Abstract
Background:
Biological agents have revolutionized care in specialties such as oncology, immunology, infectious diseases, and genetic disorders, offering targeted actions on specific molecules or select immune cells. Monoclonal antibodies, known for their high specificity and precision, represent one of the most significant and rapidly expanding categories of these agents. Understanding the drug utilization patterns of monoclonal antibodies is crucial to ensure their optimal use, especially given their high cost and potential adverse effects.
Methods:
This analytical cross-sectional study was conducted in a secondary hospital in the United Arab Emirates. Patients of either gender receiving monoclonal antibodies at the study site were included. Treatment patterns, utilization, and factors associated with the discontinuation of monoclonal antibodies were assessed.
Results:
Hyperlipidemia (136, 39.1%) was the most common indication for monoclonal antibodies, followed by prophylaxis of respiratory syncytial virus infection in congenital heart disease (104, 29.9%) and osteoporosis (42, 12.1%). Evolocumab was the most commonly prescribed monoclonal antibody (135, 38.8%), followed by palivizumab (104, 29.9%), and dupilumab (38, 10.9%). The majority of monoclonal antibodies demonstrated a prescribed daily dose to defined daily dose ratio of 1.0, reflecting their appropriate utilization. One hundred twenty-nine patients (37.0%) discontinued their treatment during the study. Patient’s level of education (OR: 0.416, 95% CI: 0.183–0.943, p = 0.036), BMI (OR: 2.358, 95% CI: 1.164–4.777, p = 0.017), number of concomitant medications (OR: 2.457, 95% CI: 1.202–5.025, p = 0.014), and treatment duration (OR: 9.180, 95% CI: 4.909–17.165, p < 0.001) were identified as predictors of discontinuation of monoclonal antibodies.
Conclusion:
This study represents the first comprehensive investigation in the United Arab Emirates focused on treatment patterns, utilization, and discontinuation of monoclonal antibodies among the local population. Monoclonal antibodies were prescribed for the management of a wide range of clinical conditions. The study reports appropriate utilization of most monoclonal antibodies and identifies factors such as patient education level, BMI, concomitant medications, and treatment duration as independent predictors of monoclonal antibody treatment discontinuation.
Keywords: monoclonal antibodies, biological agents, treatment pattern, utilization
Introduction
In recent years, the medical landscape has undergone a profound transformation, shifting from conventional pharmacological interventions to the novel utilization of biological agents. 1 These agents have revolutionized care in specialties such as oncology, immunology, infectious diseases, and genetic disorders, offering targeted actions on specific molecules or select immune cells. 2 Several biological agents, including monoclonal antibodies (mAbs), growth factors, vaccines, receptor fusion proteins, cytokine modulators, kinase inhibitors, Chimeric Antigen Receptor (CAR)-T cell, and enzyme replacement therapies, have been utilized for the management of a wide spectrum of diseases.3,4 Monoclonal antibodies, known for their high specificity and precision, are among the most significant and rapidly expanding categories of these agents. Their ability to be engineered for specific targets provides a distinctive advantage in targeted therapeutic approaches, minimizing off-target interactions and establishing their pivotal role across diverse clinical domains.5,6
To date, U.S. Food and Drug Admistration has approved well over 100 novel mAbs for treating a variety of diseases, including rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, asthma, and various forms of cancer. 7 Monoclonal antibodies can classified based on their source and protein composition into categories such as murine, chimeric, humanized, and fully human antibodies. 8 Additionally, they can be grouped based on their target and mechanism of action into categories such as anti-tumor necrosis factor (anti-TNF), anti-receptor activator of nuclear factor kappa-B ligand (anti-RANKL), anti-interleukin (anti-IL), anti-calcitonin gene-related peptide (anti-CGRP), anti-IgE, anti-respiratory syncytial virus (anti-RSV), and anti-proprotein convertase subtilisin/kexin type 9 (anti-PCSK9).8,9
Anti-TNF mAbs, like adalimumab and infliximab, have substantially enhanced autoimmune disease therapy in rheumatology, particularly in RA and ankylosing spondylitis.10,11 Anti-inflammatory mAbs, such as certolizumab and infliximab, have revolutionized the treatment of IBD, including Crohn’s disease and ulcerative colitis. 12 Management of dermatological disorders such as psoriasis and atopic dermatitis have also witnessed a transformation with mAbs such as secukinumab, ustekinumab, and dupilumab. 13 In the field of oncology, mAbs play a pivotal role in precision medicine, with rituximab, cetuximab, and immune checkpoint inhibitors such as pembrolizumab and nivolumab, serving as cornerstones for targeting specific tumor antigens and bolstering the immune response. 14 Patients with hematologic malignancies have benefited from mAbs such as rituximab and brentuximab, which provides targeted therapy for lymphomas, while romiplostim and eltrombopag have improved thrombocytopenia management. 15 Moreover, omalizumab has been effective in mitigating severe allergic asthma attacks and providing relief for those suffering from allergic rhinitis unresponsive to conventional treatments. 16 While mAbs promise a brighter therapeutic future, they also present challenges, including potential adverse effects, high costs, and the need for stringent monitoring.11,17
Many studies have been conducted worldwide to explore the drug utilization and treatment patterns of mAbs for an array of disease conditions, ranging from RA10,18 and psoriasis, 19 to IBD, 12 asthma, 20 hyperlipidemia, 21 RSV infection, 22 and osteoporosis. 23 Understanding the drug utilization patterns of mAbs is crucial to ensure their optimal use, especially given their high cost and potential adverse effects. Studies have shown varying prescription trends based on disease prevalence, drug availability, physician familiarity, and healthcare guidelines in different regions. Monitoring these patterns can provide insights into the current clinical practice and pave the way for better patient care.18 –21,23 Interestingly, while mAbs are being studied extensively globally, there is a notable lack of studies focusing on their utilization in the United Arab Emirates (UAE). Given the unique healthcare context of the region, this study was conducted to gain insights into the local utilization patterns of mAbs.
Material and methods
Study design and population
This analytical cross-sectional study was conducted between November 2022 and August 2023 in Dibba Hospital, Al Fujairah, United Arab Emirates. All patients of either gender receiving mAbs at the study site were included in the study. Patients with preexisting hepatic or renal dysfunction, those who were pregnant or lactating, and those with incomplete data were excluded from the study. The minimum sample size, considering the anticipated discontinuation rates for mAbs, 24 with a 95% confidence level and a 5% margin of error, the required sample size was determined to be 228. Three-hundred forty-eight patients were included in the study.
Patients’ characteristics
Patients’ demographics and clinical characteristics such as age, gender, weight, height, body mass index, social history, educational status, onset of the disease, disease severity, and comorbid conditions were recorded.
