Abstract
The American black bear, Ursus americanus, is a widespread and ecologically important species in North America. In California, the black bear plays an important role in a variety of ecosystems and serves as an important species for recreational hunting. While research suggests that the populations in California are currently healthy, continued monitoring is critical, with genomic analyses providing an important surveillance tool. Here we report a high-quality, near chromosome-level genome assembly from a U. americanus sample from California. The primary assembly has a total length of 2.5 Gb contained in 316 scaffolds, a contig N50 of 58.9 Mb, a scaffold N50 of 67.6 Mb, and a BUSCO completeness score of 96%. This U. americanus genome assembly will provide an important resource for the targeted management of black bear populations in California, with the goal of achieving an appropriate balance between the recreational value of black bears and the maintenance of viable populations. The high quality of this genome assembly will also make it a valuable resource for comparative genomic analyses among black bear populations and among bear species.
Keywords: California Conservation Genomics Project, CCGP, Conservation Genomics, wildlife management
Introduction
The American black bear, Ursus americanus, is an ecologically and economically important species in North America (Fig. 1). Historically, black bears were widely distributed, but loss of habitat has restricted that range, particularly in the United States (Pelton et al. 1999). In California, the species plays a critical role in many ecosystems, while also serving as an important species for recreational hunting (California Department of Fish and Game 1998). While research suggests that the California populations are currently healthy, continued monitoring is critical to developing targeted management plans in order to achieve an appropriate balance between the recreational value of black bears and the maintenance of viable populations across the state (California Department of Fish and Game 1998).
Fig. 1.
The American black bear, Ursus americanus, is a widespread species that can be found in a variety of habitats, from dense forests to open grasslands. Photos from California Department of Fish and Wildlife (left, CC BY 2.0), Florida Fish and Wildlife Conservation Commission (top right, public domain), and David Wasserman (bottom right, CC BY-SA 4.0).
Genomic resources, including high-quality genome assemblies, will provide valuable tools for the assessment of black bear populations. Genomic analyses will enable the development of population-specific management strategies by assessing population connectivity, inbreeding depression, and local adaptation. The results of these analyses will aid managers in maintaining healthy black bear populations across their range. This genome, generated from a sample from California, will be instrumental in understanding genetic variation unique to populations in the western United States and can also be used in pangenomic analyses with existing assemblies to better represent the diversity of black bears throughout their native range. There are publicly available genome assemblies from two samples, both from the eastern United States. One is contig-level (NCBI accession GCF_020975775.1); the other is scaffold-level (GCA_003344425.1, Srivastava et al. 2019). Multiple reference genomes from divergent lineages enable the identification of structural variants, which may play a critical role in local adaptation and population health.
High-quality genome assemblies will also enable comparative genomics analyses across bear species. Recent advances in multiple reference genome alignment have enabled the discovery of genetic characteristics important to species conservation (Wilder et al. 2023), as well as the evolutionary innovations unique to various lineages (Christmas et al. 2023). Ongoing efforts to generate high-quality genome assemblies for all extant bear lineages will enable the identification of deleterious and adaptive genetic variation, both within the lineage and at broader taxonomic levels.
Here we report a high-quality, near chromosome-level genome assembly generated from a California black bear as part of the California Conservation Genomics Project (CCGP; Shaffer et al. 2022). This genome assembly will be a valuable resource for management of the black bears across California and the rest of North America.
Methods
Biological materials
We captured and sedated an adult male black bear (L20-20) for relocation in September 2020 at Kings Beach, Placer County, California (39.2377°N, 120.0266°W). California Department of Fish and Wildlife (CDFW) staff captured the bear under the department’s jurisdiction as the trustee for wildlife management in the state of California, CA Fish & Game Code § 1802 (2015). While the bear was sedated, CDFW staff collected a whole-blood sample into a tube containing EDTA.
DNA extraction
We isolated high molecular weight (HMW) genomic DNA (gDNA) from the whole blood sample. We added 3 ml of RBC lysis solution (Qiagen, Germany; Cat # 158445) to 1 ml of whole blood and incubated the reaction at room temperature for 5 min. We centrifuged the sample at 2,000 x g for 5 min to pellet white blood cells. We discarded the supernatant and added 2 ml of lysis buffer containing 100 mM NaCl, 10 mM Tris-HCl pH 8.0, 25 mM EDTA, 0.5% (w/v) SDS, and 100 µg/ml Proteinase K to the cell pellet. We incubated the reaction at room temperature for a few hours until the solution was homogenous. We then treated the lysate with 20 µg/ml RNase A at 37 °C for 30 min and cleaned with equal volumes of phenol/chloroform using phase lock gels (Quantabio, Beverly, MA; Cat # 2302830). We precipitated the DNA by adding 0.4× volume of 5M ammonium acetate and 3× volume of ice-cold ethanol. We washed the DNA pellet twice with 70% ethanol and resuspended it in an elution buffer (10 mM Tris, pH 8.0). We assessed the purity of gDNA using a NanoDrop ND-1000 spectrophotometer and observed a 260/280 ratio of 1.85 and a 260/230 ratio of 2.13. We quantified the DNA yield at 15 µg with a Qbit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA). We verified the integrity of the HMW gDNA on a Femto pulse system (Agilent Technologies, Santa Clara, CA), confirming that 70% of the DNA was in fragments over 100 kb.
