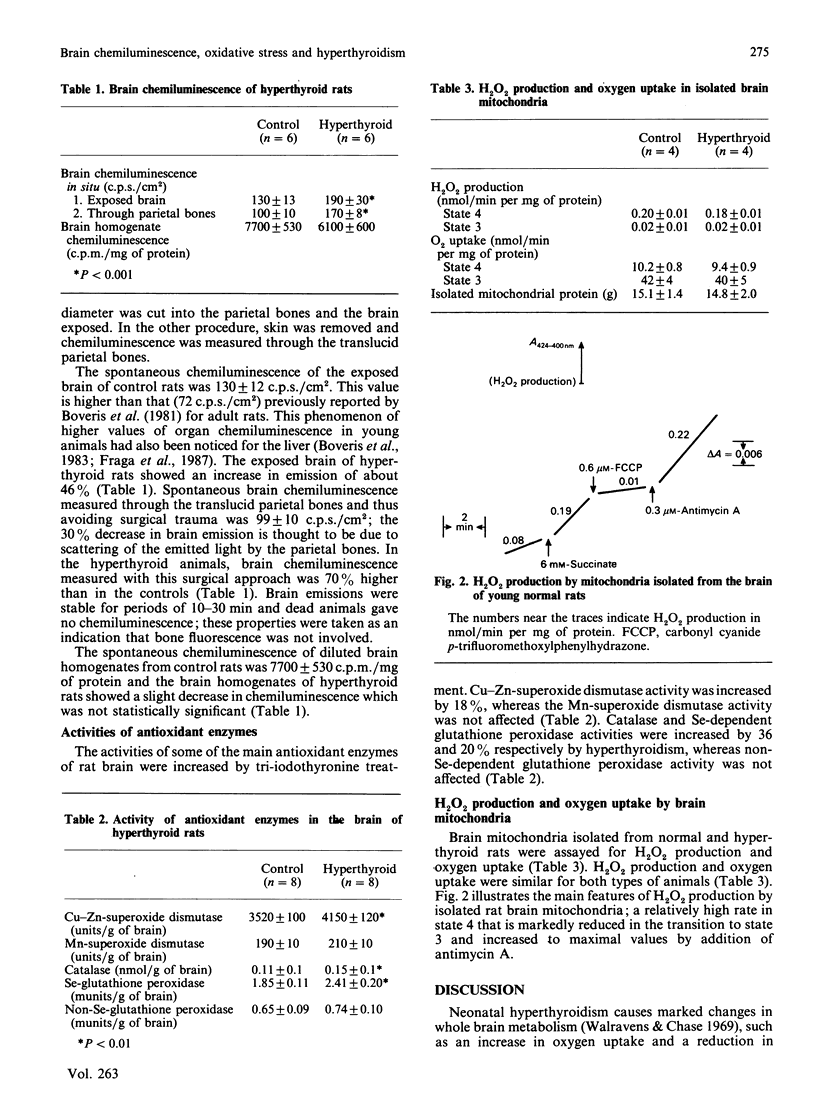
Newborn Wistar rats were made hyperthyroid by injection of tri-iodothyronine and assayed for survival, brain oxygen uptake, brain chemiluminescence and activity of antioxidant enzymes. Brain chemiluminescence was measured (1) by removing the parietal bones or (2) through the translucid parietal bones. Control animals showed a brain chemiluminescence of 130 +/- 12 c.p.s./cm2 and 99 +/- 10 c.p.s./cm2 for procedures (1) and (2) respectively. Hyperthyroid rats showed increases in the spontaneous brain photoemission of 46 and 70% compared with controls, measured by procedures 1 and 2 respectively. The hyperthyroid state did not modify the oxygen-dependent chemiluminescence of brain homogenates. The hyperthyroid animals showed a 30% increase in the oxygen uptake of brain slices and a dramatic shortening of life-span to about 16 weeks. Superoxide dismutase (the Cu-Zn enzyme), catalase and Se-dependent glutathione peroxidase activities of brain homogenates were increased by 18, 36 and 30% respectively in the hyperthyroid animals. Isolated brain mitochondria produced 0.18-0.20 nmol of H2O2/min per mg of protein in state 4 in the presence of succinate as substrate. No difference was observed between control and hyperthyroid animals. It is concluded that hyperthyroidism leads to hypermetabolism and oxidative stress in the brain. The increased levels of oxygen and peroxyl radicals may contribute to premature ageing in these animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat N. R., Rao G. S., Pieringer R. A. Investigations on myelination in vitro. Regulation of sulfolipid synthesis by thyroid hormone in cultures of dissociated brain cells from embryonic mice. J Biol Chem. 1981 Feb 10;256(3):1167–1171. [PubMed] [Google Scholar]
- Bhat N. R., Sarlieve L. L., Rao G. S., Pieringer R. A. Investigations on myelination in vitro. Regulation by thyroid hormone in cultures of dissociated brain cells from embryonic mice. J Biol Chem. 1979 Oct 10;254(19):9342–9344. [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Reiter R., Filipkowski M., Nakase Y., Chance B. Organ chemiluminescence: noninvasive assay for oxidative radical reactions. Proc Natl Acad Sci U S A. 1980 Jan;77(1):347–351. doi: 10.1073/pnas.77.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- Boveris A., Fraga C. G., Varsavsky A. I., Koch O. R. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys. 1983 Dec;227(2):534–541. doi: 10.1016/0003-9861(83)90482-4. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Sies H. Low-level chemiluminescence as an indicator of singlet molecular oxygen in biological systems. Methods Enzymol. 1984;105:221–231. doi: 10.1016/s0076-6879(84)05029-1. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Varsavsky A. I., Boveris A., Chance B. Oxygen- or organic hydroperoxide-induced chemiluminescence of brain and liver homogenates. Biochem J. 1981 Sep 15;198(3):645–654. doi: 10.1042/bj1980645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Yurko M., Fishman R. A. Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J Neurochem. 1982 Feb;38(2):525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Moscarello M. A. Changes in lipid phase behaviour in human myelin during maturation and aging. Involvement of lipid peroxidation. FEBS Lett. 1983 Jun 27;157(1):155–158. doi: 10.1016/0014-5793(83)81136-3. [DOI] [PubMed] [Google Scholar]