Monoclonal antibodies treatment pattern and utilization
Treatment patterns and utilization were assessed according to the specified study variables such as indications for mAbs, types of mAbs, dose/dosage regimen of mAbs, routes of administration, duration of therapy, monotherapy and combination therapy, number of mAbs prescribed, and concomitant medications. The prescribed daily doses (PDDs) and defined daily doses (DDDs) of mAbs were also to be collected and documented. The DDDs were taken from the WHO Anatomical Therapeutic Chemical/ Defined Daily Doses (ATC/DDD) classification system. 25 The PDDs of the mAbs were compared with their respective DDDs and PDD to DDD ratios were calculated.
Discontinuation of mAbs
Treatment discontinuation was defined when patients on mAbs did not receive a subsequent prescription within the grace period, calculated from the date of the last prescription received. Grace period corresponded to twofold the length of the prescribing interval as per the drug monograph for the mAb.
Data analysis
Data analysis was performed using SPSS version 27.0 (IBM Corporation, Armonk, New York, USA). Categorical variables were described using frequencies and percentages, while continuous variables were presented with median and interquartile range (IQR) along with 95% CIs. Categorical variable compared with the Pearson’s chi-square test, Fisher’s exact test, or Monte Carlo test, where appropriate. Continuous variables were compared using the Mann–Whitney U test. Logistic regression models (univariate and multivariate) were used to identify factors associated with mAbs discontinuation. Results were reported as odds ratios (OR) with 95% CIs. Statistical significance was defined as p < 0.05, with two-tailed tests.
Ethical consideration
The study was approved by the Research and Ethics Committee of RAK Medical and Health Sciences University (RAKMHSU-REC-030-2022/23-PG-P), Ministry of Health and Prevention Research Ethics Committee/RAK Subcommittee (MOHAP/REC/2022/43-2022-PG-P). Formal consent was not required for this type of study as it was an observational, noninterventional study without any direct involvement of the patients. The requirement of written informed consent was waived. All patient data were de-identified prior to analysis, and authors had all necessary administrative permissions to access and publish the data.
Results
Patients’ characteristics
A total of 348 patients (male, 150 (43.1%); female, 198 (56.9%)) with median age of 43.5 years (IQR, 5.0–62.0) years were included in the analysis. Majority of the patients were less than 65 years of age (273, 78.4%) with median BMI of 25.9 kg/m2 (IQR, 19.5–31.0) and with no history of allergies (314, 90.2%). One hundredtwenty-five patients (35.9%) had more than two comorbidities with hypertension being the most common comorbid condition (119, 34.2%). Antihyperlipidemics were the most common (130, 37.4%) concomitant medications followed by antihypertensives (105, 30.2%) and antidiabetics (86, 24.7%). The characteristics of patients overall and stratified by gender are reported in Table 1.
Table 1.
Characteristics of patients overall and stratified by gender.
| Variable | Overall | Gender | p-Value* | |
|---|---|---|---|---|
| Female (n = 198) | Male (n = 150) | |||
| Age, years, median (IQR) | 43.5 (5.0–62.0) | 47.5 (6.0–67.0) | 40.0 (5.0–54.0) | 0.001 (U = 11780.0) |
| Age group, n (%) | ||||
| <65 year | 273 (78.4) | 136 (68.7) | 137 (91.3) | <0.001 |
| ⩾65 year | 75 (21.6) | 62 (31.3) | 13 (8.7) | |
| Ethnicity, n (%) | ||||
| Emirati | 258 (74.1) | 165 (83.3) | 93 (62.0) | <0.001 |
| Non-Emirati | 90 (25.9) | 33 (16.7) | 57 (38.0) | |
| Education status, n (%) | ||||
| Graduation | 90 (25.9) | 60 (30.3) | 30 (20.0) | 0.045 |
| Secondary | 66 (19.0) | 29 (14.6) | 37 (24.7) | |
| Primary | 84 (24.1) | 48 (24.2) | 36 (24.0) | |
| Not available | 108 (31.0) | 61 (30.8) | 47 (31.3) | |
| Tobacco use, n (%) | 34 (9.8) | 4 (2.0) | 30 (20.0) | <0.001 |
| Alcohol use, n (%) | 7 (2.0) | 2 (1.0) | 5 (3.3) | 0.126 |
| Previous allergy, n (%) | 34 (9.8) | 26 (13.1) | 8 (5.3) | 0.015 |
| Type of allergy, n (%) | ||||
| Drug allergy | 22 (6.3) | 19 (9.6) | 3 (2.0) | 0.003 |
| Food allergy | 9 (2.6) | 7 (3.5) | 2 (1.3) | |
| Environmental allergy | 3 (0.9) | 0 (0.0) | 3 (2.0) | |
| BMI, kg/m2, median [IQR] | 25.9 [19.5–31.0] | 27 [20.8–32.4] | 25 [17.9–29.0] | 0.018 (U = 12643.500) |
| Comorbidities, n (%) | ||||
| Diabetes | 107 (30.7) | 66 (33.3) | 41 (27.3) | 0.230 |
| Hypertension | 119 (34.2) | 69 (34.8) | 50 (33.3) | 0.768 |
| Obesity | 19 (5.5) | 14 (7.1) | 5 (3.3) | 0.129 |
| Cardiovascular disease | 54 (15.5) | 28 (14.1) | 26 (17.3) | 0.415 |
| Renal disease | 17 (4.9) | 12 (6.1) | 5 (3.3) | 0.242 |
| Respiratory disease | 25 (7.2) | 17 (8.6) | 8(5.3) | 0.245 |
| Autoimmune disease | 39 (11.2) | 37 (18.7) | 2 (1.3) | <0.001 |
| Psychological disease | 8 (2.3) | 4 (2) | 4 (2) | 0.690 |
| Immunosuppressive disease | 1 (0.3) | 1 (0.5) | 0 (0.0) | 0.383 |
| No. of comorbidities, median (IQR) | 1 (0.0–3.0) | 1 (0.0–4.0) | 1 (0.0–3.0) | 0.107 (U = 13401.500) |
| No. of comorbidities, n (%) | ||||
| None | 134 (38.5) | 72 (36.4) | 62 (41.3) | 0.511 |
| One to two | 89 (25.6) | 50 (25.3) | 39 (26.0) | |
| More than two | 125 (35.9) | 76 (38.4) | 49 (32.7) | |
| Concomitant medications, n (%) | ||||
| Antidiabetics | 86.0 (24.7) | 56 (28.3) | 30 (34.9) | 0.015 |
| Antihypertensives | 105.0 (30.2) | 60 (30.3) | 45 (30) | |
| Antihyperlipidemics | 130.0 (37.4) | 75 (37.9) | 55 (36.7) | |
| Nonsteroidal anti-inflammatory | 64.0 (18.4) | 35 (17.7) | 29 (19.3) | |
| Anticoagulants | 52.0 (14.9) | 23 (11.6) | 29 (19.3) | |
*Pearson’s chi-square test, Fisher’s exact test, Monte Carlo test, Mann–Whitney U test as applicable. Statistically significant values are in bold.