PacBio HiFi library preparation and sequencing
We constructed a HiFi SMRTbell library using the SMRTbell Express Template Prep Kit v2.0 (Pacific Biosciences [PacBio], Menlo Park, CA; Cat. #100-938-900) according to the manufacturer’s instructions. We sheared HMW gDNA to a target DNA size distribution of 15 to 20 kb using Diagenode’s Megaruptor 3 system (Diagenode, Belgium; Cat. B06010003) and then concentrated the sheared DNA using 0.45× of AMPure PB beads (PacBio; Cat. #100-265-900). We then processed the DNA through a series of enzymatic reactions: removal of single-strand overhangs at 37 °C for 15 min, DNA damage repair at 37 °C for 30 min, end repair and A-tailing at 20 °C for 10 min and 65 °C for 30 min, ligation of overhang adapters v3 at 20 °C for 60 min followed by 65 °C for 10 min to inactivate the ligase, and nuclease treatment at 37 °C for 1 h. We then purified and concentrated the SMRTbell library with 0.45× Ampure PB beads for size selection using the BluePippin/PippinHT system (Sage Science, Beverly, MA; Cat #BLF7510/HPE7510) to collect fragments greater than 9 kb, with a resulting average fragment size of 16 kb. We sequenced the HiFi SMRTbell library at UC Davis DNA Technologies Core (Davis, CA) using three SMRT Cell 8M trays (PacBio, Cat #101-389-001), Sequel II sequencing chemistry 2.0, and 30-h movies for each cell on a PacBio Sequel II sequencer.
Omni-C library preparation and sequencing
We prepared an Omni-C library from whole blood (ID:AMBB2020-038-001) using a Dovetail Omni-C Kit (Dovetail Genomics, Scotts Valley, CA) according to the manufacturer’s protocol with slight modifications. We crosslinked the chromatin in the nucleus, digested the chromatin with DNase I, repaired chromatin ends and ligated a biotinylated bridge adapter to the repaired ends, reversed the crosslinks, and purified the DNA. We treated purified DNA to remove biotin that was not internal to ligated fragments. We generated a short-read sequencing library using an NEB Ultra II DNA Library Prep kit (New England Biolabs, Ipswich, MA) with an Illumina-compatible y-adaptor. We captured biotin-containing fragments using streptavidin beads. We split the post-capture product into two replicates prior to polymerase chain reaction (PCR) enrichment to preserve library complexity, with each replicate receiving a unique dual index. We sequenced the library at the Vincent J. Coates Genomics Sequencing Laboratory (Berkeley, CA) on the Illumina NovaSeq 6000 platform with an S4 flow cell (Illumina, San Diego, CA).
Nuclear genome assembly
We assembled the genome of U. americanus following the CCGP assembly pipeline version 4.0 (https://github.com/ccgproject/ccgp_assembly). Table 1 lists the software and non-default parameters used in the assembly. First, we removed the remnant adapter sequences from the PacBio HiFi dataset using HiFiAdapterFilt (Sim et al. 2022). We then generated the initial, partially phased, diploid assembly using HiFiasm (Cheng et al. 2022) in Hi-C mode using the adapter-trimmed PacBio HiFi reads and the Omni-C data. Next, we aligned the Omni-C data to the primary assembly following the Arima Genomics Mapping Pipeline (https://github.com/ArimaGenomics/mapping_pipeline) and then scaffolded it with SALSA (Ghurye et al. 2017, 2019).
Table 1.
Assembly pipeline and software used.
| Assembly step | Software and non-default options | Version | References |
|---|---|---|---|
| Filtering PacBio HiFi adapters | HiFiAdapterFilt | Commit 64d1c7b | Sim et al. (2022) |
| K-mer counting | Meryl (k = 21) | 1 | https://github.com/marbl/meryl |
| Estimation of genome size and heterozygosity | GenomeScope | 2 | Ranallo-Benavidez et al. (2020) |
| De novo assembly (contiging) | HiFiasm (HiC Mode, –primary, output hic.p_ctg, hic.a_ctg) | 0.16.1-r375 | Cheng et al. (2022) |
| Scaffolding | |||
| Omni-C data alignment | Arima Genomics Mapping Pipeline | Commit 2e74ea4 | https://github.com/ArimaGenomics/mapping_pipeline |
| Arima Genomics Mapping Pipeline (AGMP) | BWA-MEM | 0.7.17-r1188 | Li (2013) |
| samtools | 1.11 | Danecek et al. (2021) | |
| filter_five_end.pl (AGMP) | Commit 2e74ea4 | https://github.com/ArimaGenomics/mapping_pipeline | |
| two_read_bam_combiner.pl ((AGMP)) | Commit 2e74ea4 | https://github.com/ArimaGenomics/mapping_pipeline | |
| picard | 2.27.5 | https://broadinstitute.github.io/picard/ | |
| Omni-C Scaffolding | SALSA (-DNASE, -i 20, -p yes) | 2 | Ghurye et al. (2017, 2019) |
| Omni-C Contact map generation | |||
| Short-read alignment | BWA-MEM (-5SP) | 0.7.17-r1188 | Li (2013) |
| SAM/BAM processing | samtools | 1.11 | Danecek et al. (2021) |
| SAM/BAM filtering | pairtools | 0.3.0 | Open2C et al. (2023) |
| Pairs indexing | pairix | 0.3.7 | Lee at al. (2022) |
| Matrix generation | cooler | 0.8.10 | Abdennur and Mirny (2020) |
| Matrix balancing | hicExplorer (hicCorrectmatrix correct --filterThreshold -2 4) | 3.6 | Ramírez et al. (2018) |
| Contact map visualization | HiGlass | 2.1.11 | Kerpedjiev et al. (2018) |
