Abstract
Background
Despite increasing demand for breast capsular surgery to treat various benign and malignant implant-related pathologies, high-quality evidence elucidating complication profiles of capsulectomy and capsulotomy is lacking.
Objectives
The aim of this study was to provide the largest-scale analysis of associated outcomes and complications using the Tracking Operations and Outcomes for Plastic Surgeons (TOPS) database, and to investigate clinical scenarios that may subject patients to increased risks for complications, most notably extent of capsular surgery (complete vs partial) and index indication of implantation (aesthetic vs reconstructive).
Methods
An analysis of the TOPS database from 2008 to 2019 was performed. CPT codes were used to identify complete capsulectomy and partial capsulectomy/capsulotomy cases. Breast implant exchange procedures constituted procedural controls.
Results
In total, 7486 patients (10,703 breasts) undergoing capsulectomy or capsulotomy were assessed. Relative to controls, capsulectomy (4.40% vs 5.79%), but not capsulotomy (4.40% vs 4.50%), demonstrated higher overall complication rates. Both capsulectomies (0.83% vs 0.23%) and capsulotomies (0.56% vs 0.23%) also had greater rates of seroma relative to controls. Subgroup analyses demonstrated that reconstructive patients, relative to aesthetic patients, experienced greater overall complications (6.76% vs 4.34%), and increased risks for seroma (1.06% vs 0.47%), dehiscence (0.46% vs 0.14%), surgical site infections (1.03% vs 0.23%), and implant loss (0.52% vs 0.23%). A detailed synthesis of 30-day outcomes, including all patient- and breast-specific complications, for both capsulectomy and capsulotomy, stratified according to all potential confounders, is presented herein.
Conclusions
Surgeries on the breast capsule are safe overall, although complete capsulectomies and reconstructive patients are associated with significantly increased operative risks. The present findings will enhance patient selection, counseling, and informed consent.
Level of Evidence: 3

See the Commentary on this article here.
The demand for breast implant capsulectomy has been increasing in recent years.1,2 Beyond capsular contracture and breast implant rupture, capsulectomy is also extensively discussed in the face of increasingly prevalent breast implant illness (BII), breast implant–associated anaplastic large cell lymphoma (BIA-ALCL), and more recently, breast implant–associated squamous cell carcinoma.3-13
In this context, high-quality outcome data are necessary for proper preoperative counseling and informed consent. However, studies to date are limited by modest sample sizes, narrow time intervals, and single-center perspectives, and fail to consider procedural variations (complete capsulectomy, partial capsulectomy, capsulotomy), as well as key differences between aesthetic and reconstructive breast surgery.14-17 There thus remains a need for further detailed and evidence-based insight into the complication rate profile of capsulectomy and its variants in order to better elucidate their associated risks for patients seeking these procedures.18
Tracking Operations and Outcomes for Plastic Surgeons (TOPS), overseen by the American Society of Plastic Surgeons, is a national plastic surgery–specific database hosting more than 1.6 million surgeon-reported procedures and their associated outcomes, constituting the largest available aggregation of plastic surgery data.19 By providing information specific to plastic surgery, it allows for rigorous investigations of the outcomes of plastic surgery procedures. Our study seeks to leverage this database to provide a complete and thorough description of the early (30-day) complication profile of capsulectomy and its variants, in both aesthetic and reconstructive patients. We hypothesize that complication rates are greater for capsulectomy vs capsulotomy and in reconstructive compared with aesthetic patients. As an evidence-based reference on outcomes associated with this procedure, these findings will enhance clinical decision-making, patient counseling, and informed consent associated with capsulectomy and its variants.
METHODS
Study Population
The TOPS database was retrospectively queried for dates ranging between January 1, 2008 and December 31, 2019. Patients undergoing complete capsulectomy (hereafter, “capsulectomy”) or partial capsulectomy/capsulotomy (hereafter, “capsulotomy”), with or without concurrent implant exchange, were included. All patients undergoing concomitant procedures were excluded. A cohort of control patients undergoing solely breast implant exchange were included and assessed separately as suitable surgical controls.
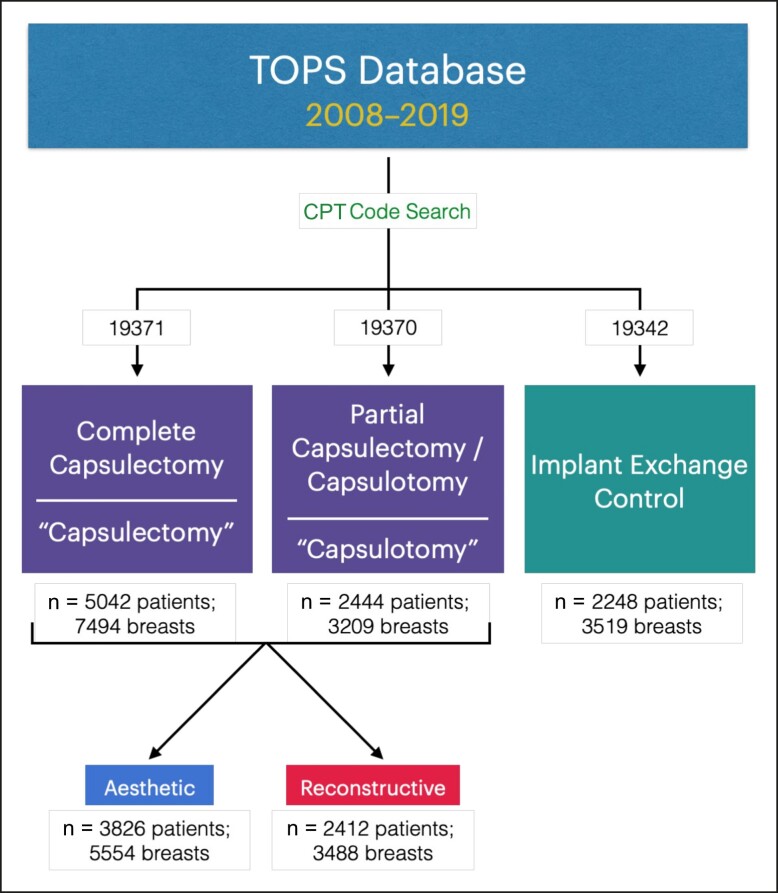
Relevant patient entries were identified using Current Procedural Terminology (CPT) codes.20,21 Capsulectomy and capsulotomy were captured with CPT codes 19371 and 19370, respectively, with concurrent implant reinsertion represented by 19340, 19342, or 19357.20,21 Implant exchange was represented by CPT 19342. TOPS's internal classification system served to stratify reconstructive and aesthetic patients, although a minority of entries were unclassified and thus precluded from subgroup analysis (Figure 1).