BMI: body mass index; IQR: interquartile range.
Monoclonal antibodies treatment pattern and utilization
The study patients were prescribed mAbs for the management of various disease conditions. Hyperlipidemia (136, 39.1%) was the most common indication for mAbs in our study followed by prophylaxis of RSV infection in congenital heart disease (CHD) (104, 29.9%) and osteoporosis (42, 12.1%). Other indications included atopic dermatitis (16, 4.6%), nasal polyposis (12, 3.4%), osteoarthritis, sinusitis (7, 2%), urticaria (4, 1.1%), RA (3, 0.9%), asthma (3, 0.9%), migraine (1, 0.3%), and bilateral sacroiliitis (1, 0.3%). The median age of the patients at the time of diagnosis (for condition managed by mAb) was 40 years (IQR = 0.5–58.0) and majority presented with severe form of the disease (346, 99.4%).
Regarding the type of mAbs, 219 patients (62.9%) in the study were prescribed fully human mAbs, while the remaining 129 (37.1%) patients were on humanized mAbs. The majority of patients (244, 70.1%) received mAbs subcutaneously, while 104 (29.9%) patients received them intramuscularly. Most of the patients were on short-term (<6 months) treatment (218, 62.6%) with the median treatment length of 4 months (IQR, 1.0–12.0). Related concomitant treatments included nonsteroidal anti-inflammatory drugs (64, 18.4%), corticosteroids (9, 2.6%), and bisphosphonates (2, 0.6%), among others (Supplemental Table 1).
The most commonly prescribed mAb was evolocumab (135, 38.8%) followed by palivizumab (104, 29.9%), dupilumab (38, 10.9%), and denosumab (30, 8.6%) among others (Figure 1). Evolocumab was prescribed for the management of hyperlipidemia among the study patients. The use of palivizumab was specifically associated with the prophylaxis of RSV infections in infants with CHD. Dupilumab was prescribed for various allergic and atopic conditions within the study population, including atopic dermatitis, asthma, sinusitis, and nasal polyposis. Denosumab’s utilization was done for osteoporosis management, with romosozumab also being used for this indication in the study. Secukinumab was administered to patients with psoriasis and RA, while omalizumab’s use in our study spanned indications for asthma and urticaria. The details of indications and dosage regimen of mAbs are reported in Table 2.
Figure 1.
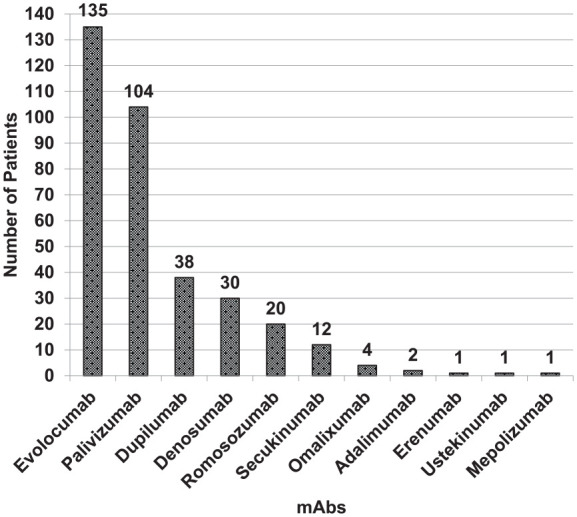
Monoclonal antibodies prescribed in the study.
Table 2.
Monoclonal antibodies, their indications, and dosage regimen.
| Monoclonal antibody | Indication | Dose | Frequency | N (%), (n = 348) |
|---|---|---|---|---|
| Evolocumab | Hyperlipidemia | 140 mg | Q2W | 87 (25.0) |
| Once | 48 (13.8) | |||
| Palivizumab | Congenital heart disease (CHD) respiratory syncytial virus (RSV) infection | 15 mg/kg | Q1M | 99 (28.4) |
| Once | 5 (1.4) | |||
| Dupilumab | Eczema, asthma, sinusitis, nasal polyposis | 200 mg, 300 mg | Q2W | 37 (10.6) |
| Q3W | 1 (0.3) | |||
| Denosumab | Osteoporosis | 60 mg | Once | 12 (3.4) |
| Q6M | 18 (5.2) | |||
| Romosozumab | Osteoporosis | 105 mg, 210 mg | Q1M | 20 (5.7) |
| Secukinumab | Psoriasis, rheumatoid arthritis | 300 mg | Q1W | 1 (0.3) |
| Q4W | 11 (3.2) | |||
| Omalizumab | Asthma, urticaria | 300 mg | Q2W | 1 (0.3) |
| Q6M | 3 (0.9) | |||
| Adalimumab | Rheumatoid arthritis psoriasis | 40 mg | Q2W | 2 (0.6) |
| Erenumab | Migraines | 70 mg | Q1M | 1 (0.3) |
| Ustekinumab | Psoriasis | 45 mg | Q3M | 1 (0.3) |
| Mepolizumab | Asthma | 100 mg | Q4W | 1 (0.3) |
Q1W: every 1 week; Q2W: every 2 weeks; Q4W: every 4 weeks; Q1M: every 1 month; Q6M: every 6 months.
The PDDs for mAbs in our study were determined as follows: evolocumab at 10 mg, palivizumab at 2.93 mg, dupilumab at 21.4 mg, denosumab at 0.33 mg, romosozumab at 7 mg, secukinumab at 10 mg, omalizumab at 21.4 mg, adalimumab at 2.85 mg, erenumab at 2.5 mg, ustekinumab at 0.5 mg, and mepolizumab at 3.60 mg. The majority of mAbs demonstrated a PDD to DDD ratio of 1.0, reflecting their appropriate utilization in our setting. However, notable deviations were observed with adalimumab and ustekinumab, which showed ratios of 0.98 and 0.92, respectively, suggesting a trend toward underutilization. Conversely, omalizumab exhibited a ratio of 1.33, indicative of slight overutilization (Table 3, Figure 2, Supplemental Figure 1).
Table 3.