| PretextMap | 0.1.4 | https://github.com/wtsi-hpag/PretextView | |
| PretextView | 0.1.5 | https://github.com/wtsi-hpag/PretextMap | |
| PretextSnapshot | 0.0.3 | https://github.com/wtsi-hpag/PretextSnapshot | |
| Manual curation tools | Rapid curation pipeline (Wellcome Trust Sanger Institute, Genome Reference Informatics Team) | Commit 7acf220c | https://gitlab.com/wtsi-grit/rapid-curation |
| Genome quality assessment | |||
| Basic assembly metrics | QUAST (--est-ref-size) | 5.0.2 | Gurevich et al. (2013) |
| Assembly completeness | BUSCO (-m geno, -l mammalia) | 5.0.0 | Manni et al. (2021) |
| Merqury | 2020-01-29 | Rhie et al. (2020) | |
| Contamination screening | |||
| Local alignment tool | BLAST + (-db nt, -outfmt “6 qseqid staxids bitscore std,” -max_target_seqs 1, -max_hsps 1, -evalue 1e-25) | 2.10 | Camacho et al. (2009) |
| General contamination screening | BlobToolKit (PacBIo HiFi Coverage, NCBI Taxa ID = 9643, BUSCODB = mammalia) | 2.3.3 | Challis et al. (2020) |
| Mitochondrial assembly | |||
| Mitochondrial genome assembly | MitoHiFi (-r, -p 90, -o 1) | 2.2 | Uliano-Silva et al. (2023) |
| Synteny analysis | |||
| Sequence alignment tool | mummer (nucmer) | 3.1 | Kurtz et al. (2004) |
We manually curated the primary haplotype by analyzing its Omni-C contact map. To generate the contact map, we aligned the Omni-C data with BWA-MEM (Li 2013), identified ligation junctions, and generated Omni-C pairs (Lee et al. 2022) using pairtools (Open2C et al. 2023). We generated a multi-resolution Omni-C matrix with cooler (Abdennur and Mirny 2020) and balanced it with hicExplorer (Ramírez et al. 2018). We used HiGlass (Kerpedjiev et al. 2018) and the PretextSuite (https://github.com/wtsi-hpag/PretextView; https://github.com/wtsi-hpag/PretextMap; https://github.com/wtsi-hpag/PretextSnapshot) to visualize the contact map in order to identify and break contigs at major misassemblies and misjoin locations. We modified the assembly using the Rapid Curation pipeline (https://gitlab.com/wtsi-grit/rapid-curation). Lastly, we checked for contamination using the BlobToolKit Framework (Challis et al. 2020).
We identified the X chromosome in our assembly using synteny with the domestic dog genome. We used Nucmer (Kurtz et al. 2004) to align a domestic dog X chromosome (NCBI RefSeq GCF_011100685.1, scaffold NC_049260.1) to our assembly and examined hits longer than 10 kb with greater than 80% identity. We also attempted to identify the Y chromosome in our assembly using the same process with a domestic dog Y chromosome (NCBI GenBank KP081776.1).
Genome assembly assessment
We generated k-mer counts from the adapter-trimmed PacBio HiFi reads using Meryl (https://github.com/marbl/meryl). We used these k-mer counts in GenomeScope2.0 (Ranallo-Benavidez et al. 2020) to estimate genome features, including genome size, heterozygosity, and repeat content. To obtain general contiguity metrics, we ran QUAST (Gurevich et al. 2013). To evaluate genome quality and functional completeness we used BUSCO (Manni et al. 2021) with the Mammalia ortholog database (mammalia_odb10), which contains 9,226 genes. We assessed base-level accuracy and k-mer completeness using Merqury (Rhie et al. 2020) with the previously generated Meryl database. We further estimated genome assembly accuracy using a frameshift analysis of the BUSCO gene set, as described in Korlach et al. (2017). We determined the size of the phased blocks based on the size of the contigs generated by HiFiasm in HiC mode. Following the quality metric nomenclature established by Rhie et al. (2021), we calculated the genome quality code x.y.P.Q.C, where, x = log10[contig NG50]; y = log10[scaffold NG50]; P = log10 [phased block NG50]; Q = Phred base accuracy QV (quality value); C = % genome represented by the first “n” scaffolds, following a karyotype of 2n = 74 (Nash and O’Brien 1987). We calculated these quality code metrics for the primary assembly.
We compared genome statistics for our assembly (mUrsAme1) to two other black bear genome assemblies available: ASM334442v1 (NCBI Genome: GCA_003344425.1) and gsx_jax_bbear_1 (NCBI RefSeq GCF_020975775.1). We generated the contiguity metrics using QUAST and the functional completeness metrics using BUSCO with the Mammalian ortholog database.
Mitochondrial genome assembly
We assembled the mitochondrial genome of U. americanus from the PacBio HiFi reads using the reference-guided pipeline MitoHiFi (Uliano-Silva et al. 2023). We used an existing mitochondrial sequence of U. americanus (NCBI:AF303109.1; Delisle and Strobeck 2002) as the starting reference sequence. We searched for matches of the resulting mitochondrial assembly sequence in the nuclear genome assembly using BLAST+ (Camacho et al. 2009) and filtered out contigs and scaffolds from the nuclear genome assembly with a sequence identity >99% and size smaller than the mitochondrial assembly sequence. We annotated the mitochondrial genome using MitoFinder (Allio et al. 2020).