Figure 1.
Study selection and stratification flowchart. TOPS, Tracking Operations and Outcomes for Plastic Surgeons; CPT, Current Procedural Terminology.
Data Analysis
Python (version 3.9.7, Python Software Foundation, Wilmington, DE) and Microsoft Excel (version 16.67, Microsoft Corp., Redmond, WA) were used for data processing and analysis. Data extracted comprised demographic, patient-related, procedure-related, implant-related, as well as 30-day outcome-related information. Outcomes were stratified as either systemic or breast-specific, calculated on a per-patient basis or a per-breast basis, respectively. Since TOPS reports on all complications at the patient level, breast-specific complications stemming from bilateral procedures were assumed to only affect a single breast. Statistical analyses by Pearson's chi-squared or Fisher's exact test, t-test, and the Mann-Whitney U test were performed on GraphPad Prism (version 9.4.0, GraphPad Software Inc.). P-values <.05 were considered statistically significant.
RESULTS
A total of 5042 patients (7494 breasts) undergoing capsulectomy and 2444 patients (3209 breasts) undergoing capsulotomy were assessed; the control group comprised 2248 patients (3519 breasts) undergoing breast implant exchange without capsulectomy or capsulotomy.
Demographics
Study groups had comparable mean ages (capsulectomy, 51.37 years [range, 16-78 years]; capsulotomy, 46.40 years [range, 14-80 years]; control, 52.01 years [range, 13-80 years]), weight, and BMI, with patients being predominantly Caucasians, nonsmokers, and nondiabetics. Most patients were of female gender (capsulectomy, n = 5017/5042; capsulotomy, n = 2431/2444; control, n = 2239/2248). Most capsulectomy and capsulotomy patients were American Society of Anesthesiologists (ASA) Class I, whereas the control group saw a greater proportion of ASA Class II patients. Self-pay was the most prevalent financing avenue for capsulectomy and capsulotomy, whereas private insurance was the predominant financing method for control patients (Supplemental Table 1).
Procedure-Related Information
High variability in surgical duration was reflected by high standard deviations of the mean duration of surgery across all groups examined (capsulectomy, 94.73 [60.27] minutes; capsulotomy, 74.66 [64.53] minutes; control, 82.44 [41.90] minutes). Facility type distribution was similar between capsulectomy and capsulotomy groups, with implant exchanges more frequently performed in hospitals rather than private surgical practices. Admission type was predominantly outpatient, whereas anesthesia was general and administered by anesthesiologists in all groups (Supplemental Table 2).
Outcomes and Complications
Systemic Complications
The overall rate of any adverse event, calculated on a per-patient basis, amounted to 5.79% (n = 292/5042) for the capsulectomy cohort, 4.50% (n = 110/2444) for the capsulotomy cohort, and 4.40% (n = 99/2248) for the control, implant exchange cohort. A significant difference in complication rates was observed when comparing capsulectomy with both control (P = .0154) and capsulotomy procedures (P = .0216). However, no significant differences in complications were observed when comparing capsulotomy with controls (P = .8876). Systemic complications included thromboembolic events (DVT/PE) with an incidence of 0.06% in the capsulectomy group, 0% in the capsulotomy group, and 0.04% in controls. Incidence of pneumothorax was 0.06% for capsulectomy, 0% for capsulotomy, and 0.04% for controls; it should be noted that rates may be underestimated given that pneumothorax may also be reported as “other respiratory occurrence” in the TOPS database, of which an incidence of 0.04% was provided for capsulectomy, and 0% for capsulotomy and controls.22 This could thus result in a pneumothorax rate of up to 0.10% in the capsulectomy cohort. Two further cases (0.04%) of cardiac events and 2 cases (0.04%) of pneumonia were also reported for patients receiving capsulectomies, whereas only 1 case of a cardiac event (0.04%) was reported in the control cohort (Table 1).
Table 1.