Defined daily doses and prescribed daily doses of monoclonal antibodies prescribed to the study population.
| Monoclonal antibody | ATC codes | DDD (in mg, parenteral route) | PDD (in mg parenteral route) | PDD/DDD ratio |
|---|---|---|---|---|
| Evolocumab | C10AX13 | 10.0 | 10.0 | 1.0 |
| Palivizumab | J06BD01 | — | 2.93 | — |
| Dupilumab | D11AH05 | 21.4 | 21.4 | 1.0 |
| Denosumab | M05BX04 | 0.33 | 0.33 | 1.0 |
| Romosozumab | M05BX06 | 7.0 | 7.0 | 1.0 |
| Secukinumab | L04AC10 | 10.0 | 10.0 | 1.0 |
| Omalizumab | R03DX05 | 16.0 | 21.4 | 1.33 |
| Adalimumab | L04AB04 | 2.90 | 2.85 | 0.98 |
| Erenumab | N02CD01 | 2.50 | 2.50 | 1.0 |
| Ustekinumab | L04AC05 | 0.54 | 0.50 | 0.92 |
| Mepolizumab | R03DX09 | 3.60 | 3.60 | 1.0 |
DDD: defined daily dose; PDD: prescribed daily dose; ATC: anatomical therapeutic chemical classification.
Figure 2.
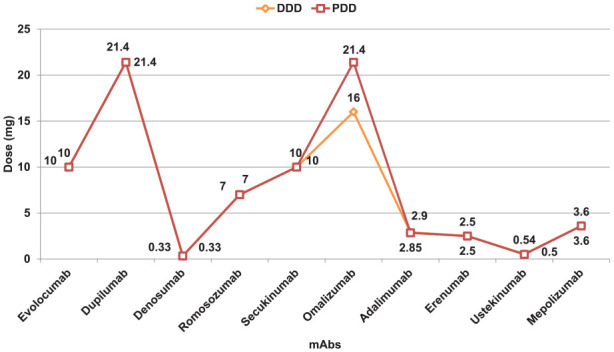
Prescribed daily dose (PDD) and defined daily dose (DDD) comparison of monoclonal antibodies.
Discontinuation of mAbs
In this study, out of 348 patients, 129 (37.0%) discontinued their treatment, while 105 patients (30.2%) completed the prescribed treatment course, and 114 (32.8%) were continuing treatment up to the final data collection point. The reasons for treatment discontinuation varied: 55 patients (15.8%) were lost to follow-up, 24 (6.9%) had changes in their treatment plans, 21 (6.0%) exhibited nonadherence to the treatment, 12 (3.4%) died, 8 (2.3%) showed clinical improvement, 6 (1.7%) discontinued due to personal reasons, and 3 (0.9%) experienced adverse drug reactions. The primary reasons of hospitalization during the treatment were related to infection and acute pain, each accounting for 6.9% of patients followed by hospital admissions for surgical interventions (14, 4.0%), and due to exacerbations of chronic diseases (13, 3.7%).
Factors associated with discontinuation of mAbs
Univariate logistic regression analysis identified several factors potentially associated with the discontinuation of mAbs treatment. Age was a significant factor associated with discontinuation of the treatment. Patients under the age of 65 years had a 2.478 times higher odds of discontinuing the monoclonal antibody treatment compared to those aged 65 years or older (OR: 2.478, 95% CI: 1.468–4.185, p = 0.001). Furthermore, gender differences were associated with discontinuation of treatment. Males had a 2.058 times higher odds of discontinuation compared to females (OR: 2.058, 95% CI: 1.285–3.296, p = 0.003). Education level also appeared to play a role in discontinuation, with those having a graduation level of education less likely to discontinue the treatment compared to those with primary education (OR: 0.345, 95% CI: 0.183–0.650, p = 0.001). BMI was another significant factor. In addition, patients with a BMI less than 25 kg/m2 had a 3.522-fold increased odds of discontinuation compared to those with a BMI of 25 kg/m2 or more (OR: 3.522, 95% CI: 2.141–5.795, p < 0.001).
Among comorbidities, the presence of diabetes, respiratory diseases, and autoimmune diseases were significantly associated with varying odds of discontinuation. Patients with diabetes and respiratory diseases had significantly lower odds of discontinuation compared to those without (diabetes–OR: 0.492, 95% CI: 0.306–0.790, p=0.003; respiratory diseases—OR: 0.352, 95% CI: 0.155–0.803, p = 0.013). Notably, concomitant medications and duration of treatment were strongly associated with discontinuation, with patients on more than two concomitant medications (OR: 4.554, 95% CI: 2.826–7.336, p < 0.001) and receiving short-term treatment (OR: 10.653, 95% CI: 6.321–17.954, p < 0.001) being 4.554 times and 10.653 times, respectively, more likely to discontinue mAbs (Table 4).
Table 4.
Univariate logistic regression model for factors associated with discontinuation of treatment.
| Variable | OR | B | 95% CI | p-Value |
|---|---|---|---|---|
| Age, years | ||||
| ⩾65 | Ref | |||
| <65 | 2.478 | 0.908 | 1.468–4.185 | 0.001 |
| Gender | ||||
| Female | Ref | |||
| Male | 2.058 | 0.722 | 1.285–3.296 | 0.003 |
| Ethnicity | ||||
| Emirati | Ref | |||
| Non-Emirati | 1.369 | 0.314 | 0.808–2.321 | 0.243 |
| Education status | ||||
| Primary | Ref | |||
| Secondary | 0.754 | −0.282 | 0.374–1.521 | 0.430 |
| Graduation | 0.345 | −1.064 | 0.183–0.650 | 0.001 |
| Not available | 1.474 | 0.388 | 0.754–2.881 | 0.257 |
| Allergic history | ||||
| No | Ref | |||
| Yes | 0. 766 | −0.267 | 0.369–1.591 | 0.475 |
| BMI, kg/m2 | ||||
| ⩾25 | Ref | |||
| <25 | 3.522 | 1.259 | 2.141–5.795 | <0.001 |
| Comorbidities | ||||
| Diabetes | Ref | |||
| Absent | 0. 492 | −0.710 | 0.306–0.790 | 0.003 |
| Present | ||||
| Hypertension | ||||
| Absent | Ref | |||
| Present | 0. 634 | −0.456 | 0.398–1.009 | 0.054 |
| Cardiovascular disease | ||||
| Absent | Ref | |||
| Present | 0. 799 | −0.224 | 0.437–1.462 | 0.467 |
| Renal disease | ||||
| Absent | Ref | |||
| Present | 0. 413 | −0.884 | 0.155–1.100 | 0.077 |
| Respiratory disease | ||||
| Absent | Ref | |||
| Present | 0. 352 | −1.043 | 0.155–0.803 | 0.013 |
| Autoimmune disease | ||||
| Absent | Ref | |||
| Present | 0. 328 | −1.116 | 0.166–0.646 | 0.001 |
| No. of comorbidities | ||||
| ⩽2 | Ref | |||
| >2 | 1.955 | 0. 670 | 1.233–3.100 | 0.004 |
| No. of concomitant medications | ||||
| ⩽2 | Ref | |||
| >2 | 4.554 | 1.516 | 2.826–7.336 | <0.001 |
| Age at diagnosis | ||||
| Late onset (⩾40 years) | Ref | |||
| Early onset (40 years) | 2.979 | 1.091 | 1.864–4.761 | <0.001 |
| Type of monoclonal antibody | ||||
| Fully human monoclonal antibody | Ref | |||
| Humanized monoclonal antibody | 5.238 | 1.656 | 2.946–9.315 | <0.001 |
| Length of treatment | ||||
| Long term (⩾6 months) | Ref | |||
| Short term (<6 months) | 10.653 | 2.366 | 6.321–17.954 | <0.001 |
| Hospitalizations during treatment | ||||
| No | Ref | |||
| Yes | 1.880 | 0. 631 | 1.037–3.408 | 0.037 |
OR: odds ratio; CI: confidence interval; B: regression coefficient; BMI: body mass index.