Results
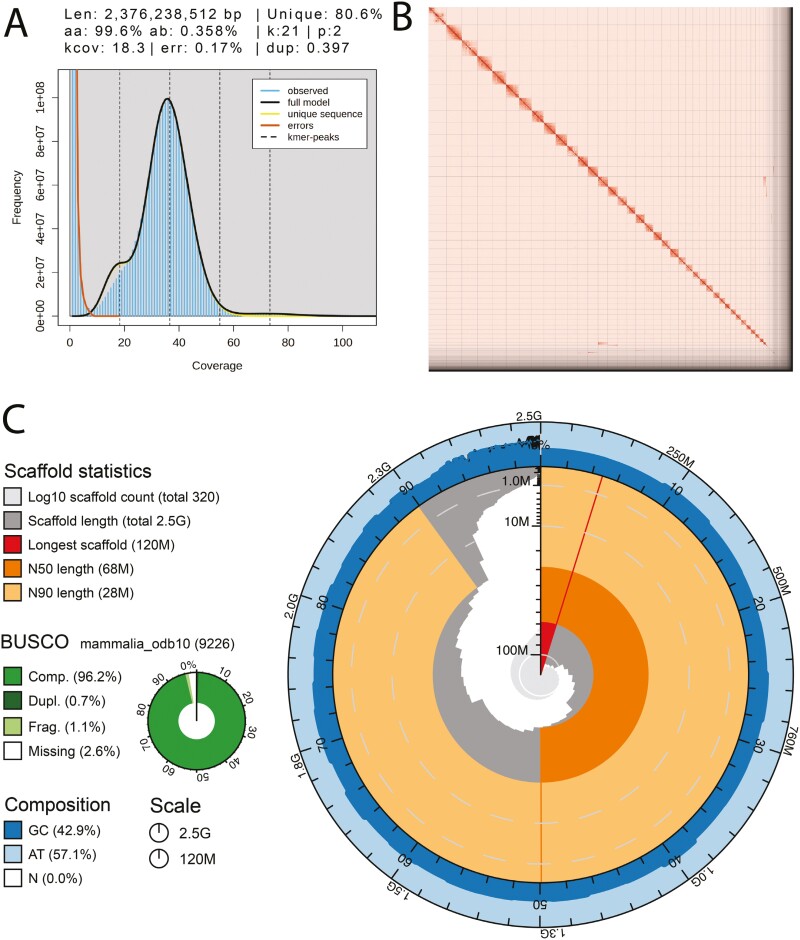
The Omni-C library generated 130.9 million read pairs and the PacBio HiFi library generated 6.1 million reads. The PacBio HiFi sequences yielded ~38× genome coverage and had an N50 read length of 15,293 bp; a minimum read length of 43 bp; a mean read length of 14,915 bp; and a maximum read length of 52,231 bp (see Supplementary Fig. S1 for read length distribution). Based on the PacBio HiFi data, Genomescope 2.0 estimated a genome size of 2.37 Gb, a 0.17% sequencing error rate, and 0.358% heterozygosity. The k-mer spectrum shows a bimodal distribution with a major coverage peak at ~37× coverage and a minor coverage peak at ~18× coverage (Fig. 2A).
Fig. 2.
Visualization of assembly metrics. (A) K-mer frequencies from the adapter-trimmed PacBio HiFi data used to estimate genome size, sequencing error rate, and heterozygosity. The main peak at ~37× coverage corresponds to homozygous regions of the genome, while the slight peak at ~18× corresponds to heterozygous regions of the genome. The peak around zero corresponds to probable sequencing errors. (B) The omni-C contact map for the primary assembly after manual curation shows the 3D organization of the genome, with darker areas indicating closer proximity. (C) Snail plot displaying assembly metrics for the primary assembly.
The final genome assembly (mUrsAme1) consists of two partially phased haplotypes. Both assemblies are similar in size, but not equal to the estimated genome size from GenomeScope2.0, as has been observed in other taxa (see Pflug et al. 2020, for example). The primary assembly (mUrsAme1.0.p) consists of 316 scaffolds spanning 2.52 Gb with a contig N50 of 58.85 Mb, a scaffold N50 of 67.55 Mb, the largest contig size of 107.13 Mb, and the largest scaffold size of 122.37 Mb. Given the level of fragmentation of the alternate assembly, we kept it as a contig-level assembly. The alternate assembly (mUrsAme1.0.a) consists of 77,310 contigs spanning 2.88 Gb with a contig N50 of 60.74 kb and the largest contig size of 831.37 kb. The fragmentation of the alternate assembly is likely due to the low heterozygosity of the sampled individual because the alternate assembly represents heterozygous regions of the genome.
The primary assembly has a BUSCO completeness score for the Mammalia gene set of 96.30%, a base pair QV of 63.01, k-mer completeness of 98.18%, and a frameshift indel QV of 43.13. The alternate assembly has a BUSCO completeness score for the Mammalia gene set of 62.6%, a base pair QV of 56.97, a k-mer completeness of 75.54%, and a frameshift indel QV of 42.81.