Systemic Complications per Patient
| Capsulectomy (N = 5042) | Capsulotomy (N = 2444) | Exchange control (N = 2248) | P-value | |||
|---|---|---|---|---|---|---|
| Capsulectomy vs control | Capsulotomy vs control | Capsulectomy vs capsulotomy | ||||
| Any adverse event | 5.79% (292) | 4.50% (110) | 4.40% (99) | .0154a | .8876 | .0216a |
| Mortality | 0.02% (1) | 0.00% (0) | 0.00% (0) | 1.0000 | 1.0000 | 1.0000 |
| DVT or PE | 0.06% (3) | 0.00% (0) | 0.04% (1) | 1.0000 | .4791 | .5556 |
| Pneumothorax | 0.06% (3) | 0.00% (0) | 0.04% (1) | 1.0000 | .4791 | .5556 |
| Bleeding requiring transfusion | 0.06% (3) | 0.04% (1) | 0.00% (0) | .5574 | 1.0000 | 1.0000 |
| Cardiac complications | 0.04% (2) | 0.00% (0) | 0.04% (1) | 1.0000 | .5577 | 1.0000 |
| Respiratory complications | 0.08% (4) | 0.00% (0) | 0.00% (0) | .0515 | 1.0000 | .3108 |
| Need for oral antibiotics | 1.23% (62) | 1.27% (31) | 1.16% (26) | .9076 | .7902 | .9115 |
| Need for intravenous antibiotics | 0.46% (23) | 0.49% (12) | 0.49% (11) | .8533 | 1.0000 | .8574 |
| Unplanned ER visit | 0.28% (14) | 0.04% (1) | 0.13% (3) | .3009 | .3553 | .0291a |
| Unplanned readmission | 0.61% (31) | 0.45% (11) | 0.67% (15) | .8728 | .3324 | .4137 |
| Unplanned return to OR | 1.88% (95) | 1.60% (39) | 2.00% (45) | .7129 | .3220 | .4040 |
Values are % (n). DVT, deep vein thrombosis; ER, emergency room; OR, operating room; PE, pulmonary embolism. aStatistically significant.
Postoperatively, 3 (0.06%) capsulectomy patients, 1 (0.04%) capsulotomy patient, and no (0.00%) control patient experienced serious bleeding requiring transfusion. Incidences of oral and intravenous antibiotic administration were consistent across all 3 groups. Unplanned emergency room (ER) visits were less frequent following capsulotomy than capsulectomy (P = .0291), while rates of unplanned readmission and reoperation were similar across the board (Table 1). One case of mortality (0.02%) was reported in the capsulectomy cohort, attributable to pre-existing comorbidities rather than to capsular surgery itself.
Breast-Specific Complications
Breast-specific complications observed in the capsulectomy cohort included seroma at 0.83%, hematoma at 1.00%, wound dehiscence at 0.24%, superficial surgical site infection at 0.20%, and deep surgical site infection at 0.31%. The capsulotomy cohort experienced seroma at 0.56%, hematoma at 0.56%, wound dehiscence at 0.44%, superficial surgical site infection at 0.19%, and deep surgical site infection at 0.44% (Table 2). Only hematoma rates significantly differed between capsulectomy and capsulotomy (P = .0231).
Table 2.
Breast-Related Complications per Breast
| Capsulectomy (N = 7494) | Capsulotomy (N = 3209) | Exchange control (N = 3519) | P value | |||
|---|---|---|---|---|---|---|
| Capsulectomy vs control | Capsulotomy vs control | Capsulectomy vs capsulotomy | ||||
| Seroma | 0.83% (62) | 0.56% (18) | 0.23% (8) | <.0001b | .0307b | .1772 |
| Hematoma | 1.00% (75) | 0.56% (18) | 0.48% (17) | .0048b | .7354 | .0231b |
| Wound dehiscence | 0.24% (18) | 0.44% (14)a | 0.71% (25) | .0004b | .1505 | .1198 |
| Superficial | 0.15% (11) | 0.16% (5) | 0.34% (12) | .0445b | .1502 | 1.0000 |
| Deep | 0.09% (7) | 0.25% (8) | 0.37% (13) | .0029b | .5126 | .0850 |
| Surgical site infection | 0.61% (46)a | 0.69% (22)a | 0.51% (18) | .5914 | .4278 | .6909 |
| Superficial | 0.20% (15) | 0.19% (6) | 0.14% (5) | .6346 | .7663 | 1.0000 |
| Deep | 0.31% (23) | 0.44% (14) | 0.28% (10) | 1.0000 | .3135 | 0.2866 |
Values are % (n). aSome occurrences were not classified as “superficial” or “deep” within the Tracking Operations and Outcomes for Plastic Surgeons database. bStatistically significant.
In comparison to implant exchange, seroma rates were significantly greater for both capsulectomy (P < .0001) and capsulotomy (P = .0307). Hematoma rates of capsulectomy were significantly greater than the control (P = .0048) and capsulotomy cohorts (P = .0231); however, no differences were noted between capsulotomy and controls (P = .7354). Interestingly, controls had significantly higher rates of dehiscence when compared to capsulectomy (P = .0004), but not to capsulotomy (P = .1505). Similar surgical site infection profiles were observed across all groups. A comprehensive summary of outcomes and statistical analyses is presented in Tables 1 and 2.
Subgroup Analysis: Aesthetic vs Reconstructive Surgical Context
Capsulectomy and capsulotomy patients were further stratified based on whether breast implants were initially placed for aesthetic or reconstructive indications. Comparative breakdowns of demographic and procedure-related characteristics in this context are presented in Supplemental Tables 3 and 4.
Systemic Complications
The overall complication rate was found to be 4.34% (n = 166/3826) in aesthetic patients and 6.76% (n = 163/2412) in reconstructive patients; this difference was statistically significant (P < .0001). Reconstructive capsulectomies and capsulotomies were associated with greater needs for postoperative antibiotics (P < .0001), with a greater proportion of antibiotics administered intravenously (P = .0229). Significantly greater incidences of unplanned ER visits (P < .0001) and unplanned readmissions (P < .0001) were seen among reconstructive patients, whereas unplanned reoperation rates did not significantly differ between reconstructive and aesthetic patients (P = .1163). Other major systemic complications, such as pneumothoraces, DVT/PE, significant bleeding requiring transfusion, as well as cardiac, renal, neurologic, and septic events, remained low (< 0.10%), and were not significantly different between the 2 patient populations (Table 3).