- Cocks J. A., Balázs R., Johnson A. L., Eayrs J. T. Effect of thyroid hormone on the biochemical maturation of rat brain: conversion of glucose-carbon into amino acids. J Neurochem. 1970 Aug;17(8):1275–1285. doi: 10.1111/j.1471-4159.1970.tb03376.x. [DOI] [PubMed] [Google Scholar]
- Faryna de Raveglia I., Gómez C. J., Ghittoni N. E. Effects of thyroxine and growth hormone on the lipid composition of the cerebral cortex and the cerebellum of developing rats. Neurobiology. 1973;3(3):176–184. [PubMed] [Google Scholar]
- Fernandez V., Llesuy S., Solari L., Kipreos K., Videla L. A., Boveris A. Chemiluminescent and respiratory responses related to thyroid hormone-induced liver oxidative stress. Free Radic Res Commun. 1988;5(2):77–84. doi: 10.3109/10715768809066914. [DOI] [PubMed] [Google Scholar]
- Flohé L., Günzler W. A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Kennedy J. Dihydroorotate-dependent superoxide production in rat brain and liver. A function of the primary dehydrogenase. Arch Biochem Biophys. 1976 Mar;173(1):219–224. doi: 10.1016/0003-9861(76)90252-6. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Arias R. F., Llesuy S. F., Koch O. R., Boveris A. Effect of vitamin E- and selenium-deficiency on rat liver chemiluminescence. Biochem J. 1987 Mar 1;242(2):383–386. doi: 10.1042/bj2420383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHMAN R., GILBERT D. L., NYE S. W., DWYER P., FENN W. O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954 May 7;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ooka H., Fujita S., Yoshimoto E. Pituitary-thyroid activity and longevity in neonatally thyroxine-treated rats. Mech Ageing Dev. 1983 Jun;22(2):113–120. doi: 10.1016/0047-6374(83)90104-5. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Dillmann W. H., Schwartz H. L., Towle H. C. Nuclear receptors and thyroid hormone action: a progress report. Fed Proc. 1979 Jul;38(8):2154–2161. [PubMed] [Google Scholar]
- Pasquini J. M., Kaplún B., García Argiz A., Gómez C. J. Hormonal regulation of brain development. I. The effect of neonatal thyroidectomy upon nucleic acids, protein and two enzymes in developing cerebral cortex and cerebellum of the rat. Brain Res. 1967 Dec;6(4):621–634. doi: 10.1016/0006-8993(67)90120-5. [DOI] [PubMed] [Google Scholar]
- Patole M. S., Swaroop A., Ramasarma T. Generation of H2O2 in brain mitochondria. J Neurochem. 1986 Jul;47(1):1–8. doi: 10.1111/j.1471-4159.1986.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Sánchez R. A., Boveris A. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol. 1988 Feb;86(2):626–630. doi: 10.1104/pp.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Bücher T., Oshino N., Chance B. Heme occupancy of catalase in hemoglobin-free perfused rat liver and of isolated rat liver catalase. Arch Biochem Biophys. 1973 Jan;154(1):106–116. doi: 10.1016/0003-9861(73)90039-8. [DOI] [PubMed] [Google Scholar]
- Walravens P., Chase H. P. Influence of thyroid on formation of myelin lipids. J Neurochem. 1969 Oct;16(10):1477–1484. doi: 10.1111/j.1471-4159.1969.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Walters S. N., Morell P. Effects of altered thyroid states on myelinogenesis. J Neurochem. 1981 May;36(5):1792–1801. doi: 10.1111/j.1471-4159.1981.tb00433.x. [DOI] [PubMed] [Google Scholar]