During manual curation, we made 11 joins and 1 break on the primary assembly based on the initial Omni-C contact map. We filtered out 4 contigs corresponding to mitochondrial contamination, one from the primary assembly and 3 from the alternate assembly. No further contigs were removed. The Omni-C contact map for the final primary assembly shows a highly contiguous assembly (Fig. 2B). Assembly statistics are reported in Table 2 and represented graphically in Fig. 2C. We have deposited the genome assembly on NCBI GenBank (see Table 2 and Data Availability for details).
Table 2.
Assembly statistics and data availability.
| Bio Projects and Vouchers | CCGP NCBI BioProject | PRJNA720569 | |||||
| Genera NCBI BioProject | PRJNA765883 | ||||||
| Species NCBI BioProject | PRJNA777227 | ||||||
| NCBI BioSample | SAMN29046565 | ||||||
| Specimen identification | L20-20 | ||||||
| NCBI Genome accessions | Primary | Alternate | |||||
| Assembly accession | JANIGQ000000000 | JANIGR000000000 | |||||
| Genome sequences | GCA_024610735.1 | GCA_024610745.1 | |||||
| Genome Sequence | PacBio HiFi reads | Run | 1 PACBIO_SMRT (Sequel II) run: | ||||
| 6.1M spots, 90.4G bases, 48.9G bytes | |||||||
| Accession | SRX17388741 | ||||||
| Omni-C Illumina reads | Runs | 2 ILLUMINA (Illumina NovaSeq 6000) runs: | |||||
| 130.9M spots, 39.5G bases, 13.9G bytes | |||||||
| Accessions | SRX23638327, SRX23638328 | ||||||
| Genome Assembly Quality Metrics | Assembly identifier (Quality codea) | mUrsAme1(7.7.P7.Q58.C91) | |||||
| HiFi Read coverageb | 38.02× | ||||||
| Primary | Alternate | ||||||
| Number of contigs | 339 | 77,310 | |||||
| Contig N50 (bp) | 58,859,121 | 43,280 | |||||
| Contig NG50b | 59,189,856 | 60,742 | |||||
| Longest Contigs | 107,133,695 | 831,372 | |||||
| Number of scaffolds | 316 | 77,310 | |||||
| Scaffold N50 | 67,550,933 | 43,280 | |||||
| Scaffold NG50b | 68,367,985 | 60,742 | |||||
| Largest scaffold | 122,379,270 | 831,372 | |||||
| Size of final assembly | 2,524,264,886 | 2,885,111,500 | |||||
| Phased block NG50b | 59,189,856 | 60,747 | |||||
| Gaps per Gbp (# Gaps) | 9 (22) | 0 (0) | |||||
| Indel QV (Frame shift) | 43.1369853 | 42.81941933 | |||||
| Base pair QV | 63.0115 | 56.9775 | |||||
| Full assembly = 58.8514 | |||||||
| k-mer completeness | 98.187 | 75.5469 | |||||
| Full assembly = 99.6329 | |||||||
| BUSCO completeness (mammalia_odb10) n = 9226 | C c | S c | D c | F c | M c | ||
| P d | 96.30% | 95.60% | 0.70% | 1.10% | 2.60% | ||
| A d | 62.60% | 58.00% | 4.60% | 7.80% | 29.60% | ||
| Organelles | 1 Partial mitochondrial sequence | JANIGQ010000317.1 | |||||
aAssembly quality code x.y.P.Q.C derived notation, from Rhie et al. (2021). x = log10[contig NG50]; y = log10[scaffold NG50]; P = log10 [phased block NG50]; Q = Phred base accuracy QV (quality value); C = % genome represented by the first “n” scaffolds, following a known karyotype for U. amerianus of 2n = 74 (Nash and O’Brien 1987). Quality code metrics were calculated from the primary assembly (mUrsAme1.0.p).
bRead coverage and NGx statistics have been calculated based on the estimated genome size of 2.37 Gb.
cBUSCO Scores. Complete BUSCOs (C). Complete and single-copy BUSCOs (S). Complete and duplicated BUSCOs (D). Fragmented BUSCOs (F). Missing BUSCOs (M).
d(P)rimary and (A)lternate assembly values.
Our assembly shows improved contiguity compared to other available assemblies for black bears (Table 3). Our primary assembly is represented in fewer contigs and scaffolds and has higher N50 statistics. The BUSCO scores for both our primary assembly and a previously assembled genome (GCF_020975775.1) are >95%, indicating that both assemblies are complete and single copies in these conserved regions of the genome.
Table 3.
Comparison of genome assembly statistics.