Table 3.
Aesthetic vs Reconstructive Subgroup Analysis: Systemic Complications per Patient
| Aesthetic (N = 3826) | Reconstructive (N = 2412) | P-value | |
|---|---|---|---|
| Any adverse event | 4.34% (166) | 6.76% (163) | <.0001b |
| DVT or PE | 0.03% (1) | 0.08% (2) | .5635 |
| Pneumothorax | 0.05% (2) | 0.00% (0) | .5256 |
| Bleeding requiring transfusion | 0.03% (1) | 0.08% (2) | .5635 |
| Cardiac complications | 0.00% (0) | 0.08% (2) | .1495 |
| Respiratory complications | 0.03% (1) | 0.08% (2) | .5635 |
| Need for antibiotics | 0.94% (36) | 2.20% (53) | <.0001b |
| Oral | 88.89% (32) | 66.04% (35) | .0229b,a |
| Intravenous | 11.11% (4) | 33.96% (18) | |
| Unplanned ER visit | 0.03% (1) | 0.54% (13) | <.0001b |
| Unplanned hospital admission | 0.16% (6) | 1.04% (25) | <.0001b |
| Unplanned return to OR | 1.57% (60) | 2.11% (51) | .1163 |
Values are % (n). DVT, deep vein thrombosis; ER, emergency room; OR, operating room; PE, pulmonary embolism. aComparison of oral/intravenous distribution between patient cohorts. bStatistically significant.
Breast-Specific Complications
Regarding breast-specific complications (Table 4), capsulectomies and capsulotomies in reconstructive patients were associated with significantly greater rates of seroma (1.06% vs 0.47%; P = .0016), wound dehiscence (0.46% vs 0.14%; P = .0059), surgical site infection (1.03% vs 0.23%; P < .0001), and breast implant loss (0.52% vs 0.23%; P = .0401). Hematoma rates, however, did not differ between aesthetic and reconstructive patients (P > .9999).
Table 4.
Aesthetic vs Reconstructive Subgroup Analysis: Breast-Related Complications per Breast
| Aesthetic (N = 5554) | Reconstructive (N = 3488) | P-value | |
|---|---|---|---|
| Seroma | 0.47% (26) | 1.06% (37) | .0016c |
| Hematoma | 0.90% (50) | 0.92% (32) | >.9999 |
| Wound dehiscence | 0.14% (8)a | 0.46% (16) | .0059c |
| Superficial | 57.14% (4) | 50.00% (8) | 1.0000b |
| Deep | 42.86% (3) | 50.00% (8) | |
| Surgical site infection | 0.23% (13) | 1.03% (36)a | <.0001c |
| Superficial | 30.77% (4) | 29.41% (10) | 1.0000b |
| Deep | 69.23% (9) | 70.59% (24) | |
| Implant/prosthesis loss | 0.23% (13) | 0.52% (18) | .0401c |
Values are % (n). aSome occurrences were not classified as “superficial” or “deep” within the Tracking Operations and Outcomes for Plastic Surgeons database. bComparison of superficial/deep distribution between patient cohorts. cStatistically significant.
DISCUSSION
TOPS-derived patient cohorts provide evidence-based data elucidating 30-day outcome profiles of capsulectomies and capsulotomies. In contrast to other large-scale reports on capsulectomy complication rates,16,17 this study benefits from a substantial population pool across a wide time interval, and is the first to stratify and report on outcomes according to the surgical context (reconstructive vs aesthetic) and specific capsulectomy type (total vs partial), to better inform surgeons and their patients on the risks associated with these procedures.
Nationally, nearly 365,000 breast augmentations were performed in 2021, representing a 44% increase from 2020.23 The number of implant-based breast reconstructions is also on the rise, concordant with ongoing paradigm shifts in breast reconstruction practices favoring an alloplastic approach.24,25 Consequently, an increasingly large pool of patients—reconstructive and aesthetic alike—are faced with a myriad of benign and malignant implant-related pathologies, the management of which virtually always involves capsulectomy or capsulotomy.26 Gaining insight into the complication profile of these procedures is therefore imperative, specifically with respect to the initial indication for breast implant placement that may affect incidence, as is the case with reconstructive implant use and capsular contracture.27
Our analyses revealed higher overall complication rates for capsulectomies than presently reported in the literature. Herein, we report an overall complication rate of 5.37% (n = 402/7486) across all capsulectomy procedure variants examined; previous studies examining data from the NSQIP and the CosmetAssure databases estimated the incidence at 3.0% and 2.8%, respectively.16,17 In contrast to the NSQIP database, TOPS captures a more extensive collection of outcome variables.16,28 CosmetAssure is limited by reports on major complications of aesthetic surgeries necessitating inpatient management only, potentially overlooking minor yet clinically relevant complications, such as seroma and hematoma, thus leading to underestimation of the incidence of complications.17,29 Complication rate estimates from TOPS are thus more likely to capture outcome incidences encountered in practice, and thus have greater applicability for patient counseling and informed consent.
Complete Capsulectomy vs Partial Capsulectomy/Capsulotomy
It has repeatedly been theorized that technical variants of capsulectomy could have distinct complication rates,16-18,30 and data from our study support this theory. Capsulectomy was associated with a significantly greater overall complication rate than capsulotomy (5.79% vs 4.50%, P = .0216), likely attributed to the more extensive pericapsular dissection entailed. Additional dissection risks greater lymphatic and vascular injury, and greater shearing of tissues—all of which may contribute to greater seroma and hematoma rates. Indeed, capsulectomy had a 1.8-fold increase in hematoma rates (1.00% vs 0.56%) compared with capsulotomy, and a 3.6-fold increase in seroma rates (0.83% vs 0.23%) compared with controls. Capsulotomy, however, did not exhibit significantly higher overall complication rates in comparison to implant exchange controls.