In the multivariate logistic regression model, level of education, BMI, number of concomitant medications, and length of treatment emerged as significant predictors of discontinuation of mAbs. Patients with a graduation level of education were less likely to discontinue treatment compared to those with primary education (OR: 0.416, 95% CI: 0.183–0.943, p = 0.036). Additionally, patients with a BMI less than 25 kg/m2 were 2.358 times more likely to discontinue treatment compared to those with a higher BMI (OR: 2.358, 95% CI: 1.164–4.777, p = 0.017). Furthermore, patients taking more than two concomitant medications (OR: 2.457, 95% CI: 1.202–5.025, p = 0. 014) and receiving short-term treatment (OR: 9.180, 95% CI: 4.909–17.165, p < 0.001) were significantly more likely to their discontinue mAbs treatment (Table 5).
Table 5.
Multivariate logistic regression model for factors associated with discontinuation of treatment.
| Variable | OR | B | 95% CI | p-Value |
|---|---|---|---|---|
| Age, years | ||||
| ⩾65 | Ref | |||
| <65 | 1.115 | 0.109 | 0.464–2.679 | 0.808 |
| Gender | ||||
| Female | Ref | |||
| Male | 1.257 | 0.229 | 0.658–2.402 | 0.489 |
| Education status | ||||
| Primary | Ref | |||
| Secondary | 0.598 | -0.515 | 0.238–1.503 | 0.274 |
| Graduation | 0.416 | -0.878 | 0.183–0.943 | 0.036 |
| Not available | 1.446 | 0.369 | 0.611–3.421 | 0.402 |
| BMI, kg/m2 | ||||
| ⩾25 | Ref | |||
| <25 | 2.358 | 0.858 | 1.164–4.777 | 0.017 |
| Comorbidities | ||||
| Diabetes | Ref | |||
| Absent | 0.826 | -0.191 | 0.358–1.907 | 0.654 |
| Present | ||||
| Respiratory disease | ||||
| Absent | Ref | |||
| Present | 0.534 | -0.627 | 0.184–1.554 | 0.250 |
| Autoimmune disease | ||||
| Absent | Ref | |||
| Present | 0.759 | -0.276 | 0.290–1.987 | 0.574 |
| No. of comorbidities | ||||
| ⩽2 | Ref | |||
| >2 | 2.159 | 0.770 | 0.883–5.279 | 0.092 |
| No. of concomitant medications | ||||
| ⩽2 | Ref | |||
| >2 | 2.457 | 0.899 | 1.202–5.025 | 0.014 |
| Age at diagnosis | ||||
| Late onset (⩾40 years) | Ref | |||
| Early onset (40 years) | 1.555 | 0.441 | 0.719–3.363 | 0.262 |
| Type of monoclonal antibody | ||||
| Fully human monoclonal antibody | Ref | |||
| Humanized monoclonal antibody | 1.432 | 0.359 | 0.596–3.442 | 0.422 |
| Length of treatment | ||||
| Long term (⩾6 months) | Ref | |||
| Short term (<6 months) | 9.180 | 2.217 | 4.909–17.165 | <0.001 |
| Hospitalizations during treatment | ||||
| No | Ref | |||
| Yes | 1.838 | 0.609 | 0.868–3.894 | 0.112 |
OR: odds ratio; CI: confidence interval; B: regression coefficient.
Discussion
This study represents the first comprehensive investigation in the UAE focused on the treatment patterns, utilization, and discontinuation of mAbs among the local population. As such, it provides insights into the use of these advanced therapeutic agents within a specific regional healthcare context. Our analysis captures the use of mAbs across a wide range of clinical conditions, reflecting their growing significance in contemporary clinical practice in the UAE. The findings not only offer a snapshot of current treatment patterns and utilization of mAbs but also shed light on the factors associated with their discontinuation, which is pivotal for optimizing patient outcomes in the domain of mAb treatment.
Evolocumab prescribed for the management of hyperlipidemia was the most commonly used mAb among the study patients. This use pattern of evolocumab for hyperlipidemia within our study population reflects a broader, global shift toward targeted biological therapies for lipid disorders. Evolocumab, a PCSK9 inhibitor, has been recognized for its efficacy in significantly lowering LDL cholesterol levels, especially in patients who are statin-intolerant or have familial hypercholesterolemia. 26 Furthermore, the prevalent use of palivizumab for RSV prophylaxis in infants with CHD in our study can be attributed to several key factors. Infants with CHD are particularly susceptible to severe respiratory complications from RSV, often leading to increased hospitalization and intensive care needs. This vulnerability underscores the importance of preventive strategies, as recommended by the American Academy of Pediatrics, which advocates for the prophylactic use of palivizumab in this high-risk group. 27 Additionally, the distinct seasonality of RSV in the UAE, with peak incidences during the winter months, 28 highlights the necessity for timely and targeted RSV prophylaxis. In this context, our findings reflect the rational use of palivizumab in infants with CHD, adhering to both clinical guidelines and regional epidemiological trends.