| mUrsAme1.0.p | mUrsAme1.0.a | ASM334442v1 | gsc_jax_bbear_1 | |
|---|---|---|---|---|
| NCBI Accession | GCA_024610735.1 | GCA_024610745.1 | GCA_003344425.1 | GCF_020975775.1 |
| Number of contigs | 339 | 77,310 | 101,411 | 2,213 |
| Contig N50 (bp) | 58,859,121 | 43,280 | 190,236 | 13,882,922 |
| Contig NG50a | 59,189,856 | 60,742 | 210,302 | 13,882,922 |
| Longest Contigs | 107,133,695 | 831,372 | 2,352,914 | 95,818,817 |
| Number of scaffolds | 316 | 77,310 | 374,624 | 2,213 |
| Scaffold N50 | 67,550,933 | 43,280 | 11,835 | 13,882,922 |
| Scaffold NG50a | 68,367,985 | 60,742 | 12,107 | 13,882,922 |
| Largest scaffold | 122,379,270 | 831,372 | 141,485 | 95,818,817 |
| Size of final assembly | 2,524,264,886 | 2,885,111,500 | 2,584,460,632 | 2,351,964,450 |
| Gaps per Gbp (# Gaps) | 9 (22) | 0 (0) | 144,952 (353,480) | 0 (0) |
| BUSCO Scores (mammalia, n = 9,226) | ||||
| C b | 96.30% | 62.60% | 85.20% | 95.90% |
| S b | 95.60% | 58.00% | 84.40% | 95.30% |
| D b | 0.70% | 4.60% | 0.80% | 0.60% |
| F b | 1.10% | 7.80% | 6.00% | 1.20% |
| M b | 2.60% | 29.60% | 8.80% | 2.90% |
aNGx statistics calculated with an estimated genome size of 2.37 Gbp.
bBUSCO Scores. Complete BUSCOs (C). Complete and single-copy BUSCOs (S). Complete and duplicate BUSCOs (D). Fragmented BUSCOs (F). Missing BUSCOs (M).
We examined chromosome assignments and determined that our assembly is near-chromosome level. We identified scaffold JANIGQ010000001.1 in our assembly as the X chromosome based on synteny with the domestic dog genome. No scaffolds in our assembly had alignments to the domestic dog Y chromosome that matched our criteria of longer than 10 kb with greater than 80% identity. A handful of scaffolds had shorter alignments, indicating that the Y chromosome in our assembly is fragmented. We did not attempt to assign scaffolds in our assembly to autosomes based on the black bear karyotype (Nash and O’Brien 1987). However, we note that with a karyotype indicating 2n = 74 chromosomes, 92% of our assembly is contained in the 37 largest scaffolds (with the largest scaffold identified as the X chromosome), suggesting our assembly is near-chromosome level.
The final mitochondrial sequence has a size of 16,789 bp, with the base composition of A = 31.21%, C = 25.09%, G = 15.44%, T = 28.26%, and consisting of 2 rRNAs, 23 unique transfer RNAs, and 13 protein-coding genes. We evaluate the mitochondrial genome to be partial because while it is close to the expected size, the expected circularity is not supported. Additionally, while we annotated the expected number of rRNAs and protein-coding genes, the number of transfer RNAs differs from expected. The mitochondrial genome is scaffolded JANIGQ010000317.1 in our assembly.
Discussion
We generated a high-quality, near chromosome-level genome assembly for an American black bear from California. This new genome will serve as the foundation for landscape and population genomic analyses that will aid conservation decision-makers. Large mammals can serve as umbrella species, whose conservation can extend protections to other species in the same habitat, and healthy bear populations are often an indication of ecosystem health (Pelton et al. 1999). The genome assembly is a foundational component of studies on the effects of habitat loss and fragmentation on wildlife populations, particularly the impacts of local adaptation and inbreeding depression.
This new black bear genome assembly expands opportunities for pangenomic analyses within the species. Both previously assembled genomes are from the eastern United States, whereas our new genome is from the western United States, enabling comparisons to identify potentially adaptive genomic differences to different habitats and anthropogenic pressures. For example, it is known that hibernation length and coat color vary across the range of black bears (Gámez-Brunswick and Rojas-Soto 2020; Puckett et al. 2023).
This new black bear genome assembly also expands opportunities for comparative genomic analyses among bear species. There are 8 extant species of bears, and all of them have high-quality reference genomes available or in progress (Willey and Korstanje 2022; Beth Shapiro, personal communication). These bear species live in diverse habitats from the Arctic to the Tropics and survive on a variety of diets, including both generalist and specialist diets (Pelton et al. 1999). The availability of genome assemblies for species with divergent ecological pressures and phenotypes enables the identification of both coding and regulatory variation that may underlie ecologically important variation. The inclusion of additional individuals and/or species into taxonomically broad multi-species alignments, such as the Zoonomia alignment of placental mammals (Christmas et al. 2023), may be useful in identifying adaptations unique to bears, in addition to functional variation that may be important for black bear conservation.
Supplementary material
Supplementary material is available at Journal of Heredity Journal online.
Acknowledgments
We thank the California Department of Fish and Wildlife staff for collecting the reference sample. The DNA Technologies and Expression Analysis Cores at the UC Davis Genome Center carried out the PacBio Sequel II library prep and sequencing, supported by NIH Shared Instrumentation Grant 1S10OD010786-01. The Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley conducted the deep sequencing of the Omni-C libraries using the NovaSeq 6000 sequencing platform, supported by NIH Instrumentation Grant S10 OD018174. We thank the staff at the UC Davis DNA Technologies and Expression Analysis Cores and the UC Santa Cruz Paleogenomics Laboratory for their diligence and dedication to generating high-quality sequence data.
Contributor Information
Megan A Supple, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States; Howard Hughes Medical Institute, University of California, Santa Cruz, CA, United States.
Merly Escalona, Department of Biomolecular Engineering, University of California, Santa Cruz, CA, United States.
Jillian Adkins, Wildlife Forensic Lab, Law Enforcement Division, California Department of Fish and Wildlife, Sacramento, CA, United States.
Michael R Buchalski, Wildlife Genetics Research Unit, Wildlife Health Laboratory, California Department of Fish and Wildlife, Sacramento, CA, United States.