In absolute terms, rates and differences remained low, with an absolute risk increase from capsulotomy to capsulectomy amounting to 1.29%, translating into a number needed to harm of 77.5 patients. Additionally, these numbers largely fall within ranges reported in the literature, with a retrospective cohort of BII patients receiving capsulectomy demonstrating respective rates of overall complications, hematoma, seroma, and infection of 5.65%, 2.42%, 2.02%, and 1.21% (vs 5.79%, 1.00%, 0.83%, and 0.61% from our capsulectomy cohort, respectively).14 More extensive capsulectomy should thus still be given full consideration by the plastic surgeon under the appropriate clinical indications. For instance, for patients with BIA-ALCL, en bloc capsulectomy is warranted and indicated given its established benefit in this context.12,31 Conversely, for patients with BII, or other conditions for which a clear consensus on optimal treatment is lacking, the added risk of complete capsulectomy, relative to partial, may thus warrant more careful deliberation.5,32-35 Ultimately, preoperative risk assessment should involve appraisal of the impact of both statistical differences in complication rates and clinical implications of these procedures on the patient. Such an approach would pave way for streamlined, individualized, and evidence-based discussions during informed consent.
The higher rates of deep dehiscence in the capsulotomy cohort relative to the capsulectomy cohort may be explained by a potentially higher proportion of submuscular implants in the former group. Indeed, it has been recommended that implants placed in the deeper submuscular plane be managed preferentially with an anterior, partial capsulectomy in order to avoid inadvertent damage posterior to the chest wall.5,9,36,37 Moreover, in partial capsulectomy, capsular remnants may serve as a nidus for bacterial colonization and lead to higher infection rates than complete capsular removal. A preponderance of submuscular implant placement in the capsulotomy group could also account for its higher but statistically insignificant rates of deep surgical site infections, correlating with the slightly higher rates of postoperative antibiotic administration observed. Nonetheless, infection rates across all groups fell within the lower bounds of previously reported estimates for breast procedures.38 Unfortunately, the TOPS database currently lacks information pertaining to the plane of initial implant insertion in which capsulectomy is performed, which may also have an impact on observed outcomes.
Paradoxically higher dehiscence rates in the control cohort are likely multifactorial. Upon further investigation, 24% of dehiscences observed in the control cohort (vs 5.56% in the capsulectomy cohort and 7.69% in the capsulotomy cohort) occurred in previously dehisced or irradiated breasts, both well-established predisposing factors.39,40 Additional confounders included a greater proportion of control cases being performed in the hospital setting (63.93%, vs 36.95% for capsulectomy and 37.85% for capsulotomy), and higher control BMI and ASA scores. Future studies should attempt to elucidate the specific influence of these factors on dehiscence.
Aesthetic vs Reconstructive Capsulectomy/Capsulotomy
Breast capsular surgery performed in reconstructive patients presents with additional layers of surgical complexity, with unique challenges including thin mastectomy flaps, absent gland buffering between the skin and the capsulectomy plane, the presence of irradiation history predisposing to fibrosis, and poorer skin and tissue quality, as well as surgical trauma from the index mastectomy.
Demographic stratification demonstrated that the reconstructive population had an ASA class distribution skewed towards higher scores relative to aesthetic subjects. Furthermore, mastectomies represent a source of local tissue inflammation that may jeopardize subsequent capsular surgery at the same site.41,42 Incrementation in ASA class has been shown to engender stepwise increases in the frequency of postoperative complications and readmissions in outpatient surgeries, both of which can be seen in reconstructive capsulectomies and capsulotomies.43
Irradiation history was exclusively found within the reconstructive population (1.82% vs 0.00%, P < .0001), with a likely underreported prevalence given the nonmandatory nature of radiation status reporting in TOPS.22 Nevertheless, it is well documented that radiation induces fibrosis, leading to poor skin and soft tissue quality, contributing to complications.40 With increases in the use of radiation therapy in breast reconstruction over the years, more radiated patients are expected to present for capsular release or removal, all the more so since radiation is a long-established risk factor for capsular contracture.44-46
Mastectomy in reconstructive patients engenders tissue separation and thus dead space creation, as well as lymphatic injury in the case of axillary surgery, making these patients more prone to seromas during subsequent capsular surgery.47,48 Indeed, seroma incidence was significantly greater for patients receiving both capsulectomy and capsulotomy with a reconstructive indication of index implant placement (1.06% vs 0.47%, P = .0016). In parallel, pooled serous collections may act as a nidus for subsequent infections. Indeed, occurrence rates of surgical site infections in the reconstructive cohort saw a nearly 4.5-fold increase (1.03% vs 0.23%, P < .0001) relative to the aesthetic cohort, which may be additionally explained by the longer mean duration of surgery in the reconstructive cohort (95.60 minutes vs 82.90 minutes; P < .0001), a known predisposing factor.49 This in turn correlated with increased need for antibiotic administration following reconstructive capsulectomies and capsulotomies (P < .0001).