Dupilumab’s extensive use in treating atopic dermatitis, asthma, sinusitis, and nasal polyposis in the study is substantiated by its targeted mechanism and efficacy across these conditions. For atopic dermatitis, especially moderate-to-severe atopic dermatitis, dupilumab has become a cornerstone treatment, as recommended by the American Academy of Dermatology (AAD), due to its ability to significantly improve skin lesions and reduce pruritus. 29 In addition to improving these visible symptoms, dupilumab has shown significant impact on subclinical disease activity, a vital aspect of disease management often overlooked. Research utilizing ultra high frequency ultrasound (UHFUS) demonstrated significant decrease in the subepidermal low-echogenic band (SLEB) thickness, which correlates with subclinical inflammation. A study conducted by Dini et al. 30 reported that dupilumab effectively reduced SLEB thickness, vascular signals, and epidermal thickness, providing comprehensive improvements in both visible and subclinical aspects of the disease. These findings highlight dupilumab’s role in managing the broader inflammatory processes characteristic of atopic dermatitis
In asthma, the Global Initiative for Asthma (GINA) guidelines supports the role of dupilumab in decreasing exacerbations and enhancing lung function, particularly in eosinophilic or steroid-resistant forms. 31 Additionally, its effectiveness in chronic rhinosinusitis with nasal polyposis, demonstrated through improved symptom scores and reduced nasal polyp size, 32 aligns with international ENT guidelines.33,34 Similar dupilumab use patterns are reflected in recent drug utilization studies, highlighting its growing prominence in managing these diverse yet related conditions.35,36
Use of denosumab and romosozumab in the management of osteoporosis, as observed in the study, aligns with findings from previous drug utilization studies and international guidelines. Denosumab, recognized for its efficacy in enhancing bone density and reducing fracture risk, is recommended for high-risk osteoporosis patients, particularly postmenopausal women. Its usage patterns, reflected in drug utilization studies, underscore its role as a key therapeutic agent in osteoporosis management.37,38 Similarly, Romosozumab, with its dual action of bone formation stimulation and resorption inhibition, is endorsed for patients with severe osteoporosis or those unresponsive to other treatments. 38 Its growing adoption is supported by its demonstrated efficacy in fracture risk reduction. 39 These mAbs, fitting within the framework of modern osteoporosis management, represent a shift toward more targeted and effective strategies in osteoporosis care.
Our study’s exploration of the PDDs and their comparison with DDDs of different mAbs ventures into an area not extensively reported in literature. It provides new insights, as there are not many studies focusing on PDDs and DDDs of mAbs. In our study, the majority of mAbs showed a PDD to DDD ratio of 1.0, indicative of their appropriate utilization in our clinical setting. This suggests that the prescribing practices related to mAbs at the study site are in accordance with the recommended WHO ATC/DDD system dosing guidelines. 25 However, slight deviations were noted in the cases of adalimumab and ustekinumab, with ratios of 0.98 and 0.92, respectively, hinting at a potential trend of slight underutilization. In contrast, omalizumab showed a ratio of 1.33, indicative of slight overutilization. It is important to note that PDDs may not always correspond to the DDDs, as PDDs can vary based on individual patient characteristics and specific disease factors. These findings provide valuable insights into the real-world prescribing of these mAbs, underscoring the need for continuous evaluation and adjustment of their usage to ensure the most effective and efficient patient care.
Discontinuation of mAb treatment in clinical practice shows significant variability and is influenced by a multitude of factors including specific disease or condition being treated, the type of mAb used, patient demographics, the duration of treatment and follow-up, as well as the reasons for discontinuation, and the criteria used to define it. Previous studies have reported that the discontinuation rates for mAbs can vary widely, ranging from 30% to 75%.40–45 Such disparities underscore the complexity of mAb therapies and highlight the necessity for a comprehensive understanding of treatment adherence and persistence in diverse clinical contexts. In this study, 37.0% of the patients discontinued their treatment. This discontinuation rate is significant yet consistent with the other published studies. The reasons of discontinuation varied ranging from lost to follow-up, changes in treatment plan, nonadherence, clinical improvement to adverse drug reactions.
Regarding the factors associated with discontinuation of mAbs, univariate logistic regression analysis revealed that younger age (less than 65 years), male gender, less BMI, more than two concomitant medications, and short treatment duration were associated with higher odds of discontinuing mAb treatment. Furthermore, higher education (graduation level) and comorbid conditions such as diabetes and respiratory diseases were associated with lower odds of discontinuing mAb treatment. In the multivariate logistic regression model, level of education, BMI, concomitant medications, and short-term treatment emerged as significant predictors of discontinuation of mAb treatment. Similar and contrasting findings related to these factors of discontinuation were reported by different studies in diverse patient populations. A study conducted in patients receiving mAbs for severe asthma reported that younger and male patients were more likely to discontinue their biological treatment. 46 However, older and female patients were more likely to discontinue biologics in a study conducted in patients with IBD. 47
In contrast to our study, higher BMI or obesity was identified as a predictor for discontinuation of biological therapies in studies conducted in psoriasis patients. 48 Studies have reported that decision-making factors for switching between mAbs are crucial for optimizing patient outcomes. Margiotta et al reported that a family history of psoriasis may influence treatment dynamics, potentially serving as a protective factor against therapy switching. 49 This insight suggests that genetic background could play a role in the stability of treatment response, reducing the likelihood of switching due to inadequate control of symptoms or adverse effects. Although our study did not specifically investigate family history of patients, such insights underscore the importance of considering familial disease history when making therapeutic decisions in clinical practice.
Education level is a well-established predictor of therapeutic noncompliance and treatment discontinuation in general, 50 as well as in the context of biological treatments. 51 The observed association between higher education level and lower discontinuation of mAb treatment in our study aligns with existing literature, indicating that educated patients often have better health literacy, and, consequently, an understanding of importance of adhering to treatment regimens. Furthermore, concomitant medications are known to impact adherence and continuation of biological therapies. 52 In our study, number of concomitant medications emerged as a predictor of discontinuation of mAb treatment. This could be attributed to increased medication burden and complexity of treatment regimens associated with the use of multiple medications, which can contribute to nonadherence and subsequent discontinuation.
To enhance adherence to mAbs, particularly in light of the factors identified as predictors of treatment discontinuation in our study, targeted strategies can be implemented. First, patient education programs that improve understanding of treatment benefits and potential side effects could mitigate nonadherence and discontinuation. Second, the management of concomitant medications emerges as crucial, especially given their association with discontinuation rates. Simplifying treatment regimens, where clinically feasible, could reduce the burden of polypharmacy and enhance patient adherence. Furthermore, integrating patient support programs that offer reminders for dosing and provide platforms for patients to report and discuss side effects can also play a significant role. Lastly, involving patients in the decision-making process about their treatment options can foster a greater sense of control and commitment to the prescribed therapy regimen.
The results of this study should be interpreted in light of some limitations. First, being conducted in a single center, the findings might not be generalizable across different regions or healthcare systems. The analytical cross-sectional study design, while effective for assessing prevalence and associations, may not provide insights into temporal relationships or causality due to its inherent nature of data collection. Data collection accuracy, particularly for subjective variables might be influenced by reporting biases. Our definition of treatment discontinuation, based on the absence of a subsequent prescription within a set grace period, may not encompass all complexities of discontinuation reasons. The study’s findings, derived from a specific geographical and clinical setting, might not be universally applicable, as regional differences in patient demographics and healthcare practices could affect the results.