Nicolas Alexandre, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States; Howard Hughes Medical Institute, University of California, Santa Cruz, CA, United States.
Ruta M Sahasrabudhe, DNA Technologies and Expression Analysis Core Laboratory, Genome Center, University of California, Davis, CA, United States.
Oanh Nguyen, DNA Technologies and Expression Analysis Core Laboratory, Genome Center, University of California, Davis, CA, United States.
Samuel Sacco, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States.
Colin Fairbairn, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States.
Eric Beraut, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States.
William Seligmann, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States.
Richard E Green, Department of Biomolecular Engineering, University of California, Santa Cruz, CA, United States.
Erin Meredith, Wildlife Forensic Lab, Law Enforcement Division, California Department of Fish and Wildlife, Sacramento, CA, United States.
Beth Shapiro, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, United States; Howard Hughes Medical Institute, University of California, Santa Cruz, CA, United States.
Funding
This work was supported by the California Conservation Genomics Project, with funding provided to the University of California by the State of California, State Budget Act of 2019 [UC Award ID RSI-19-690224]; and a Pittman-Robertson Wildlife Restoration Act grant awarded by the U.S Fish and Wildlife Service to California Department of Fish and Wildlife [F23AF00999-00].
Author contributions
Megan Supple (Conceptualization, Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing), Merly Escalona (Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing), Jillian Adkins (Investigation, Writing – review & editing), Michael Buchalski (Investigation, Writing – review & editing), Nicolas Alexandre (Investigation, Writing – review & editing), Ruta Sahasrabudhe (Investigation, Writing – review & editing), Oanh Nguyen (Investigation, Writing – review & editing), Samuel Sacco (Investigation, Writing – review & editing), Colin Fairbairn (Investigation, Writing – review & editing), Eric Beraut (Investigation, Writing – review & editing), William Seligmann (Investigation, Writing – review & editing), Richard Green (Methodology, Supervision), Erin Meredith (Conceptualization, Funding acquisition, Project administration, Supervision), and Beth Shapiro (Conceptualization, Funding acquisition, Project administration, Supervision)
Data availability
Data generated for this study are available under NCBI BioProject PRJNA777227. Raw sequencing data for sample L20-20 (NCBI BioSample SAMN29046565) are deposited in the NCBI Short Read Archive (SRA) under experiment accessions SRX17388741 (PacBio HiFi sequencing data) and SRX23638327-SRX23638328 (Omni-C Illumina sequencing data). GenBank accessions for the assemblies are GCA_024610735.1 (primary) and GCA_024610745.1 (alternate), with nucleotide sequences under accessions JANIGQ000000000 and JANIGR000000000, respectively. The partial mitochondrial nucleotide sequence can be found with GenBank accession JANIGQ010000317.1. Assembly workflow is available at www.github.com/ccgproject/ccgp_assembly.
References
- Abdennur N, Mirny LA.. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020:36:311–316. doi: 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, Prosdocimi F, Nabholz B, Delsuc F.. MitoFinder: efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020:20:892–905. doi: 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Fish and Game. Black Bear Management Plan. 1998. [Accessed 14 February 2024].https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=82769&inline [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL.. BLAST+: architecture and applications. BMC Bioinf. 2009:10:421. doi: 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, Cochrane G, Blaxter M.. BlobToolKit—interactive quality assessment of genome assemblies. G3 Genes Genomes Genetics. 2020:10:1361–1374. doi: 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jarvis ED, Fedrigo O, Koepfli K-P, Urban L, Gemmell NJ, Li H.. Haplotype-resolved assembly of diploid genomes without parental data. Nature Biotechnology. 2022:40:1332–1335. doi: 10.1038/s41587-022-01261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas MJ, Kaplow IM, Genereux DP, Dong MX, Hughes GM, Li X, Sullivan PF, Hindle AG, Lindblad-Toh K, Karlsson EK; Zoonomia Consortium. Evolutionary constraint and innovation across hundreds of placental mammals. Science. 2023:380:eabn3943. doi: 10.1126/science.abn3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021:10:giab008. doi: 10.1093/gigascience/giab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle I, Strobeck C.. Conserved primers for rapid sequencing of the complete mitochondrial genome from carnivores, applied to three species of Bears. Mol Biol Evol. 2002:19:357–361. doi: 10.1093/oxfordjournals.molbev.a004090 [DOI] [PubMed] [Google Scholar]
- Gámez-Brunswick C, Rojas-Soto O.. The effect of seasonal variation on the activity patterns of the American black bear: an ecological niche modeling approach. Mammalia. 2020:84:315–322. doi: 10.1515/mammalia-2019-0017 [DOI] [Google Scholar]