Higher incidences of wound dehiscence (P = .0059) and implant loss (P = .0401) in reconstructive patients are likely consequences of absent native glandular buffering—accentuated by possible prior chest irradiation—leading to thin mastectomy skin flaps with compromised vascularity and wound healing capabilities. As a consequence, there is decreased physical support for the reinserted implant, as well as increased risk for off-plane dissection that would further degrade tissue quality. In addition, seromas and surgical site infections, to which reconstructive patients are already predisposed, may also contribute to dehiscence and implant loss. Altogether, postoperative complications exist in a largely intertwined fashion, whereby a complicated patient becomes prone to subsequently cascading complications, incurring considerable burden that disproportionately falls onto reconstructive patients.50
Special Consideration: Pneumothorax
Pneumothorax may arguably represent the most feared complication of capsulectomy by virtue of the anatomical proximity to the pleura.51-53 Afshari et al extrapolated a maximum incidence of 0.2% from CosmetAssure data without having access to pneumothorax as an explicit variable.17 In comparison, our TOPS data explicitly report pneumothorax rates of 0.06% for capsulectomy compared with 0% for capsulotomy; subgroup analysis revealed pneumothorax rates of 0.05% and 0% in reconstructive and aesthetic patients, respectively. More frequent postoperative ER visits in the capsulectomy cohort (P = .0291) and more frequent postoperative readmissions among reconstructive patients (P < .0001), may complementarily support higher occurrences in these groups, given the urgent and often inpatient nature of pneumothorax management.54,55
Study Limitations
Our study is not without its limitations. The self-reported nature of cases examined introduces room for selection bias and reporting errors.56 TOPS data also suffered from missing preoperative information in some cases, such as ASA classification, BMI, as well as smoking and diabetes status, all of which could impact demographic representation of study groups and interpretation of findings.56 Moreover, TOPS did not ensure rigorous reporting of irradiation history and pneumothorax, likely yielding underestimated incidence rates. Additionally, the TOPS database is devoid of data regarding the plane of initial implant insertion and subsequent capsular surgery, preventing further subgroup analysis in this context. The CPT codes used to identify surgical procedures, while successfully isolating complete capsulectomy (CPT 19371), failed to stratify partial capsulectomy from capsulotomy (both under CPT 19370),20,21 which are inherently different procedures. Further, CPT 19370 may be incorrectly reported in the context of incidental capsulotomy, or incorrectly omitted due to denial by insurance providers, both potentially skewing surgical case stratification.56 Indeed, the latter case may have resulted in control cases that in reality underwent capsulotomy, as suggested by the control group's elevated mean surgical duration (Supplemental Table 2). Without access to original patient charts, the true extent of capsular manipulation could not be verified. Future studies are encouraged to employ chart review or prospective observation methodologies to further elaborate our findings. Nonetheless, our study remains successful in providing the most comprehensive and evidence-based insight into capsulectomy and capsulotomy complication rates, with a comparative profile between aesthetic and reconstructive patients.
CONCLUSIONS
Surging patient demands for breast implant capsulectomy and capsulotomy reveal a need for high-quality, reliable data regarding their complication profiles for evidence-based discussions during informed consent. The present study demonstrates that, overall, surgery on the breast capsule is safe, despite the extent of capsular work, although complete capsulectomies, and capsulectomies performed on reconstructive patients, were found to carry greater risk. Reconstructive patients are at greater risk for complications than those receiving breast implants for aesthetic purposes. Plastic surgeons must thus firmly grasp procedural and population factors influencing complications in capsular surgery, as presented herein, in order to optimally navigate preoperative discussions and ensure delivery of evidence-based and patient-centered plastic surgical care.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Supplementary Material
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. Tang SYQ, Israel JS, Afifi AM. Breast implant illness: symptoms, patient concerns, and the power of social media. Plast Reconstr Surg. 2017;140(5):765e–766e. doi: 10.1097/prs.0000000000003785 [DOI] [PubMed] [Google Scholar]
- 2. Keane G, Ha CD, Myckatyn AY, M T. En bloc capsulectomy for breast implant illness: a social media phenomenon? Aesthet Surg J. 2021;41(4):448–459. doi: 10.1093/asj/sjaa203 [DOI] [PubMed] [Google Scholar]
- 3. Glicksman C, McGuire P, Kadin M, et al. Impact of capsulectomy type on post-explantation systemic symptom improvement: findings from the ASERF systemic symptoms in women-biospecimen analysis study: part 1. Aesthet Surg J. 2022;42(7):809–819. doi: 10.1093/asj/sjab417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheflan M, Gronovich Y, Maisel Lotan A, Winder G. What 736 plastic surgeons think about explantation and capsulectomy: a global opinion poll. Plast Reconstr Surg. 2022;149(6):1071e–1079e. doi: 10.1097/prs.0000000000009090 [DOI] [PubMed] [Google Scholar]