While this study provides a comprehensive snapshot of treatment patterns and discontinuation factors for mAbs, long-term follow-up would have offered deeper insights into the sustained impacts of mAb therapies and patient adherence. However, due to constraints such as the duration of the study period and resource limitations, this was not feasible. Further studies to incorporate extended follow-up phases to capture these dynamics would undoubtedly enrich our understanding of mAb therapies in chronic conditions. These limitations highlight the need for cautious interpretation of the study results and suggest areas for future research to enhance understanding of treatment patterns and utilization of mAbs.
Conclusion
The study offers insights into the current treatment patterns, utilization, and discontinuation of mAbs in a diverse array of clinical conditions. Several mAbs were prescribed to the study patients with evolocumab being the most commonly prescribed mAb, particularly for the treatment of hyperlipidemia. The study’s exploration of the PDDs and their comparison with DDDs of different mAbs ventured into an area not extensively studied. Majority of the mAbs showed a PDD to DDD ratio of 1.0, indicative of their appropriate utilization. Patient factors such as age, gender, education level, BMI, comorbid conditions, and treatment duration significantly influenced the continuation of mAb therapy. Patients’ education level, BMI, concomitant medication, and treatment duration were identified as the independent predictors of discontinuation of mAb treatment. While mAbs offer promising therapeutic benefits, their safe and effective use requires a comprehensive understanding of their utilization patterns, drug-related problems, and factors influencing treatment continuation. This study provides a fundamental understanding of these aspects, paving the way for large-scale multicenter drug utilization studies on mAbs in the region.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121241271817 for Treatment patterns and factors associated with discontinuation of monoclonal antibodies by Muzoon Matar Saleh Alkaabi, Syed Arman Rabbani, Padma GM Rao and Mai Ismail Mohamedelhassan in SAGE Open Medicine
Acknowledgments
The authors extend their gratitude to the administration of Dibba Hospital, Al Fujairah, UAE, for their invaluable support and assistance. The authors would like to acknowledge the support and encouragement from the President, Vice-President for Research, Vice-President for Academic Affairs at RAK Medical and Health Sciences University.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from Research and Ethics Committee of RAK Medical and Health Sciences University (RAKMHSU-REC-030-2022/23-PG-P) and Ministry of Health and Prevention Research Ethics Committee/RAK Subcommittee (MOHAP/REC/2022/43-2022-PG-P).
Informed consent: Formal consent was not required for this type of study as it was an observational, noninterventional study without any direct involvement of the patients. The requirement of written informed consent was waived. All patient data were de-identified prior to analysis, and authors had all necessary administrative permissions to access and publish the data.
Trial registration: Not applicable.
ORCID iD: Syed Arman Rabbani  https://orcid.org/0000-0002-8454-8158
https://orcid.org/0000-0002-8454-8158
Supplemental material: Supplemental material for this article is available online.
References
- 1. Danhof M, Klein K, Stolk P, et al. The future of drug development: the paradigm shift towards systems therapeutics. Drug Discov Today 2018; 23: 1990–1995. [DOI] [PubMed] [Google Scholar]
- 2. Zinn S, Vazquez-Lombardi R, Zimmermann C, et al. Advances in antibody-based therapy in oncology. Nat Cancer 2023; 4: 165–180. [DOI] [PubMed] [Google Scholar]
- 3. Mahler S. Safety of biologics therapy: monoclonal antibodies, cytokines, fusion proteins, hormones, enzymes, coagulation proteins, vaccines, botulinum toxins. MAbs 2017; 9: 885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepard HM, Phillips GL, Thanos CD, et al. Developments in therapy with monoclonal antibodies and related proteins. Clin Med (Northfield Il) 2017; 17: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malik B, Ghatol A. Understanding how monoclonal antibodies work, http://www.ncbi.nlm.nih.gov/pubmed/31820172 (2023, accessed 11 November 2023). [PubMed]
- 6. Kaliyaperumal R, Ranganathan S, Krishnamoorthy M, et al. Current strategy of monoclonal antibody: development, cloning, formulation and drug delivery. Recent Adv drug Deliv Formul.2023; 17(4): 264–285. [DOI] [PubMed] [Google Scholar]
- 7. Martins AC, Albericio F, de la Torre BG. FDA approvals of biologics in 2022. Biomedicines 2023; 11: 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Wang G, Lu H, et al. Development of therapeutic antibodies for the treatment of diseases. Mol Biomed 2022; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinch MS, Kraft Z, Schwartz T. Monoclonal antibodies: trends in therapeutic success and commercial focus. Drug Discov Today 2023; 28: 103415. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease-modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatol Ther 2020; 7: 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng H, Zhao Y, Kuang W, et al. Adverse events of tumor necrosis factor alpha inhibitors for the treatment of ankylosing spondylitis: a meta-analysis of randomized, placebo-controlled trials. Front Pharmacol 2023; 14: 1084614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Degli Esposti L, Daperno M, Dovizio M, et al. A retrospective analysis of treatment patterns, drug discontinuation and healthcare costs in Crohn’s disease patients treated with biologics. Dig Liver Dis 2023; 55: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 13. da Silva DLF, Secamilli EN, Beleli MV, et al. Immunobiologicals in dermatology. An Bras Dermatol 2022; 97: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Effer B, Perez I, Ulloa D, et al. Therapeutic targets of monoclonal antibodies used in the treatment of cancer: current and emerging. Biomedicines 2023; 11: 2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh K, Ghosh K. Monoclonal antibodies used for the management of hemataological disorders. Expert Rev Hematol 2022; 15: 443–455. [DOI] [PubMed] [Google Scholar]
- 16. Menzella F, Just J, Sauerbeck IS, et al. Omalizumab for the treatment of patients with severe allergic asthma with immunoglobulin E levels above >1500 IU/mL. World Allergy Organ J 2023; 16: 100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein RH, Walensky RP. The challenges ahead with monoclonal antibodies. JAMA 2020; 324: 2151. [DOI] [PubMed] [Google Scholar]
- 18. Cruz BH, Garnica IU, Parera RS, et al. Disease-modifying antirheumatic drug prescription patterns in adult rheumatoid arthritis patients in routine clinical practice in Spain. Eur J Rheumatol 2020; 7: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krantz Å, Carrero JJ, Yang Y, et al. Psoriasis/psoriatic arthritis patients’ long-term treatment patterns and adherence to systemic treatments monitoring recommendations. Acta Derm Venereol 2023; 103: adv6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menzies-Gow AN, McBrien C, Unni B, et al. Real world biologic use and switch patterns in severe asthma: data from the international severe asthma registry and the US CHRONICLE study. J Asthma Allergy 2022; 15: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zafrir B, Egbaria A, Stein N, et al. PCSK9 inhibition in clinical practice: treatment patterns and attainment of lipid goals in a large health maintenance organization. J Clin Lipidol 2021; 15: 202–211.e2. [DOI] [PubMed] [Google Scholar]
- 22. Sun M, Lai H, Na F, et al. Monoclonal antibody for the prevention of respiratory syncytial virus in infants and children: a systematic review and network meta-analysis. JAMA Netw Open 2023; 6: E230023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morkos M, Mahrous P, Casagrande A, et al. Patterns of osteoporosis medications selection after drug holiday or continued therapy: a real-world experience. Endocr Pract 2022; 28: 1078–1085. [DOI] [PubMed] [Google Scholar]
- 24. Bakalos G, Zintzaras E. Drug discontinuation in studies including a switch from an originator to a biosimilar monoclonal antibody: a systematic literature review. Clin Ther 2019; 41: 155–173.e13. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. World Health Organization collaborating centre for drug statistics methodology—purpose of the ATC/DDD system, https://www.whocc.no/atc_ddd_methodology/purpose_of_the_atc_ddd_system/ (2023, accessed 11 November 2023).