- Ghurye J, Pop M, Koren S, Bickhart D, Chin C-S.. Scaffolding of long read assemblies using long range contact information. BMC Genomics. 2017:18:527. doi: 10.1186/s12864-017-3879-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghurye J, Rhie A, Walenz BP, Schmitt A, Selvaraj S, Pop M, Phillippy AM, Koren S.. Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput Biol. 2019:15:e1007273. doi: 10.1371/journal.pcbi.1007273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G.. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013:29:1072–1075. doi: 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, McCallum C, Dinkla K, Strobelt H, Luber JM, Ouellette SB, Azhir A, Kumar N, et al. HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018:19:125. doi: 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlach J, Gedman G, Kingan SB, Chin C-S, Howard JT, Audet J-N, Cantin L, Jarvis ED.. De novo PacBio long-read and phased avian genome assemblies correct and add to reference genes generated with intermediate and short reads. GigaScience. 2017:6:gix085. doi: 10.1093/gigascience/gix085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL.. Versatile and open software for comparing large genomes. Genome Biol. 2004:5:R12. doi: 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Bakker CR, Vitzthum C, Alver BH, Park PJ.. Pairs and Pairix: a file format and a tool for efficient storage and retrieval for Hi-C read pairs. Bioinformatics. 2022:38:1729–1731. doi: 10.1093/bioinformatics/btab870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv, arXiv:1303.3997. http://arxiv.org/abs/1303.3997, preprint: not peer reviewed. [Google Scholar]
- Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM.. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021:38:4647–4654. doi: 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash WG, O’Brien SJ.. A comparative chromosome banding analysis of the Ursidae and their relationship to other carnivores. Cytogenet Cell Genet. 1987:45:206–212. doi: 10.1159/000132455 [DOI] [PubMed] [Google Scholar]
- Open2C, Abdennur N, Fudenberg G, Flyamer IM, Galitsyna AA, Goloborodko A, Imakaev M, Venev SV.. Pairtools: from sequencing data to chromosome contacts. 2023. bioRxiv 2023.02.13.528389. 10.1101/2023.02.13.528389, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton MR, Coley AB, Eason TH, Doan Martinez DL, Pederson JA, van Manen FT, Weaver KM.. American Black Bear Conservation Action Plan. In: Servheen C, Herrero S, Peyton B, editors. Bears: Status Survey and Conservation Action Plan. Cambridge, UK: IUCN; 1999. p. 144–156. [Google Scholar]
- Pflug JM, Holmes VR, Burrus C, Johnston JS, Maddison DR.. Measuring genome sizes using Read-Depth, k-mers, and flow cytometry: methodological comparisons in beetles (Coleoptera). G3 (Bethesda, Md.). 2020:10:3047–3060. doi: 10.1534/g3.120.401028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett EE, Davis IS, Harper DC, Wakamatsu K, Battu G, Belant JL, Beyer DE, Carpenter C, Crupi AP, Davidson M, et al. Genetic architecture and evolution of color variation in American black bears. Curr Biol. 2023:33:86–97.e10. doi: 10.1016/j.cub.2022.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Bhardwaj V, Arrigoni L, Lam KC, Grüning BA, Villaveces J, Habermann B, Akhtar A, Manke T.. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat Commun. 2018:9:189. doi: 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranallo-Benavidez TR, Jaron KS, Schatz MC.. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat Commun. 2020:11:1432. doi: 10.1038/s41467-020-14998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, Damas J, Formenti G, Koren S, Uliano-Silva M, Chow W, Fungtammasan A, Kim J, et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021:592:737–746. doi: 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, Phillippy AM.. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020:21:245. doi: 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HB, Toffelmier E, Corbett-Detig RB, Escalona M, Erickson B, Fiedler P, Gold M, Harrigan RJ, Hodges S, Luckau TK, et al. Landscape genomics to enable conservation actions: The California Conservation Genomics Project. J Hered. 2022:113:577–588. doi: 10.1093/jhered/esac020 [DOI] [PubMed] [Google Scholar]
- Sim SB, Corpuz RL, Simmonds TJ, Geib SM.. HiFiAdapterFilt, a memory efficient read processing pipeline, prevents occurrence of adapter sequence in PacBio HiFi reads and their negative impacts on genome assembly. BMC Genomics. 2022:23:157. doi: 10.1186/s12864-022-08375-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Kumar Sarsani V, Fiddes I, Sheehan SM, Seger RL, Barter ME, Neptune-Bear S, Lindqvist C, Korstanje R.. Genome assembly and gene expression in the American black bear provides new insights into the renal response to hibernation. DNA Res. 2019:26:37–44. doi: 10.1093/dnares/dsy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, Formenti G, Abueg L, Torrance J, Myers EW, Durbin R, Blaxter M, McCarthy SA; Darwin Tree of Life Consortium. MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinf. 2023:24:288. doi: 10.1186/s12859-023-05385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder AP, Supple MA, Subramanian A, Mudide A, Swofford R, Serres-Armero A, Steiner C, Koepfli K-P, Genereux DP, Karlsson EK, et al. ; Zoonomia Consortium‡. The contribution of historical processes to contemporary extinction risk in placental mammals. Science. 2023:380:eabn5856. doi: 10.1126/science.abn5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey C, Korstanje R.. Sequencing and assembling bear genomes: the bare necessities. Front Zool. 2022:19:30. doi: 10.1186/s12983-022-00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated for this study are available under NCBI BioProject PRJNA777227. Raw sequencing data for sample L20-20 (NCBI BioSample SAMN29046565) are deposited in the NCBI Short Read Archive (SRA) under experiment accessions SRX17388741 (PacBio HiFi sequencing data) and SRX23638327-SRX23638328 (Omni-C Illumina sequencing data). GenBank accessions for the assemblies are GCA_024610735.1 (primary) and GCA_024610745.1 (alternate), with nucleotide sequences under accessions JANIGQ000000000 and JANIGR000000000, respectively. The partial mitochondrial nucleotide sequence can be found with GenBank accession JANIGQ010000317.1. Assembly workflow is available at www.github.com/ccgproject/ccgp_assembly.