- 5. Wan D, Rohrich RJ. Revisiting the management of capsular contracture in breast augmentation: a systematic review. Plast Reconstr Surg. 2016;137(3):826–841. doi: 10.1097/01.prs.0000480095.23356.ae [DOI] [PubMed] [Google Scholar]
- 6. Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg. 2013;132(5):1128–1137. doi: 10.1097/PRS.0b013e3182a4c243 [DOI] [PubMed] [Google Scholar]
- 7. Magnusson MR, Cooter RD, Rakhorst H, McGuire PA, Adams WP Jr, Deva AK. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;143(3S):74s–81s. doi: 10.1097/prs.0000000000005573 [DOI] [PubMed] [Google Scholar]
- 8. Swanson E. Open capsulotomy: an effective but overlooked treatment for capsular contracture after breast augmentation. Plast Reconstr Surg Glob Open. 2016;4(10):e1096. doi: 10.1097/gox.0000000000001096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young VL. Guidelines and indications for breast implant capsulectomy. Plast Reconstr Surg. 1998;102(3):884–891. doi: 10.1097/00006534-199809030-00043 [DOI] [PubMed] [Google Scholar]
- 10. Metzinger SE, Homsy C, Chun MJ, Metzinger RC. Breast implant illness: treatment using total capsulectomy and implant removal. ePlasty. 2022;22:e5. [PMC free article] [PubMed] [Google Scholar]
- 11. American Society of Plastic Surgeons . ASPS statement on breast implant associated-squamous cell carcinoma (BIA-SCC). Accessed January 3, 2024. https://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/asps-statement-on-breast-implant-associated-squamous-cell-carcinoma
- 12. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Supplement_1):S3–s13. doi: 10.1093/asj/sjy331 [DOI] [PubMed] [Google Scholar]
- 13. Turton P, El-Sharkawi D, Lyburn I, et al. UK guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA) Plastic, Reconstructive and Aesthetic Surgery Expert Advisory Group (PRASEAG). J Plast Reconstr Aesthet Surg. 2021;74(1):13–29. doi: 10.1016/j.bjps.2020.10.064 [DOI] [PubMed] [Google Scholar]
- 14. Katsnelson JY, Spaniol JR, Buinewicz JC, Ramsey FV, Buinewicz BR. Outcomes of implant removal and capsulectomy for breast implant illness in 248 patients. Plastic Reconstr Surg Glob Open. 2021;9(9):e3813. doi: 10.1097/gox.0000000000003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swanson E. Evaluating the necessity of capsulectomy in cases of textured breast implant replacement. Ann Plast Surg. 2020;85(6):691–698. doi: 10.1097/sap.0000000000002301 [DOI] [PubMed] [Google Scholar]
- 16. Abi-Rafeh J, Safran T, Winocour S, Dionisopoulos T, Davison P, Vorstenbosch J. Complications of capsulectomies: an analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. Aesthet Surg J Open Forum. 2022;4:ojac025. doi: 10.1093/asjof/ojac025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Afshari A, Nguyen L, Glassman GE, Perdikis G, Grotting JC, Higdon KK. Incidence and preoperative risk factors for major complications after capsulectomy: analysis of 3048 patients. Aesthet Surg J. 2022;42:603–612. doi: 10.1093/asj/sjac004 [DOI] [PubMed] [Google Scholar]
- 18. Abi-Rafeh J, Safran T, Winocour S, Dionisopoulos T, Davison P, Vorstenbosch J. Lack of evidence on complication profile of breast implant capsulectomy: a call to action for plastic surgeons. Plast Reconstr Surg. 2021;148(1):157e–158e. doi: 10.1097/prs.0000000000008010 [DOI] [PubMed] [Google Scholar]
- 19. The Plastic Surgery Foundation . Tracking Operations and Outcomes for Plastic Surgeons. Accessed January 3, 2024. https://www.thepsf.org/research/registries/tops
- 20. American Medical Association . CPT® 2022 Professional Edition, 4th ed. American Medical Association; 2021. [Google Scholar]
- 21. Kozlow J, Adler E, French C. CPT Corner: A Look at New Changes Coming to E&M and Breast Coding in 2021. Accessed January 3, 2024. https://www.plasticsurgery.org/documents/medical-professionals/health-policy/PSN-CPT-Corner_Dec-20.pdf
- 22. The Plastic Surgery Foundation . Additional TOPS Resources: ASPS-TOPS Case Report Form. Accessed January 3, 2024. https://www.thepsf.org/documents/Research/Registries/TOPS/TOPS-Case-Report-Form.pdf
- 23. Aesthetic plastic surgery national databank statistics 2022. Aesthet Surg J. 2023; 43(Supplement_2):1–19. doi: 10.1093/asj/sjad354 [DOI] [PubMed] [Google Scholar]
- 24. American Society of Plastic Surgeons . 2020 Plastic Surgery Statistics Report. https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
- 25. Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde [DOI] [PubMed] [Google Scholar]
- 26. Vorstenbosch J, Chu JJ, Ariyan CE, McCarthy CM, Disa JJ, Nelson JA. Clinical implications and management of non–BIA-ALCL breast implant capsular pathology. Plast Reconstr Surg. 2023;151(1):20e–30e. doi: 10.1097/prs.0000000000009780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marques M, Brown SA, Oliveira I, et al. Long-term follow-up of breast capsule contracture rates in cosmetic and reconstructive cases. Plast Reconstr Surg. 2010;126(3):769–778. doi: 10.1097/PRS.0b013e3181e5f7bf [DOI] [PubMed] [Google Scholar]
- 28. McNeely ML, Binkley JM, Pusic AL, Campbell KL, Gabram S, Soballe PW. A prospective model of care for breast cancer rehabilitation: postoperative and postreconstructive issues. Cancer. 2012;118(8 Suppl):2226–2236. doi: 10.1002/cncr.27468 [DOI] [PubMed] [Google Scholar]
- 29. Valente DS, Kaye AE, Simmons CJ, Zanella RK, Pannucci CJ. Leveraging the tracking operations and outcomes for plastic surgeons database for plastic surgery research: a “How-To” guide. Plast Reconstr Surg. 2021;148(5):735e–741e. doi: 10.1097/prs.0000000000008483 [DOI] [PubMed] [Google Scholar]
- 30. McGuire P. Commentary on: complications of capsulectomies: an analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Aesthet Surg J Open Forum. 2022;4:ojac026. doi: 10.1093/asjof/ojac026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vorstenbosch J, Ghione P, Plitas G, et al. Surgical management and long-term outcomes of BIA-ALCL: a multidisciplinary approach. Ann Surg Oncol. 2024;31(3):2032–2040. doi: 10.1245/s10434-023-14636-4 [DOI] [PubMed] [Google Scholar]