- 26. Gupta M, Mancini GBJ, Wani RJ, et al. Real-world insights into evolocumab use in patients with hyperlipidemia: Canadian analysis from the ZERBINI study. CJC Open 2022; 4: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Academy of Pediatrics. Updated guidance: use of palivizumab prophylaxis to prevent hospitalization from severe respiratory syncytial virus infection during the 2022-2023 RSV season, https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-for-use-of-palivizumab-prophylaxis-to-prevent-hospitalization/ (2023, accessed 10 November 2023).
- 28. Salim S, Celiloglu H, Tayyab F, et al. Seasonal prevalence of respiratory pathogens among children in the United Arab Emirates: a multicenter cross-sectional study in the Pre-COVID-19 era. Cureus 2023; 15(9): e45204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis DMR, Drucker AM, Alikhan A, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol 2023; 90(2): e43–e56. [DOI] [PubMed] [Google Scholar]
- 30. Dini V, Iannone M, Michelucci A, et al. Ultra-high frequency ultraSound (UHFUS) assessment of barrier function in moderate-to-severe atopic dermatitis during dupilumab treatment. Diagnostics 2023; 13: 2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Global Initiative for Asthma. 2023. GINA main report—Global Initiative for Asthma—GINA, 2023. accessed 11 November 2023. [Google Scholar]
- 32. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–1650. [DOI] [PubMed] [Google Scholar]
- 33. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinol J 2020; 58: 1–464. [DOI] [PubMed] [Google Scholar]
- 34. American Academy of Otolaryngology-Head and Neck Surgery. Clinical Practice Guideline: Adult Sinusitis (Update)—American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS), https://www.entnet.org/quality-practice/quality-products/clinical-practice-guidelines/adult-sinusitis/ (2023, accessed 11 November 2023).
- 35. Sears AV, Woolf RT, Gribaleva E, et al. Real-world effectiveness and tolerability of dupilumab in adult atopic dermatitis: a single-centre, prospective 1-year observational cohort study of the first 100 patients treated. Br J Dermatol 2021; 184: 755–757. [DOI] [PubMed] [Google Scholar]
- 36. Thelen JC, van Zelst CM, van Brummelen SE, et al. Efficacy and safety of dupilumab as add-on therapy for patients with severe asthma: a real-world Dutch cohort study. Respir Med 2023; 206: 107058. [DOI] [PubMed] [Google Scholar]
- 37. Singer AJ, Liu J, Yan H, et al. Treatment patterns and long-term persistence with osteoporosis therapies in women with medicare fee-for-service (FFS) coverage. Osteoporos Int 2021; 32: 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu M, Zhang Y, Guo J, et al. Meta-analysis of the effects of denosumab and romosozumab on bone mineral density and turnover markers in patients with osteoporosis. Front Endocrinol (Lausanne) 2023; 14: 1188969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClung MR, Betah D, Deignan C, et al. Romosozumab efficacy in postmenopausal women with no prior fracture who fulfill criteria for very high fracture risk. Endocr Pract 2023; 29: 716–722. [DOI] [PubMed] [Google Scholar]
- 40. Li P, Blum MA, Von Feldt J, et al. Adherence, discontinuation, and switching of biologic therapies in Medicaid enrollees with rheumatoid arthritis. Value Heal 2010; 13: 805–812. [DOI] [PubMed] [Google Scholar]
- 41. Menne J, Delmas Y, Fakhouri F, et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol 2019; 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol 2016; 74: 1057–1065.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Charleston L, Talon B, Sullivan C, et al. Persistence to anti-CGRP monoclonal antibodies and onabotulinumtoxinA among patients with migraine: a retrospective cohort study. J Headache Pain 2023; 24: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fakhouri F, Fila M, Hummel A, et al. Eculizumab discontinuation in children and adults with atypical hemolytic-uremic syndrome: a prospective multicenter study. Blood 2021; 137: 2438–2449. [DOI] [PubMed] [Google Scholar]
- 45. Nikoloudaki M, Nikolopoulos D, Koutsoviti S, et al. Clinical response trajectories and drug persistence in systemic lupus erythematosus patients on belimumab treatment: a real-life, multicentre observational study. Front Immunol 2023; 13: 1074044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silver J, Bogart M, Molfino NA, et al. Factors leading to discontinuation of biologic therapy in patients with severe asthma. J Asthma 2022; 59: 1839–1849. [DOI] [PubMed] [Google Scholar]
- 47. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 48. Zweegers J, van den Reek JMPA, van de Kerkhof PCM, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol 2016; 175: 340–347. [DOI] [PubMed] [Google Scholar]
- 49. Manzo Margiotta F, Michelucci A, Panduri S, et al. Family history of psoriasis: a novel protective factor for therapy switch in patients treated with Secukinumab or Ixekizumab. J Eur Acad Dermatology Venereol 2022; 36: e454–e456. [DOI] [PubMed] [Google Scholar]
- 50. Li S-C. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag 2008; 4: 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumar A, Kim ES, Kozan P, et al. Patient-preferences favoring treatment discontinuation are reduced with vedolizumab and ustekinumab compared With TNF antagonists in inflammatory bowel disease. Crohn’s Colitis 360 2020; 2: otaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soliman MM, Ashcroft DM, Watson KD, et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011; 70: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121241271817 for Treatment patterns and factors associated with discontinuation of monoclonal antibodies by Muzoon Matar Saleh Alkaabi, Syed Arman Rabbani, Padma GM Rao and Mai Ismail Mohamedelhassan in SAGE Open Medicine