- 32. Collis N, Sharpe DT. Recurrence of subglandular breast implant capsular contracture: anterior versus total capsulectomy. Plast Reconstr Surg. 2000;106(4):792–797. doi: 10.1097/00006534-200009020-00006 [DOI] [PubMed] [Google Scholar]
- 33. Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg. 2016;6(2):163–168. doi: 10.21037/gs.2016.09.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGuire P, Glicksman C, Magnusson MR, Deva AK. Systemic symptoms associated with breast implants (SSBI): current evidence shows benefit of implant removal with or without capsulectomy. Aesthet Surg J. 2023;43(9):1057–1060. doi: 10.1093/asj/sjad165 [DOI] [PubMed] [Google Scholar]
- 35. McGuire P, Glicksman C, Ferenz S, et al. Symptom improvement after explantation with no capsulectomy for systemic symptoms associated with breast implants. Aesthet Surg J. 2024;44(8):820–828. doi: 10.1093/asj/sjae034 [DOI] [PubMed] [Google Scholar]
- 36. Spear SL. Capsulotomy, capsulectomy, and implantectomy. Plast Reconstr Surg. 1993;92(2):323–324. doi: 10.1097/00006534-199308000-00018 [DOI] [PubMed] [Google Scholar]
- 37. Hendricks H. Complete submuscular breast augmentation: 650 cases managed using an alternative surgical technique. Aesthetic Plast Surg. 2007;31(2):147–153. doi: 10.1007/s00266-006-0128-2 [DOI] [PubMed] [Google Scholar]
- 38. Abi-Rafeh J, Safran T, Al-Halabi B, Dionisopolous T. Death by implants: critical analysis of the FDA-MAUDE database on breast implant-related mortality. Plast Reconstr Surg Glob Open. 2019;7(12):e2554. doi: 10.1097/gox.0000000000002554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karamanos E, Osgood G, Siddiqui A, Rubinfeld I. Wound healing in plastic surgery: does age matter? An American College of Surgeons National Surgical Quality Improvement Program Study. Plast Reconstr Surg. 2015;135(3):876–881. doi: 10.1097/prs.0000000000000974 [DOI] [PubMed] [Google Scholar]
- 40. Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7(1):162. doi: 10.1186/1748-717x-7-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–S84. doi: 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 42. American Society of Anesthesiologists . Statement on ASA Physical Status Classification System. Accessed January 3, 2024. https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system
- 43. Foley C, Kendall MC, Apruzzese P, Oliveira D, S G. American society of Anesthesiologists Physical Status Classification as a reliable predictor of postoperative medical complications and mortality following ambulatory surgery: an analysis of 2,089,830 ACS-NSQIP outpatient cases. BMC Surg. 2021;21(1):253. doi: 10.1186/s12893-021-01256-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doherty C, Pearce S, Baxter N, et al. Trends in immediate breast reconstruction and radiation after mastectomy: a population study. Breast J. 2020;26(3):446–453. doi: 10.1111/tbj.13500 [DOI] [PubMed] [Google Scholar]
- 45. Rosato RM, Dowden RV. Radiation therapy as a cause of capsular contracture. Ann Plast Surg. 1994;32(4):342–345. doi: 10.1097/00000637-199404000-00002 [DOI] [PubMed] [Google Scholar]
- 46. Lipa JE, Qiu W, Huang N, Alman BA, Pang CY. Pathogenesis of radiation-induced capsular contracture in tissue expander and implant breast reconstruction. Plast Reconstr Surg. 2010;125(2):437–445. doi: 10.1097/PRS.0b013e3181c82d05 [DOI] [PubMed] [Google Scholar]
- 47. Kuroi K, Shimozuma K, Taguchi T, et al. Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol. 2006;36(4):197–206. doi: 10.1093/jjco/hyl019 [DOI] [PubMed] [Google Scholar]
- 48. Al-Hilli Z, Wilkerson A. Breast surgery: management of postoperative complications following operations for breast cancer. Surg Clin North Am. 2021;101(5):845–863. doi: 10.1016/j.suc.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 49. Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect (Larchmt). 2017;18(6):722–735. doi: 10.1089/sur.2017.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gabriel A, Gupta S, Orgill DP. Challenges and management of surgical site occurrences. Plast Reconstr Surg. 2019;143(1S):7S–10S. doi: 10.1097/prs.0000000000005305 [DOI] [PubMed] [Google Scholar]
- 51. Gascoigne AC, Malata CM. Pleural damage during capsulectomy and exchange of long-standing breast implants in Poland syndrome: a cautionary tale. Ann Plast Surg. 2012;69(2):148–151. doi: 10.1097/SAP.0b013e318226b4c4 [DOI] [PubMed] [Google Scholar]
- 52. Osborn JM, Stevenson TR. Pneumothorax as a complication of breast augmentation. Plast Reconstr Surg. 2005;116(4):1122–1126; discussion 1127-8. doi: 10.1097/01.prs.0000179182.58036.a7 [DOI] [PubMed] [Google Scholar]
- 53. Schneider LF, Albornoz CR, Huang J, Cordeiro PG. Incidence of pneumothorax during tissue expander-implant reconstruction and algorithm for intraoperative management. Ann Plast Surg. 2014;73(3):279–281. doi: 10.1097/SAP.0b013e31827e2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–ii31. doi: 10.1136/thx.2010.136986 [DOI] [PubMed] [Google Scholar]
- 55. Tschopp JM, Rami-Porta R, Noppen M, Astoul P. Management of spontaneous pneumothorax: state of the art. Eur Respir J. 2006;28(3):637–650. doi: 10.1183/09031936.06.00014206 [DOI] [PubMed] [Google Scholar]
- 56. Xu HH, Abi-Rafeh J, Davison P, Winocour S, Reece EM, Vorstenbosch J. Prompting rigor in database reporting: working towards higher quality plastic surgery database research. Plast Reconstr Surg. 2023. doi: 10.1097/prs.0000000000011157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.